Towards Construction of the “Periodic Table” of 1-Methylbenzotriazole
Abstract
:1. Introduction
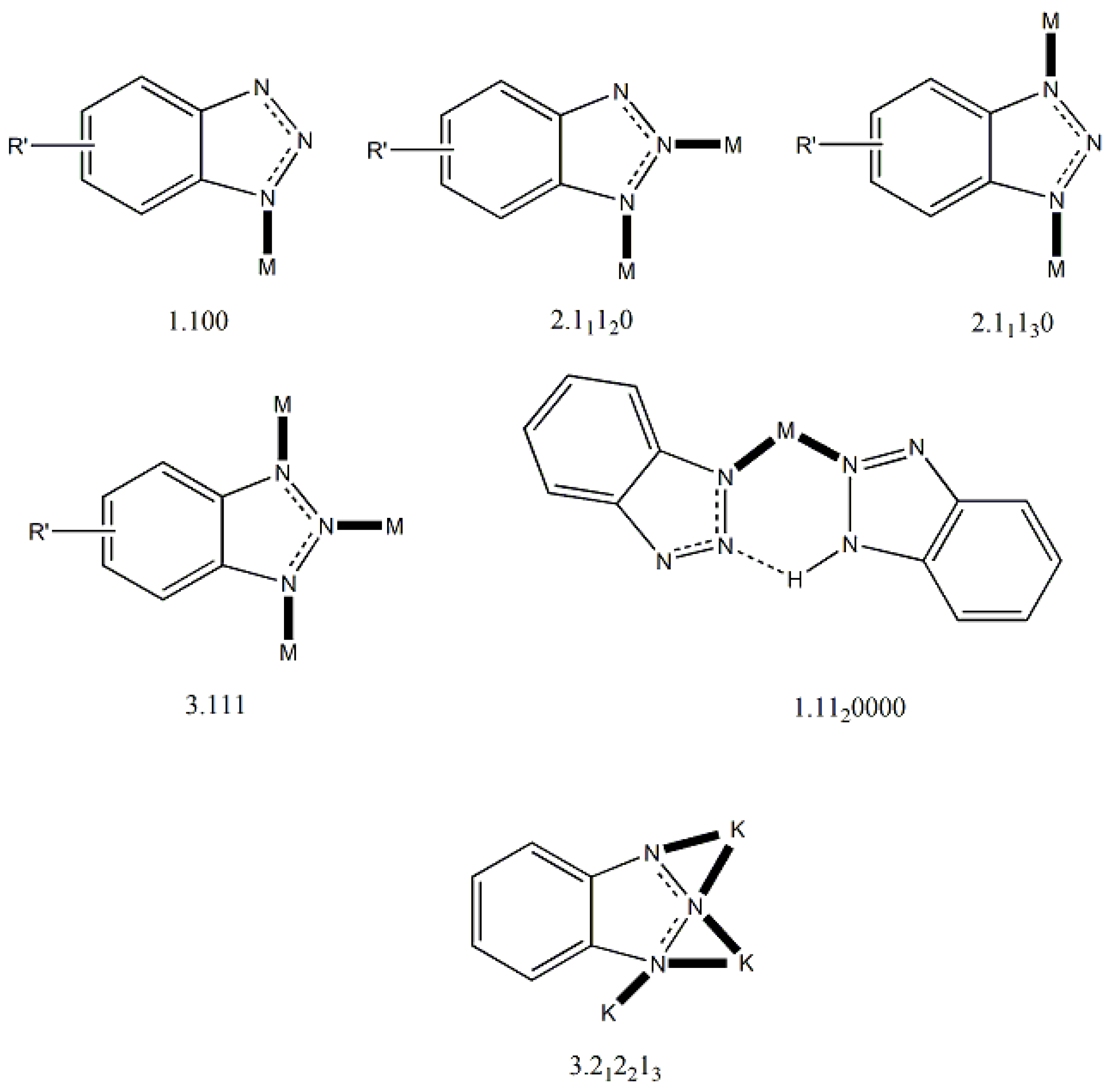
2. Ligands in Coordination Chemistry
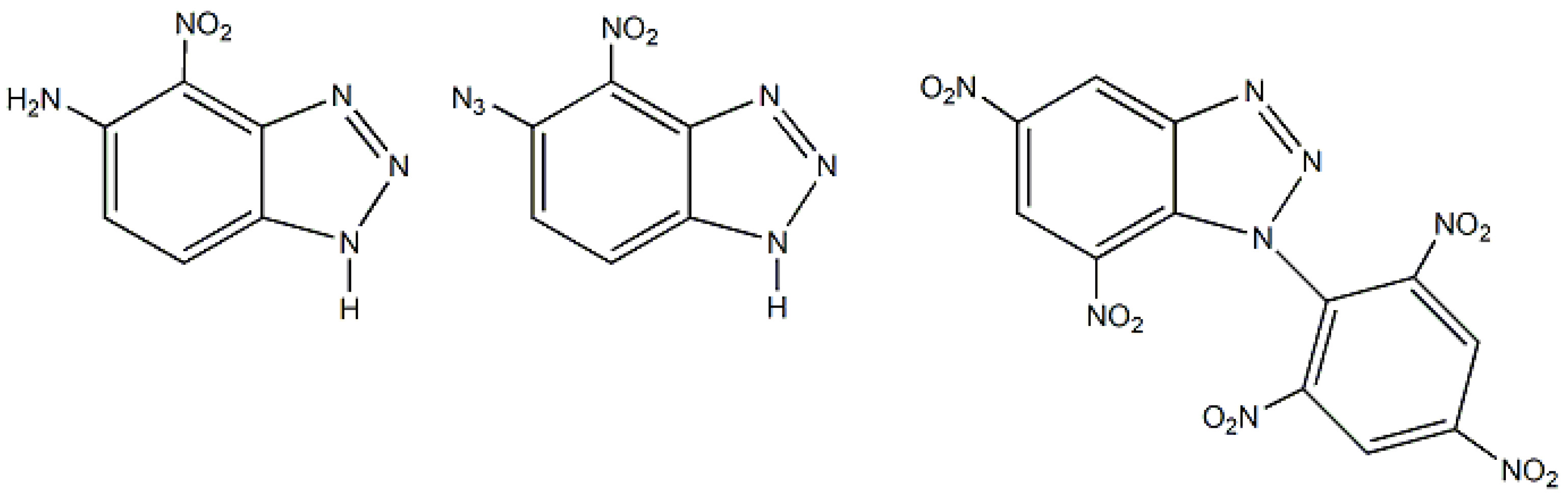
3. Benzotriazoles in Several Branches of Chemistry
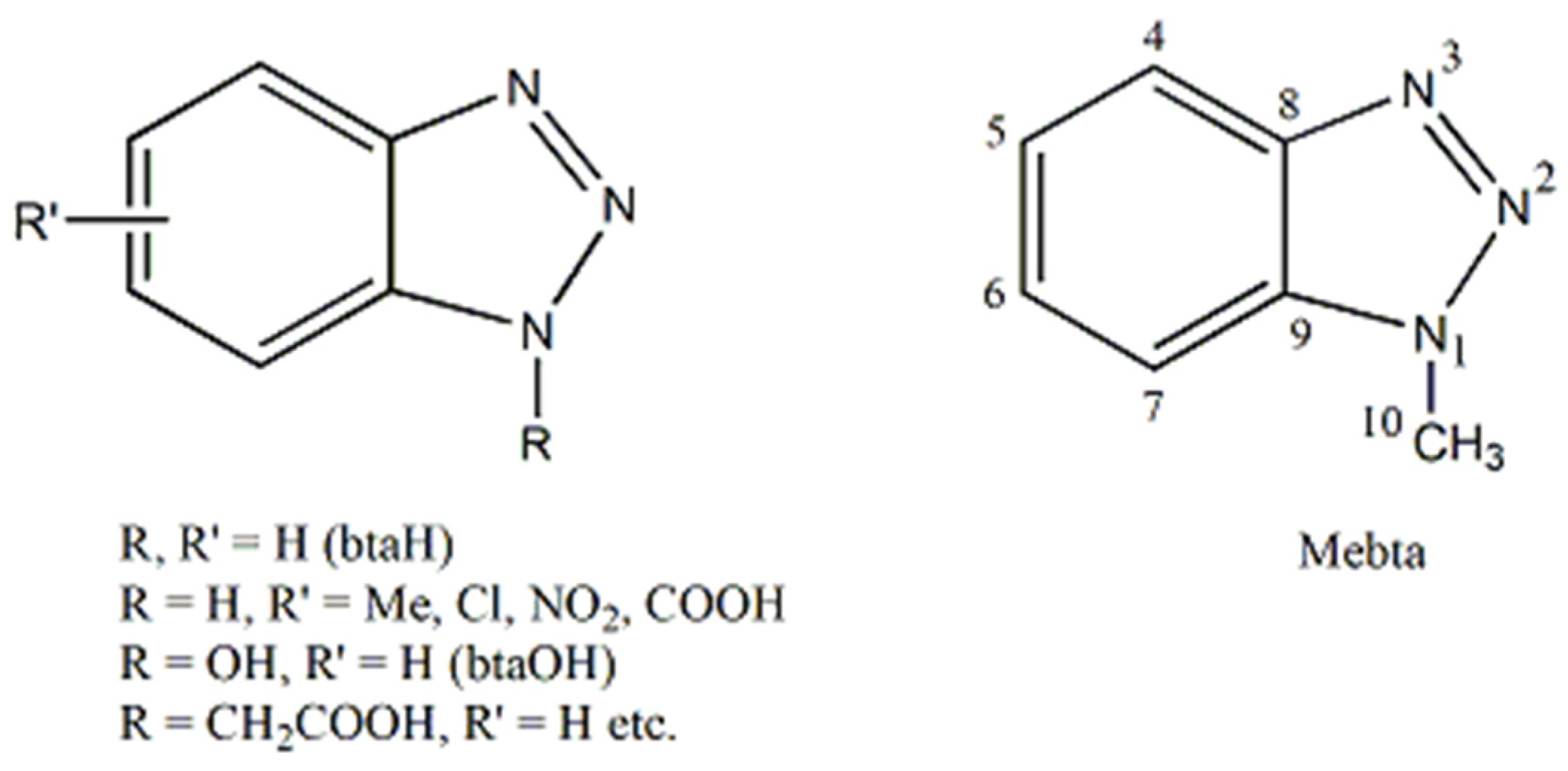
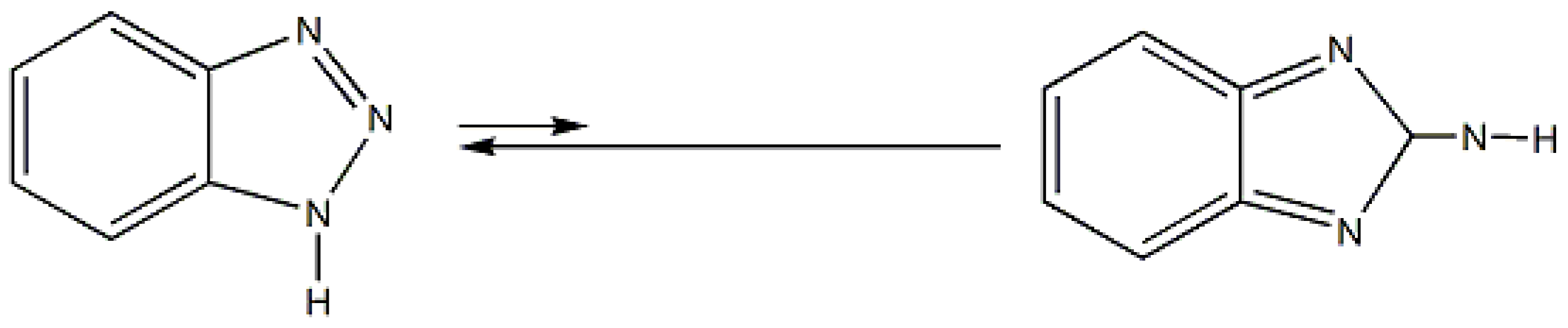
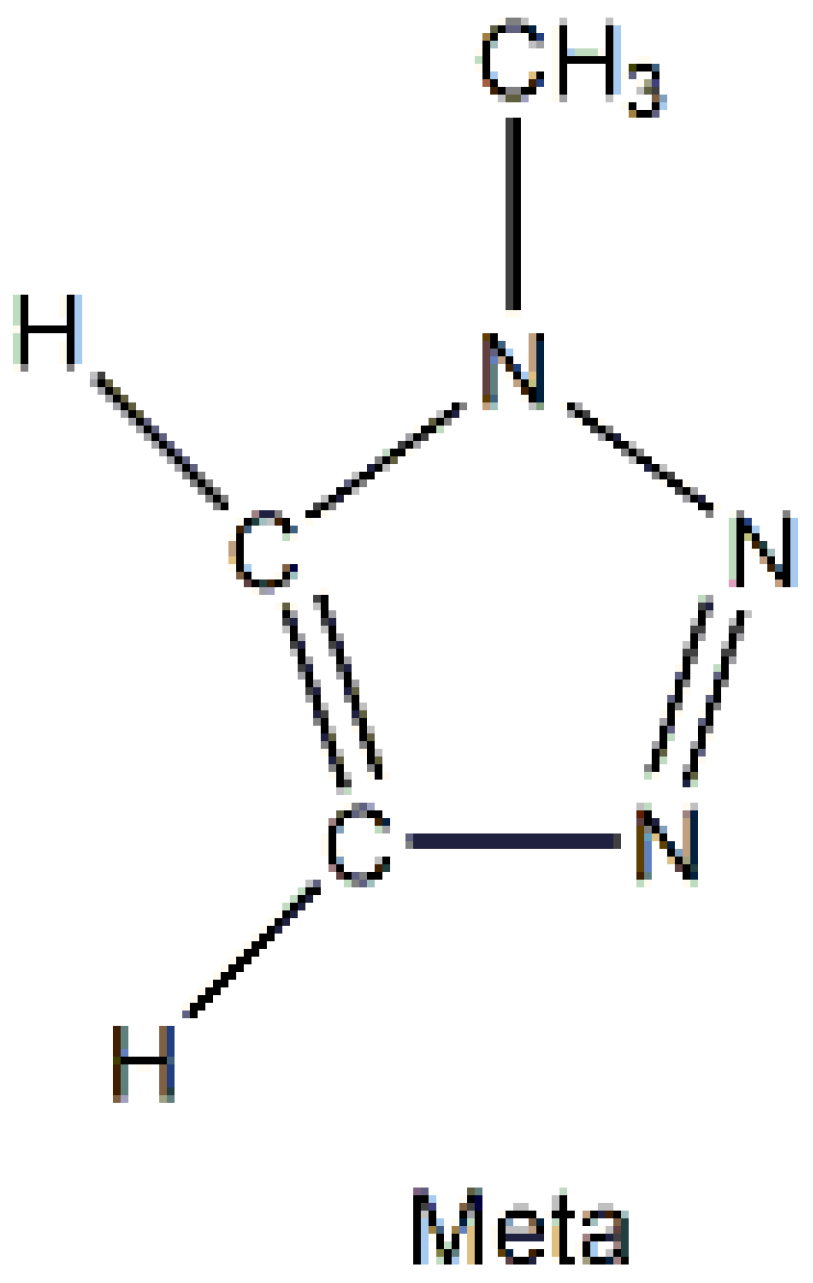
4. 1-Methylbenzotriazole: The “Free” Organic Compound
5. The Published Coordination Chemistry of 1-methylbenzotriazole
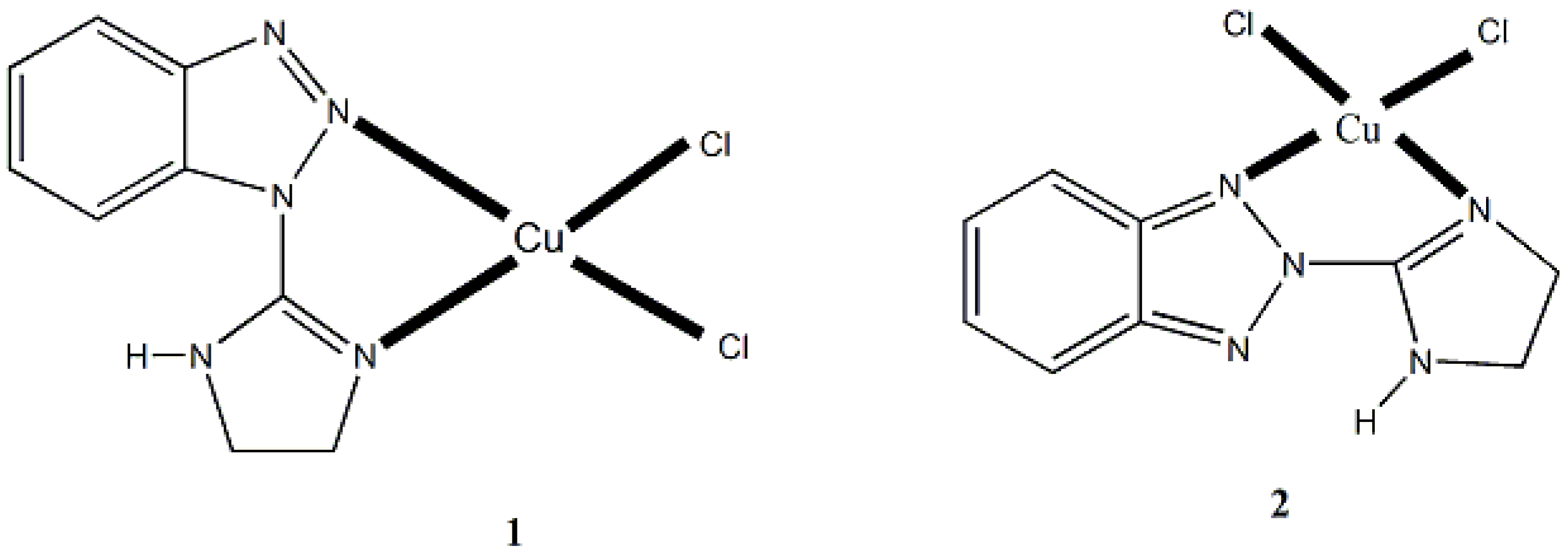
5.1. Is Mebta a Boring Ligand?
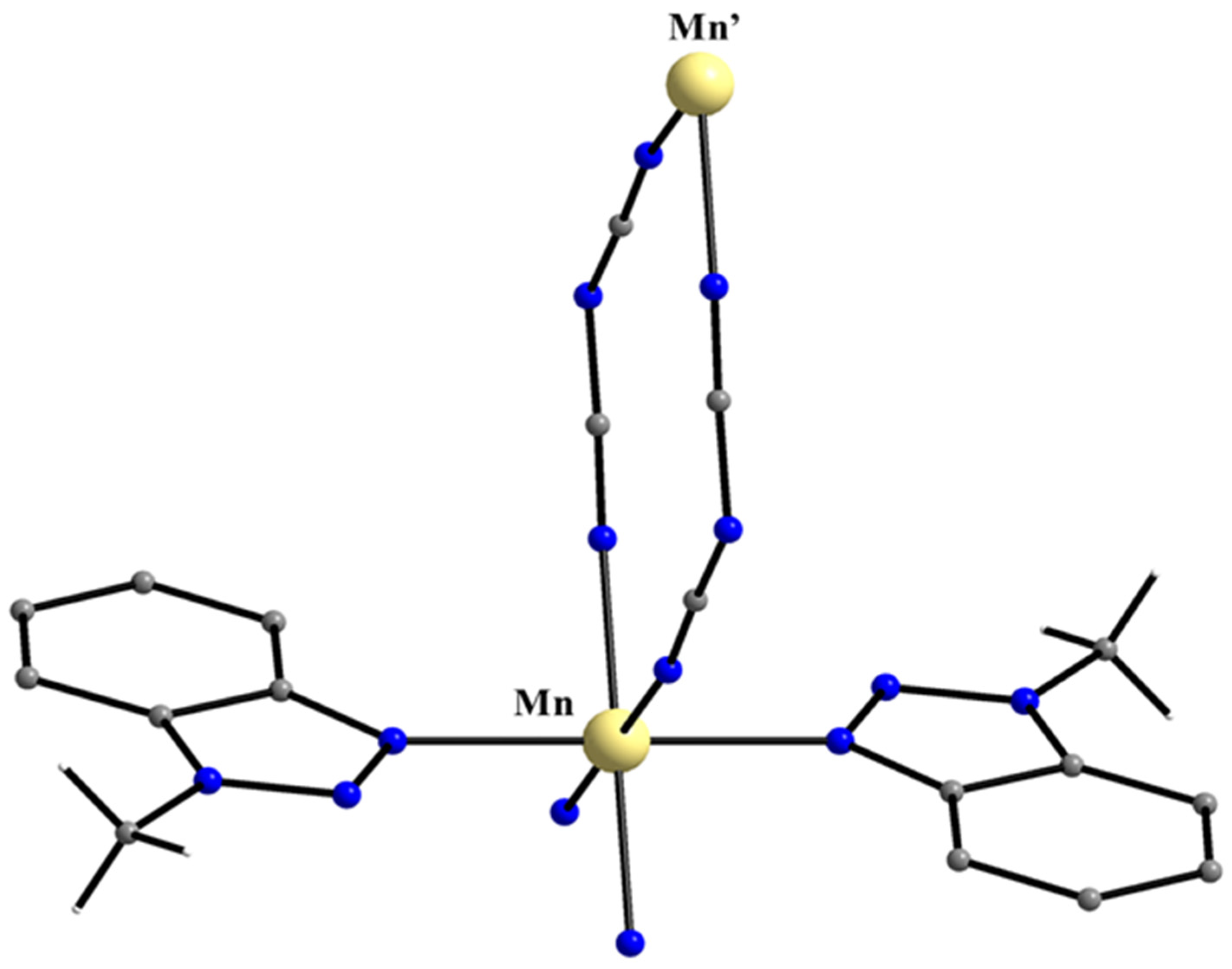
5.2. An 1D Mn(II)/Mebta Coordination Polymer from the Use of the Dicyanamido Ligand
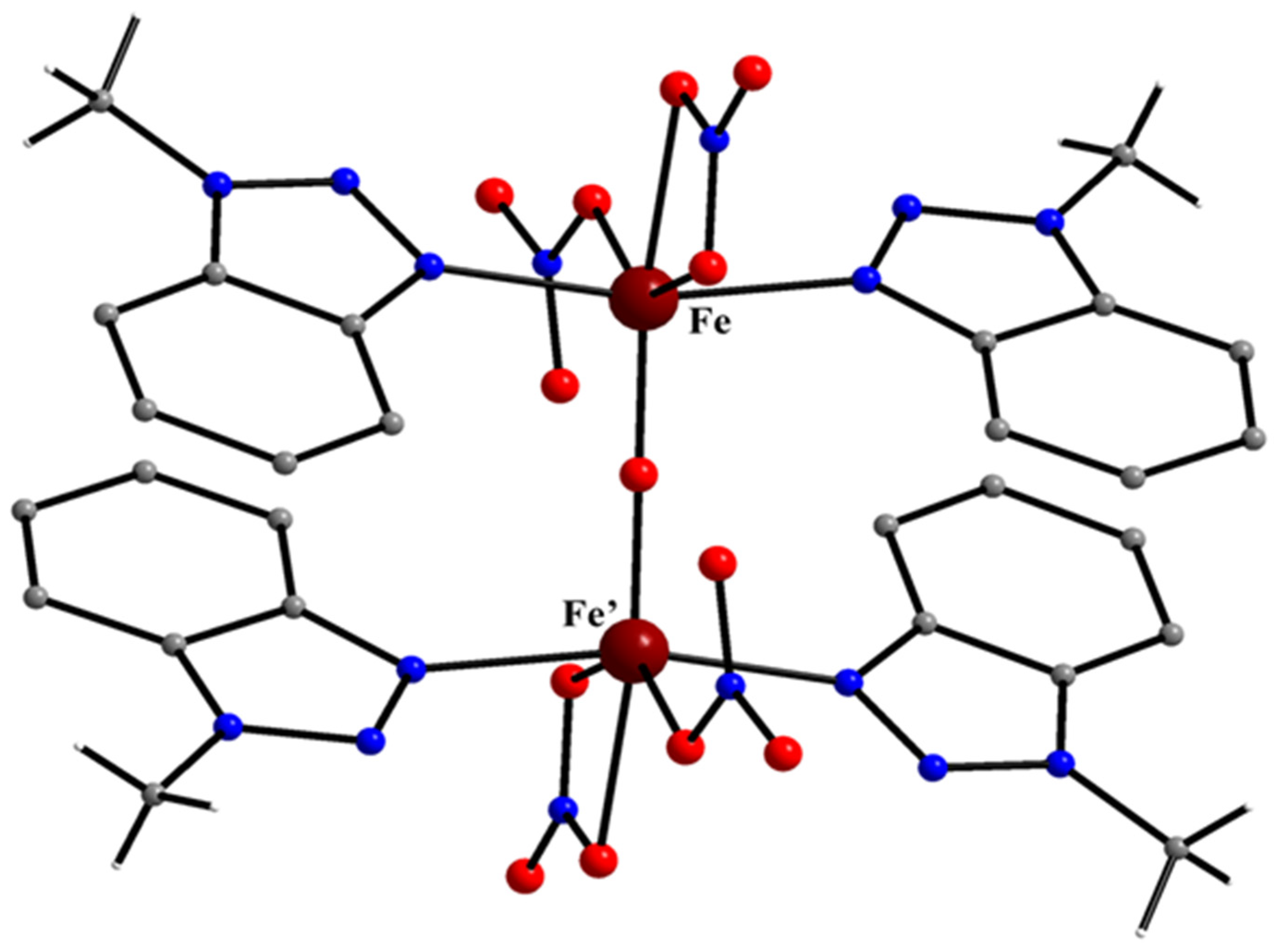
5.3. Fe(II) and Fe(III) Complexes
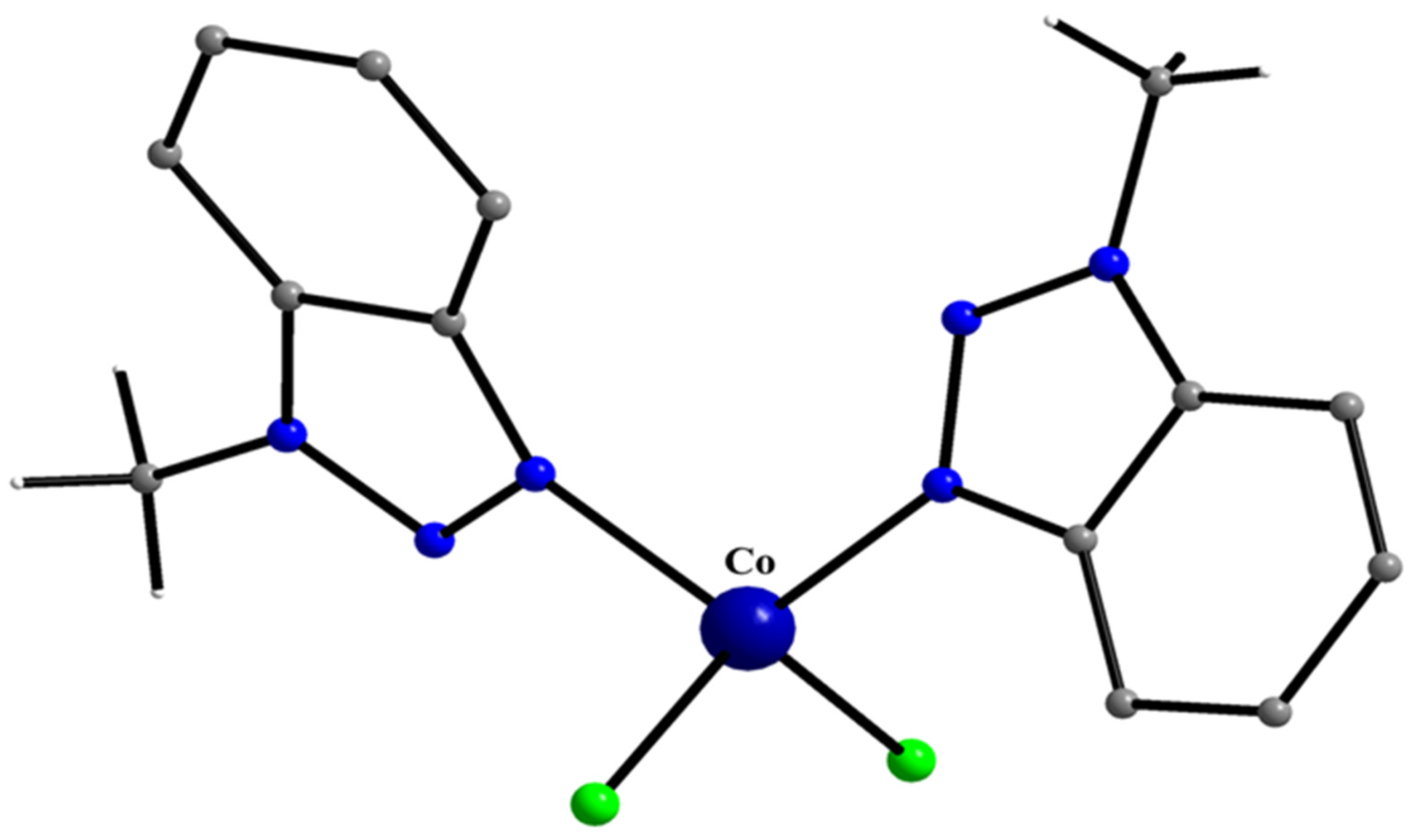
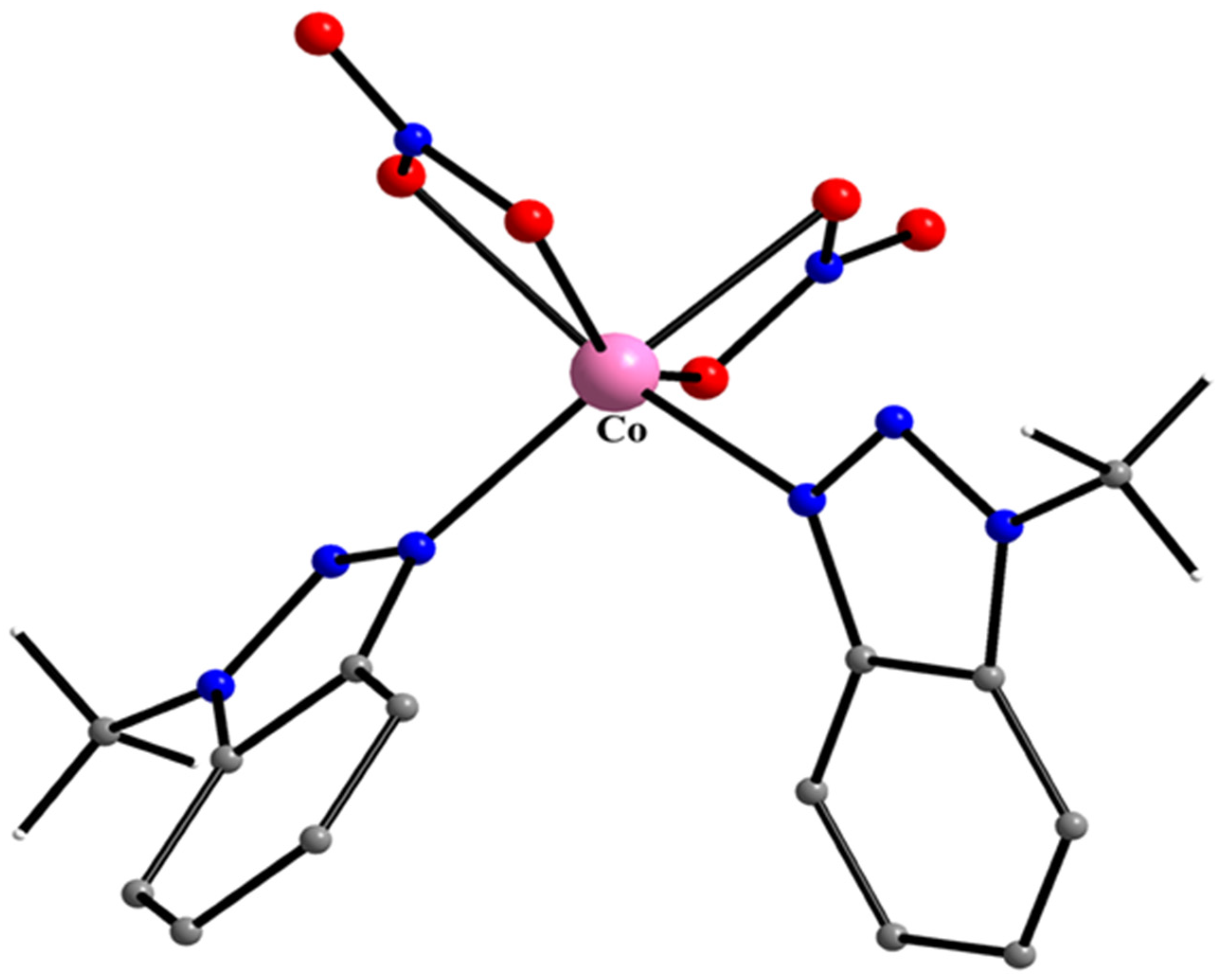
5.4. Tetrahedral and Octahedral Co(II) Complexes
10 10a
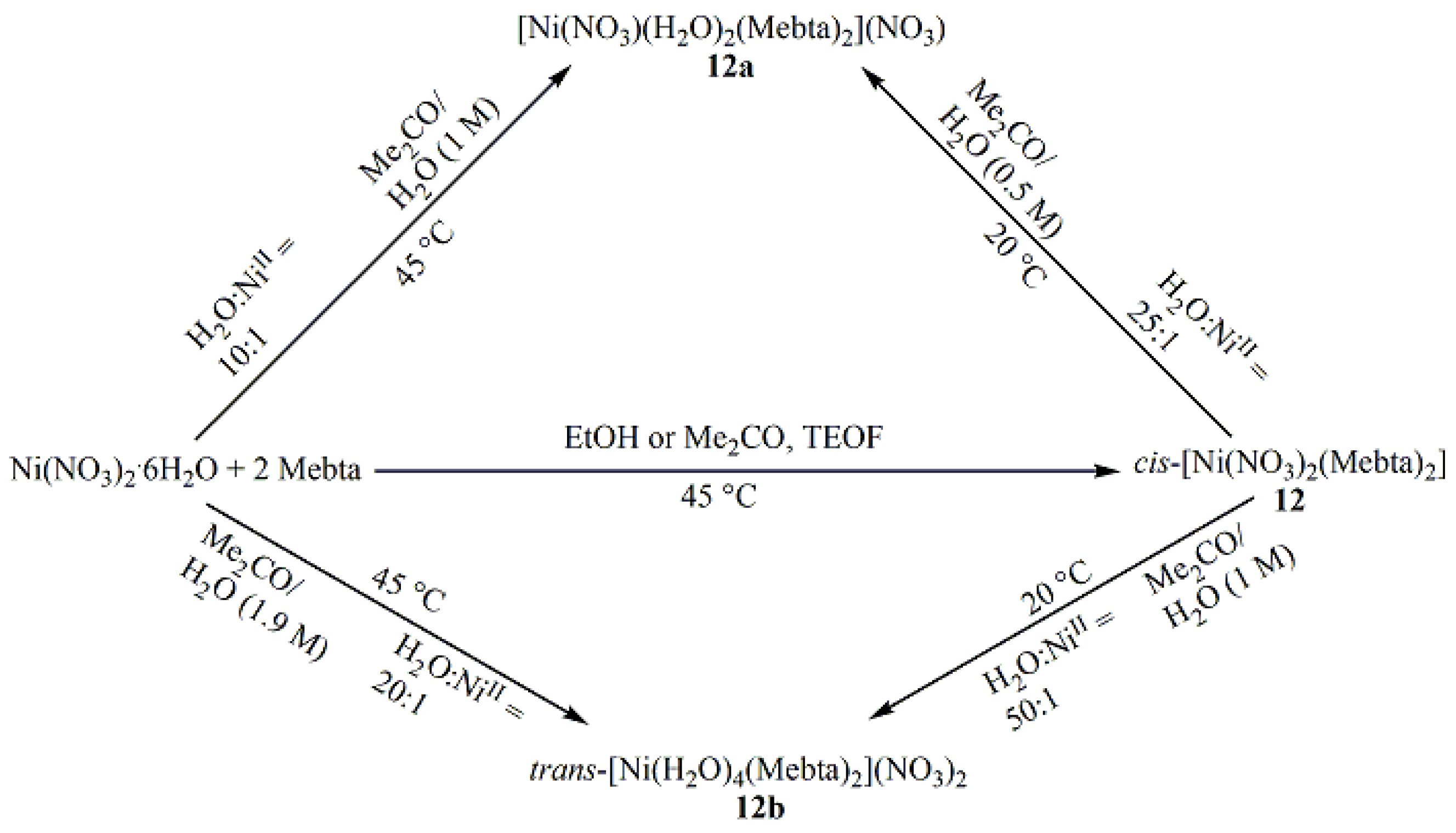
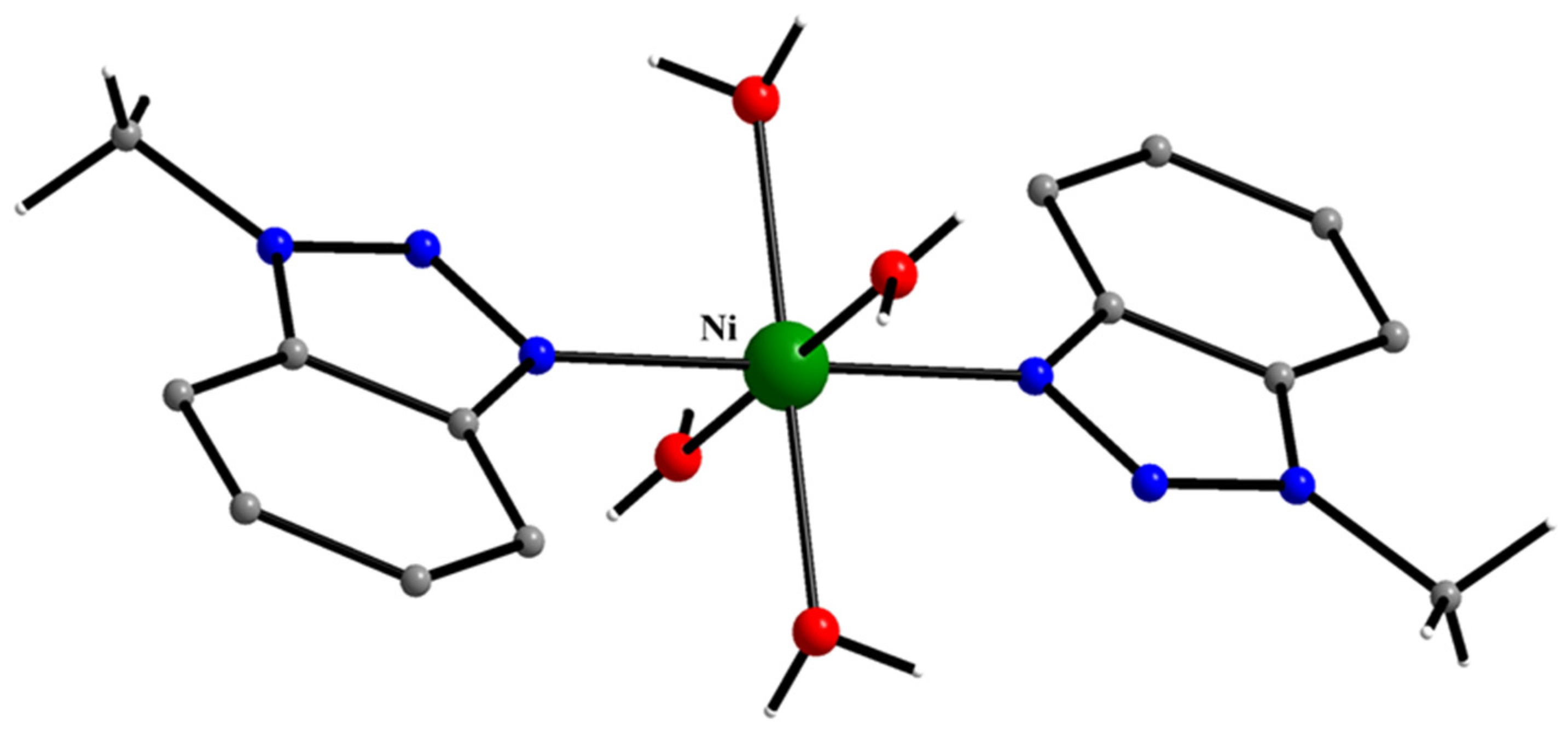
5.5. Reactions of Ni(II) Thiocyanate and Nitrate with 1-methylbenzotriazole
5.6. Square Planar Pd(II) and Pt(II) Complexes
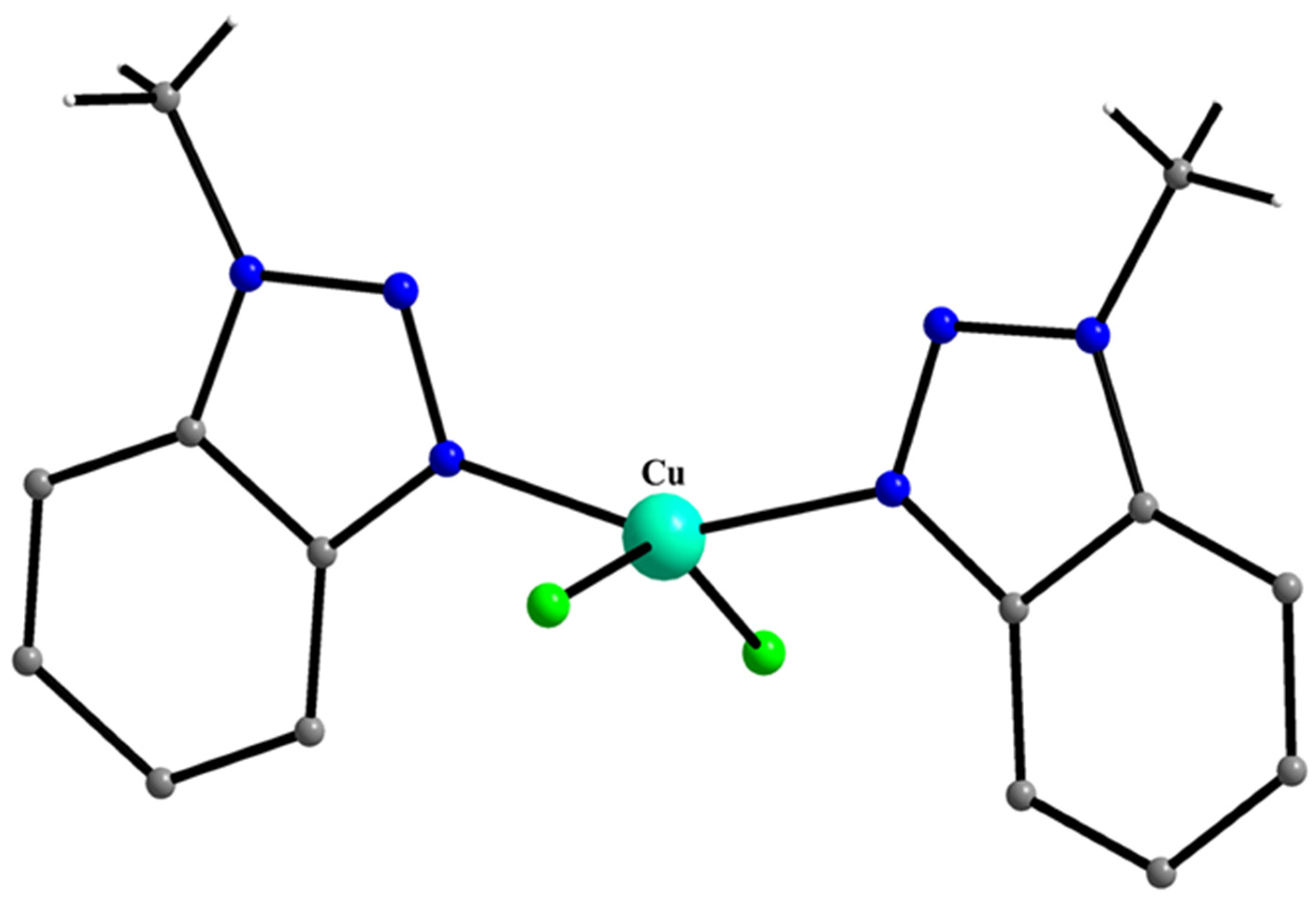
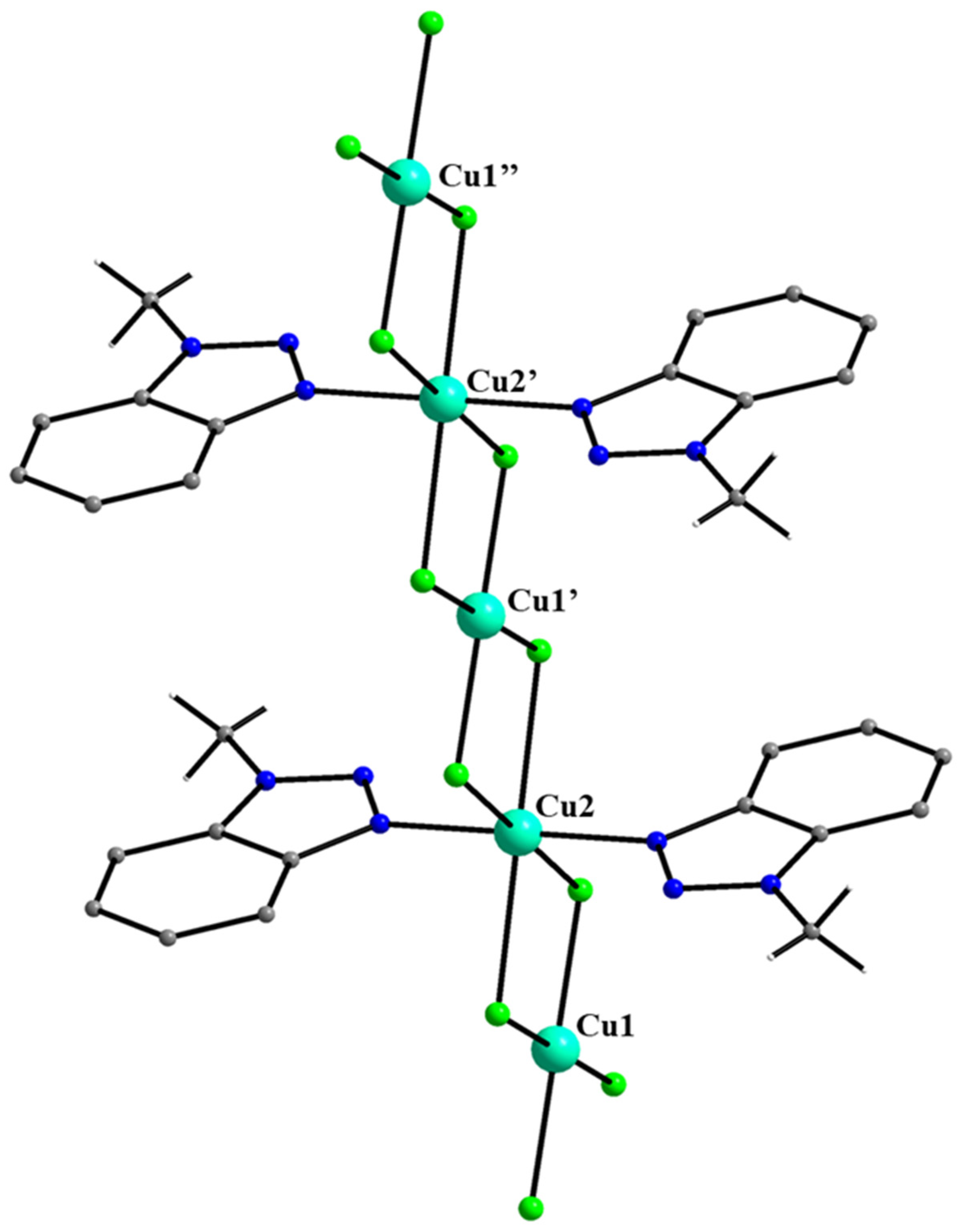
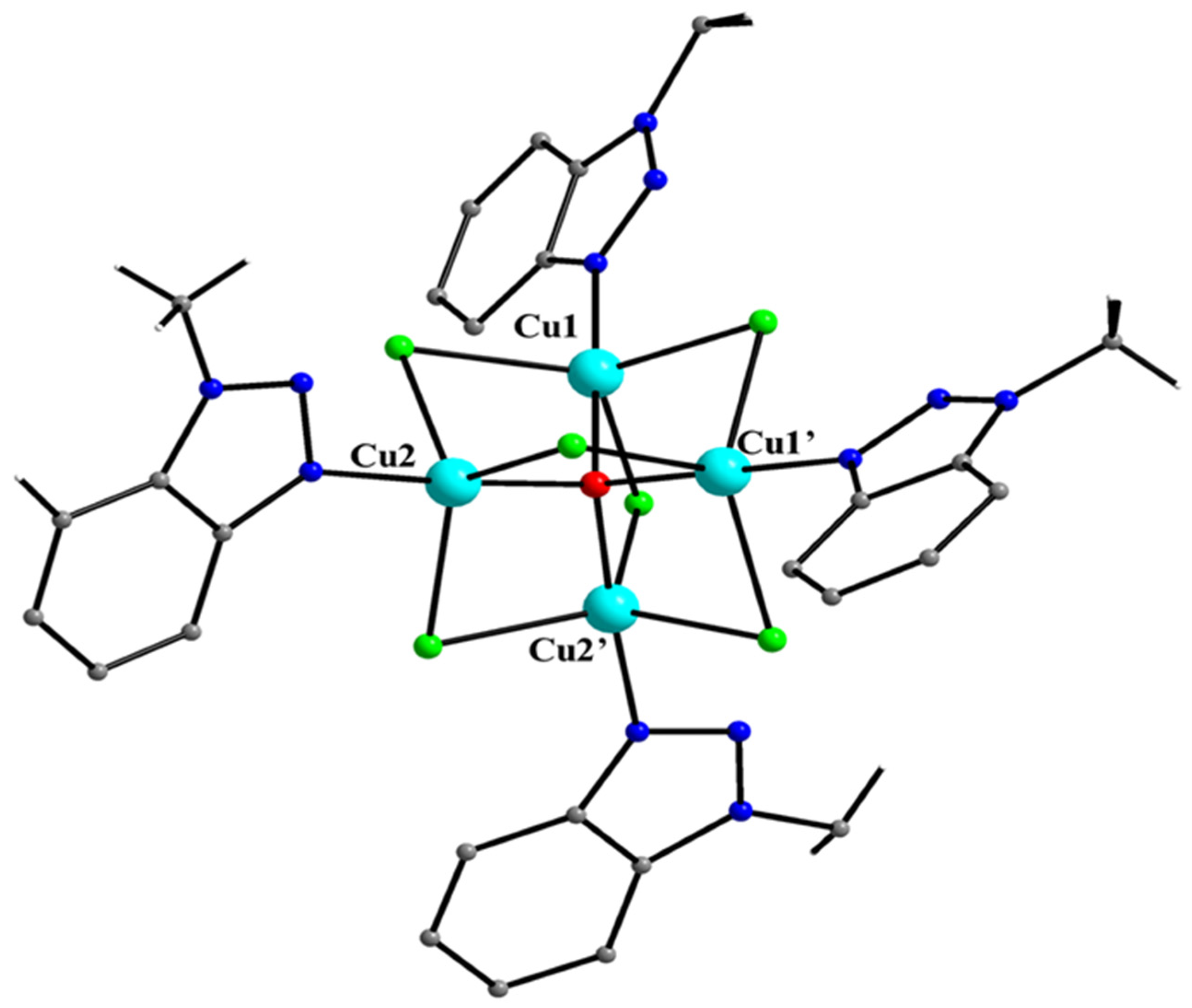
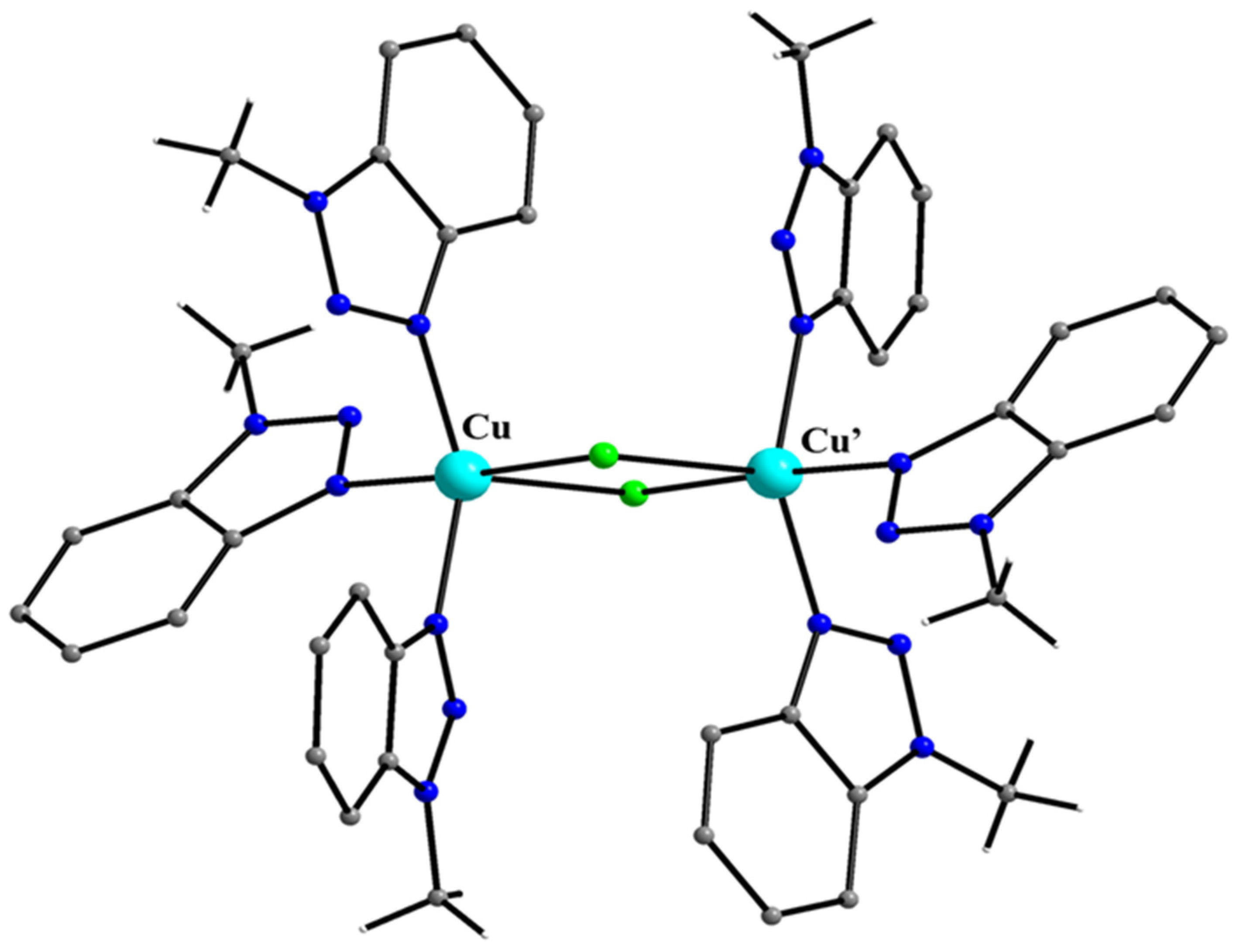
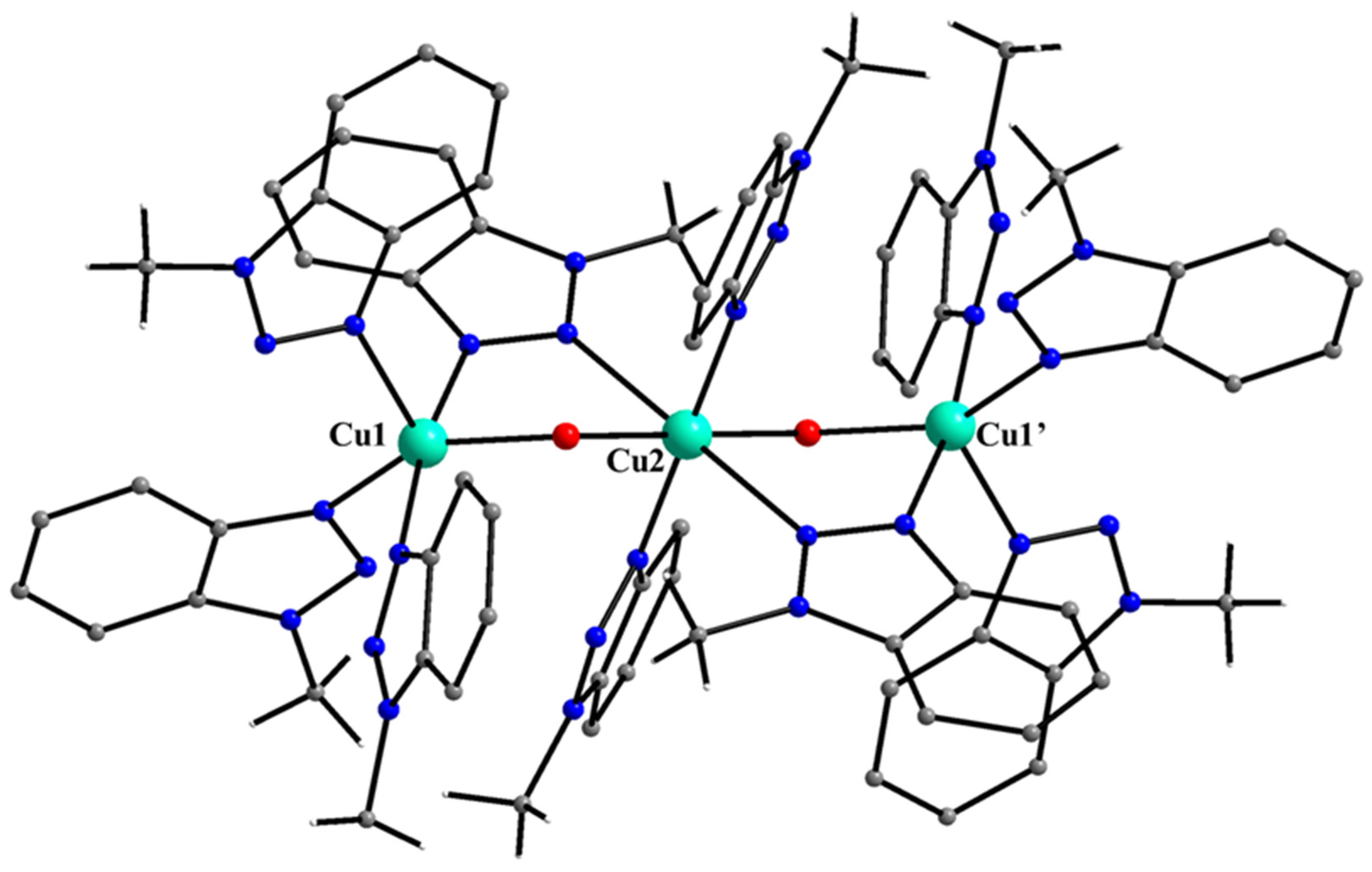
5.7. The Rich Coordination Chemistry of Mebta with Cu(II)
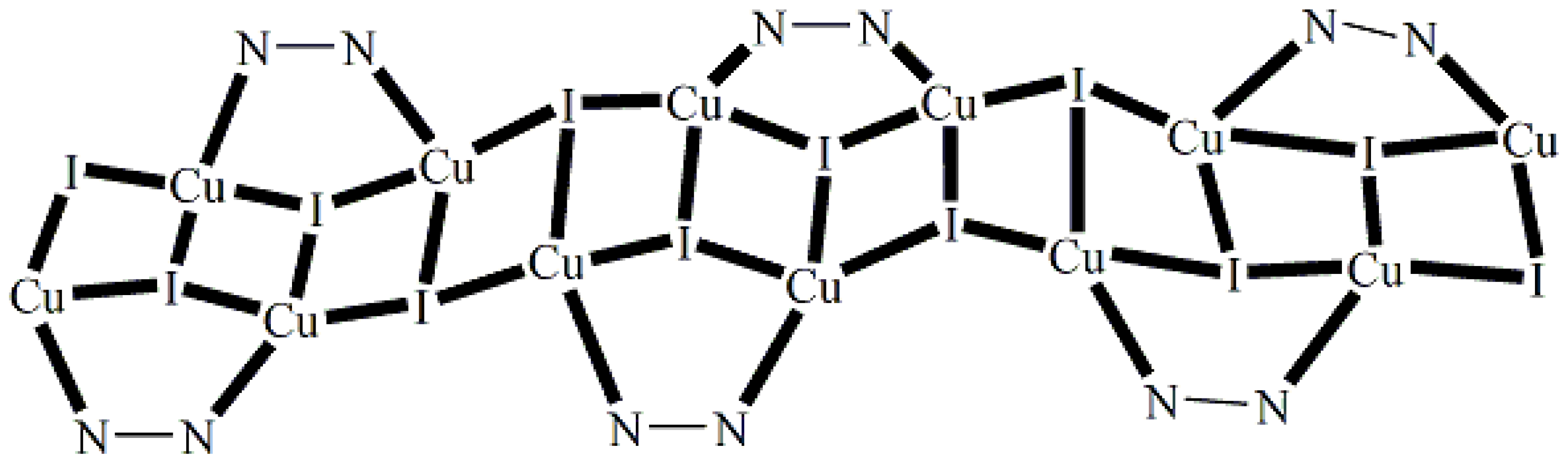
5.8. A Luminescent Copper(I) Polymer with a Unique Bridging Mode of Mebta
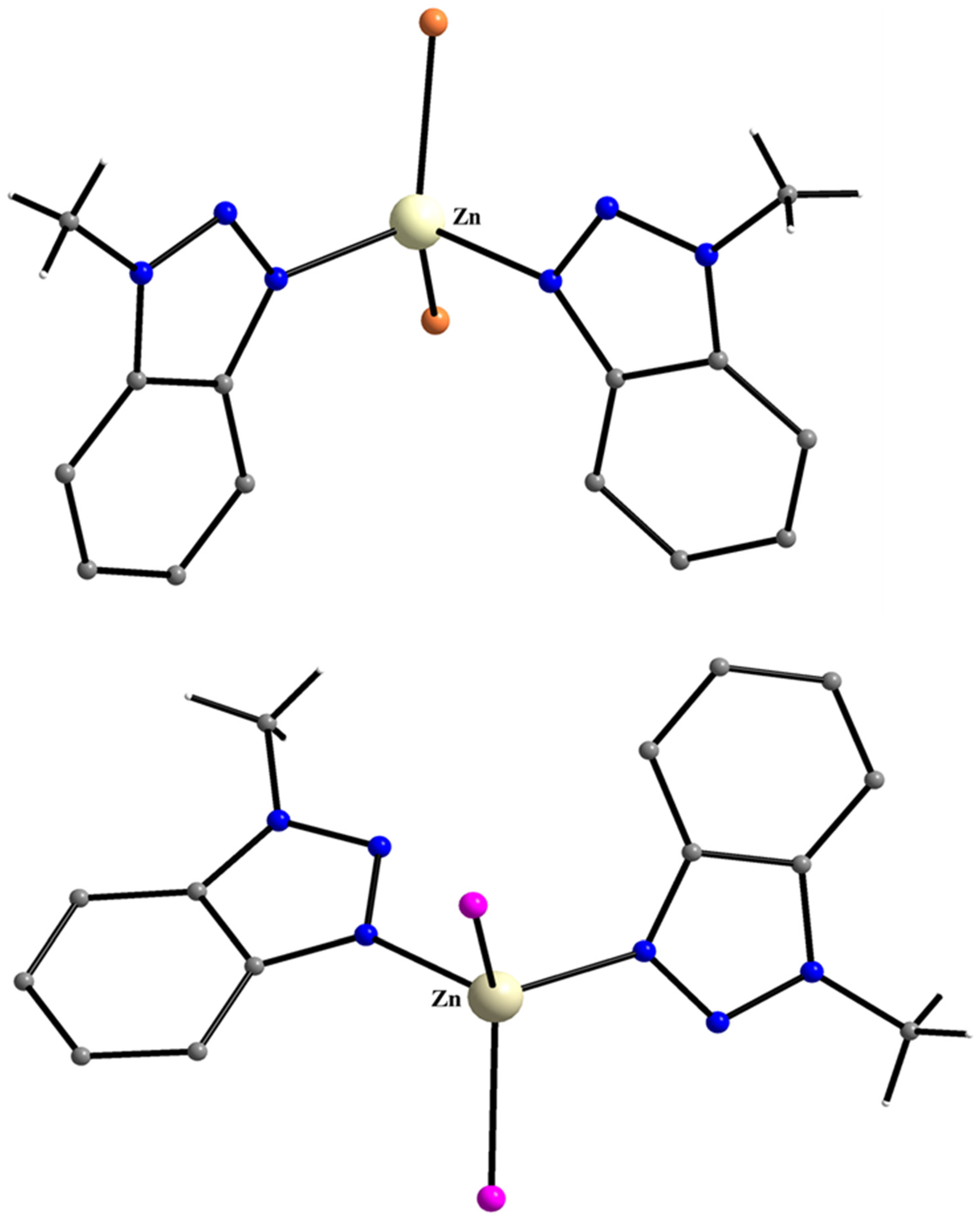
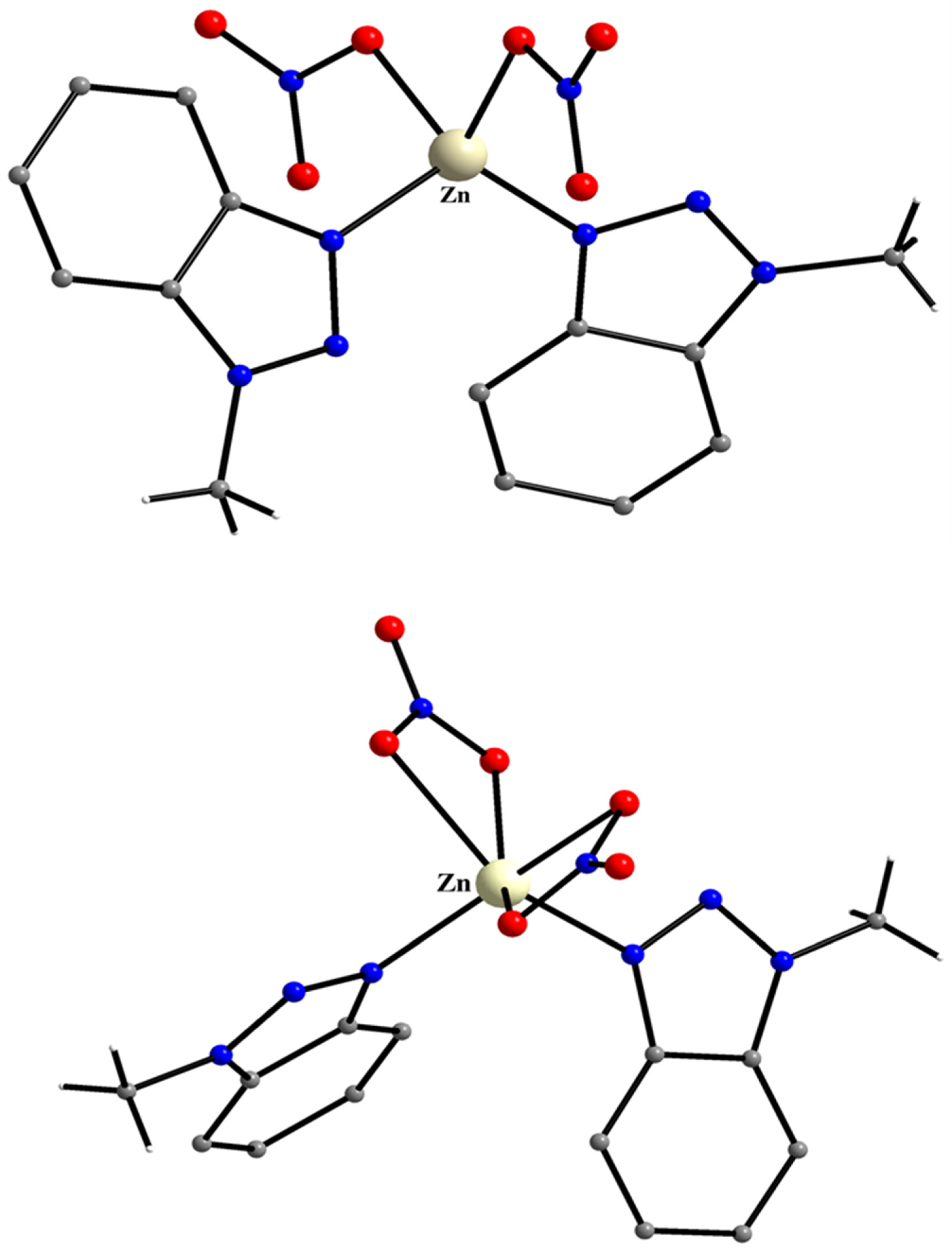
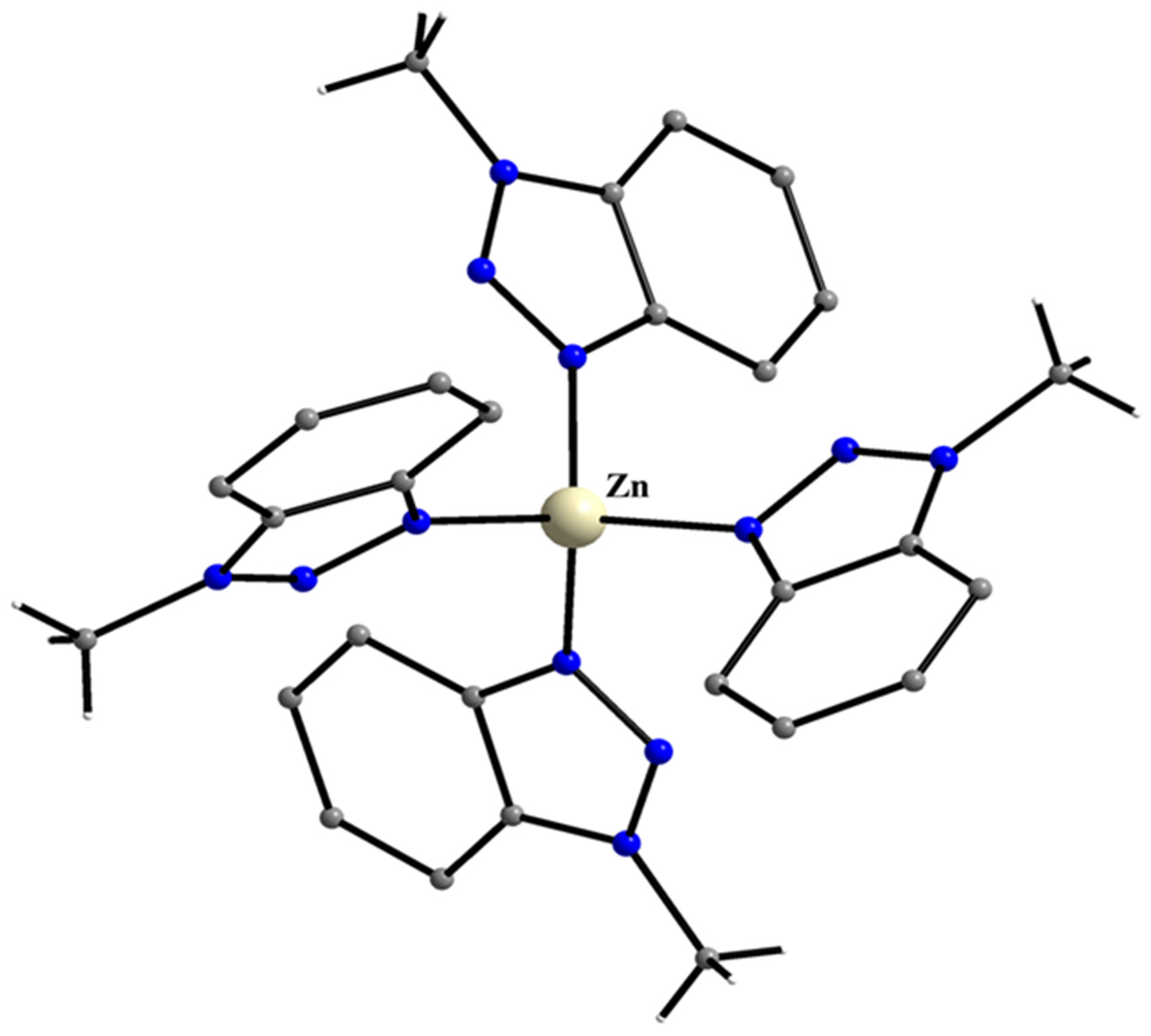
5.9. Filling in the Empty Position of Zn(II)
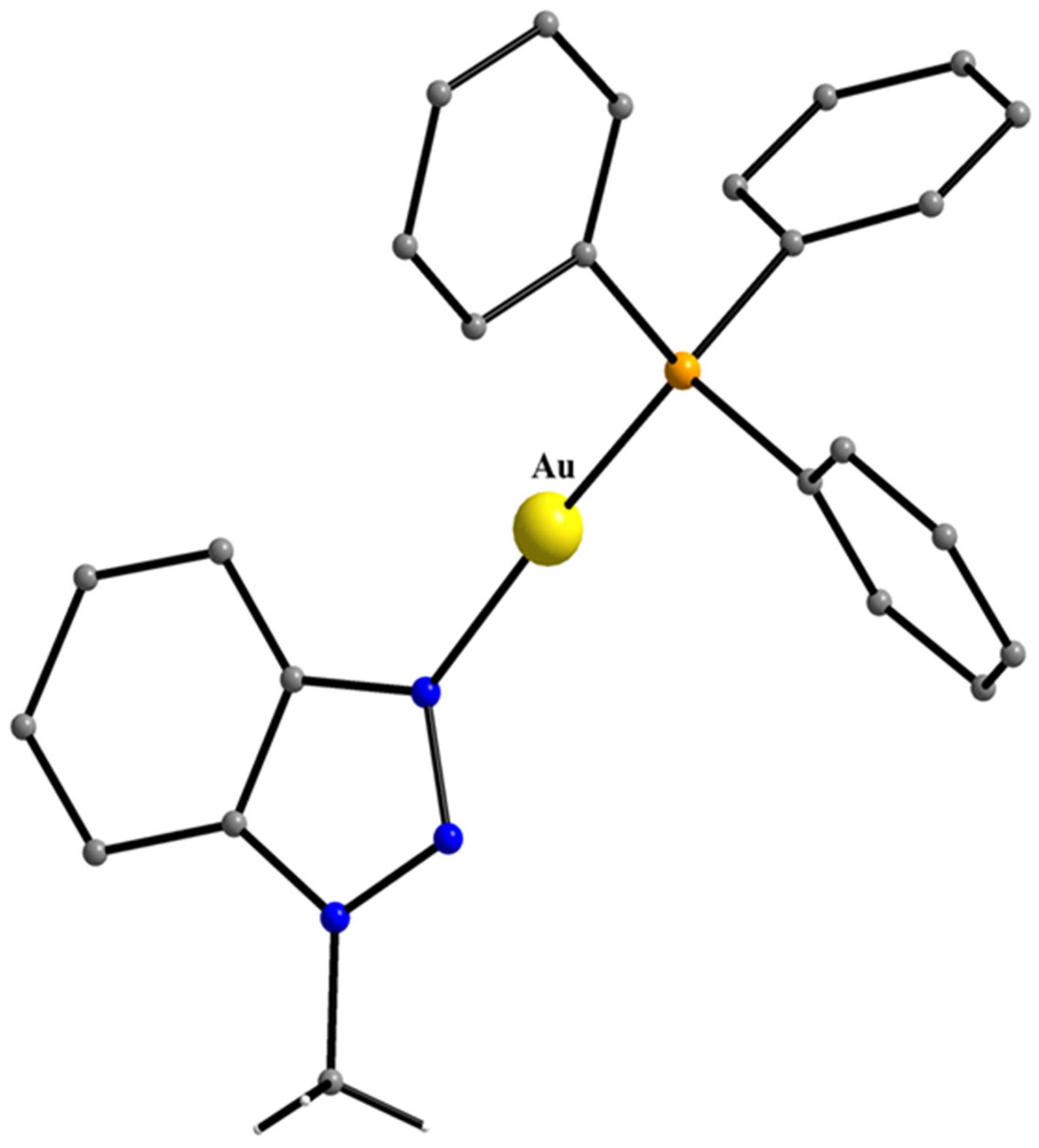
5.10. A Two-Coordinate Au(I) Complex
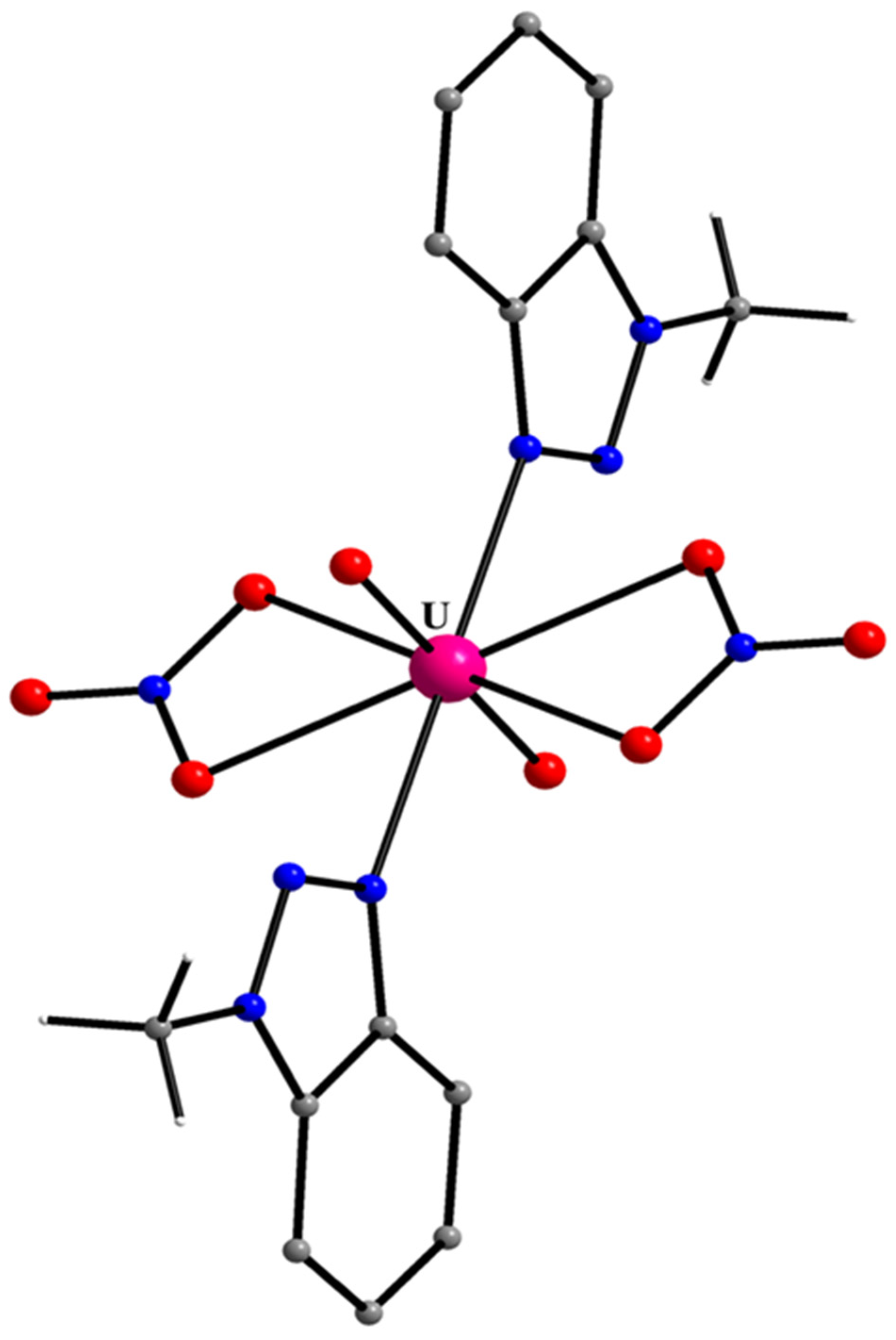
5.11. A Hexagonal Bipyramidal {UVIO2}2+/Mebta Complex
6. Unpublished Work on the Coordination Chemistry of Mebta
7. Concluding Remarks in Brief and Prognosis for the Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loukopoulos, E.; Kostakis, G. Recent advances in the coordination chemistry of benzotriazole-based ligands. Coord. Chem. Rev. 2019, 395, 193–229. [Google Scholar] [CrossRef]
- Moore, D.S.; Robinson, S.D. Catenated Nitrogen Ligands Part II. Transition Metal Derivatives of Triazoles, Tetrazoles, Pentazoles, and Hexazine. Adv. Inorg. Chem. 1998, 32, 171–239. [Google Scholar]
- Stamou, C.; Barouni, E.; Plakatouras, J.C.; Sigalas, M.M.; Raptopoulou, C.P.; Psycharis, V.; Bakalbassis, E.G.; Perlepes, S.P. The “Periodic Table” of 1-methylbenzotriazole: Zinc(II) Complexes. Inorganics 2023, 11, 356. [Google Scholar] [CrossRef]
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parsons, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-Ligand Reactions: In Situ Formation of New Polydentate Ligands. J. Chem. Soc. Dalton Trans. 2000, 14, 2349–2356. [Google Scholar] [CrossRef]
- Aromi, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles. Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Constable, E.C. What’s in a Name?—A Short History of Coordination Chemistry from Then to Now. Chemistry 2019, 1, 126–133. [Google Scholar] [CrossRef]
- Klement, R. Phosphorsäure als Ligand in Komplexen Kobaltverbindungen. Z. Anorg. Allg. Chem. 1926, 156, 237–244. [Google Scholar] [CrossRef]
- Tsuchida, R. Absorption spectra of coordination compounds. I. Bull. Chem. Soc. Jpn. 1938, 13, 388–400. [Google Scholar] [CrossRef]
- Tumaki, T. Coordinate valency rings. V. Spectrochemical researches on the inner complex metallic salts of salicylaldehyde-ethylenediimine and related compounds. Bull. Chem. Soc. Jpn. 1938, 13, 583–591. [Google Scholar]
- IUPAC. Nomenclature of Inorganic Chemistry, 1957 Report of CNIC; Butterworths Scientific Publications: London, UK, 1959. [Google Scholar]
- Constable, E.C. Metals and Ligand Reactivity; VCH: Weinheim, Germany, 1996; pp. 22–45. [Google Scholar]
- Tomãs, F.; Abboud, J.-L.M.; Laynez, J.; Notario, R.; Santos, L.; Nilsson, S.O.; Catalãn, J.; Claramunt, R.M.; Elguero, J. Tautomerism and Aromaticity in 1,2,3-Triazoles: The Case of Benzotriazole. J. Am. Chem. Soc. 1989, 111, 7348–7353. [Google Scholar] [CrossRef]
- Hall, C.D.; Panda, S.S. The Benzotriazole Story. Adv. Heterocycl. Chem. 2016, 119, 1–23. [Google Scholar]
- Katritzky, A.R.; Belyakov, S.A. Benzotriazole-Based Intermediates: Reagents for Efficient Organic Synthesis. Aldrichim. Acta 1998, 31, 35–45. [Google Scholar]
- Katritzky, A.R.; Rachwal, S.; Hitchings, G.J. Benzotriazole: A Novel Synthetic Auxiliary. Tetrahedron 1991, 47, 2683–2732. [Google Scholar] [CrossRef]
- Katritzky, A.R. N-Substituted Benzotriazoles: Properties, Reactivities and Synthetic Utility. Bull. Soc. Chim. Belg. 1992, 101, 409–413. [Google Scholar] [CrossRef]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar] [CrossRef]
- Patel, P.K.; Patel, P.D.; Patel, S. Synthesis, Characterization, Chelating Properties and Biological Activities of Benzotriazole-Salicylic Acid Combined Molecule. Int. J. Pharm. Chem. Sci. 2012, 1, 1799–1804. [Google Scholar]
- Oberley, L.W.; Buettner, G.R. Role of superoxide dismutase in cancer: A review. Cancer Res. 1979, 39, 1141–1149. [Google Scholar]
- Kraševec, I.; Prosen, H. Solid-Phase Extraction of Polar Benzotriazoles as Environmental Pollutants: A Review. Molecules 2018, 23, 2501. [Google Scholar] [CrossRef]
- Ding, J.; Yan, Z.; Feng, L.; Zhai, F.; Chen, X.; Xu, Y.; Tang, S.; Huang, C.; Li, L.; Pan, N.; et al. Benzotriazole decorated graphene oxide for efficient removal of U(VI). Environ. Pollut. 2019, 253, 221–230. [Google Scholar] [CrossRef]
- Flippen-Anderson, J.L.; Gilardi, R.D.; Pitt, A.M.; Wilson, W.S. Synthesis and Explosive Properties of Benzotriazoles. Aust. J. Chem. 1992, 45, 513–524. [Google Scholar] [CrossRef]
- Malow, M.; Wehrstedt, K.D.; Neuenfeld, S. On the explosive properties of 1H-benzotriazole and 1H, 1,2,3-triazole. Tetrahedron Lett. 2007, 48, 1233–1235. [Google Scholar] [CrossRef]
- Srinivas, D.; Ghule, V.D.; Tewari, S.P.; Muralidharan, K. Synthesis of Amino, Azido, Nitro, and Nitrogen-Rich Azole-Substituted Derivatives of 1H-Benzotriazole for High-Energy Materials Applications. Chem. Eur. J. 2012, 18, 15031–15037. [Google Scholar] [CrossRef]
- Paterson, M.J.; Robb, M.A.; Blancafort, L.; DeBellis, A.D. Theoretical Study of Benzotriazole UV Photostability: Ultrafast Deactivation through Coupled Proton and Electron Transfer Triggered by a Charge-Transfer State. J. Am. Chem. Soc. 2004, 126, 2912–2922. [Google Scholar] [CrossRef]
- Lai, H.-J.; Ying, G.-G.; Ma, Y.-B.; Chen, Z.-F.; Chen, F.; Liu, Y.-S. Field dissipation and plant uptake of benzotriazole ultraviolet stabilizers in biosolid-ammended soils. Environ. Sci. Process. Impacts 2014, 16, 558–566. [Google Scholar] [CrossRef]
- Kiejza, D.; Karpińska, J.; Kotowska, U. Degradation of Benzotriazole UV Stabilizers in PAA/d-Electron Metal Ion Systems- Removal Kinetics, Products and Mechanism Evaluation. Molecules 2022, 27, 3349. [Google Scholar] [CrossRef]
- Kokalj, A.; Peljhan, S.; Finšgar, M.; Milošev, I. What Determines the Inhibition Effectiveness of ATH, BTAH, and BTAOH Corrosion Inhibitors on Copper? J. Am. Chem. Soc. 2010, 132, 16657–16668. [Google Scholar] [CrossRef]
- Grillo, F.; Tee, D.W.; Francis, S.M.; Früchti, H.; Richardson, N.V. Initial stages of benzotriazole adsorption on the Cu(111) surface. Nanoscale 2013, 5, 5269–5273. [Google Scholar] [CrossRef]
- Kokalj, A. Ab initio modeling of the bonding of benzotriazole corrosion inhibitor to reduced and oxidized copper surfaces. Faraday Discuss. 2015, 180, 415–438. [Google Scholar] [CrossRef]
- Gattinoni, C.; Michaelides, A. Understanding corrosion inhibition with van der Waals DFT methods: The case of benzotriazole. Faraday Discuss. 2015, 180, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Törnkvist, C.; Thierry, D.; Bergman, J.; Liedberg, B.; Leygraf, C. Methyl Substitution in Benzotriazole and Its Influence on Surface Structure and Corrosion Inhibition. J. Electrochem. Soc. 1989, 136, 58–64. [Google Scholar] [CrossRef]
- Procter and Gamble Co. Compositions for Inhibiting Metal Tarnish. British Patent 652339, 8 November 1948. [Google Scholar]
- Madsen, H.B. A Preliminary Note on the Use of Benzotriazole for Stabilizing Bronze Objects. Stud. Conserv. 1967, 12, 163–166. [Google Scholar] [CrossRef]
- Sease, C. Benzotriazole: A Review for Conservators. Stud. Conserv. 1978, 23, 76–85. [Google Scholar] [CrossRef]
- Walker, R. Benzotriazole, a Corrosion Inhibitor for Antiques. J. Chem. Educ. 1980, 57, 789–791. [Google Scholar] [CrossRef]
- Catalán, J.; Claramunt, R.M.; Elguero, J.; Laynèz, J.; Menéndez, M.; Anvia, F.; Quian, J.H.; Taagepera, M.; Taft, R.W. Basicity and Acidity of Azoles: The Annelation Effect in Azoles. J. Am. Chem. Soc. 1988, 110, 4105–4111. [Google Scholar] [CrossRef]
- Begtrup, M.; Elguero, J.; Faure, R.; Camps, P.; Estopá, C.; Ilavský, D.; Fruchier, A.; Marzin, C.; Mendoza, J.d. Effects of N-substituents on the 13C NMR Parameters of Azoles. Magn. Reson. Chem. 1988, 26, 134–151. [Google Scholar] [CrossRef]
- Claramunt, R.M.; Sanz, D.; Boyer, G.; Catalán, J.; Paz, J.L.G.d. Experimental (13C and 15N Spectroscopy) and Theoretical (6-31G) Study of the Protonation of N-Methylazoles and N-Methylbenzazoles. Magn. Reson. Chem. 1993, 31, 791–800. [Google Scholar] [CrossRef]
- Novak, I.; Abu-Izneid, T.; Kovač, B.; Klasinc, L. Electronic Structure and Stability of Benzotriazoles. J. Phys. Chem. A 2009, 113, 9751–9756. [Google Scholar] [CrossRef]
- Catalán, J.; Pérez, P.; Elguero, J. Structure of Benzotriazole in the Gas Phase: A UV Experimental Study. J. Org. Chem. 1993, 58, 5276–5277. [Google Scholar] [CrossRef]
- Rondeau, R.E.; Rosenberg, H.M.; Dunbar, D.J. Nuclear Magnetic Resonance Analysis of 1- and 2-Methylbenzotriazole. J. Mol. Spectr. 1969, 29, 305–311. [Google Scholar] [CrossRef]
- Thomas, S.; Venkateswaran, S.; Kapoor, S.; D’Cunha, R.; Mukherjee, T. Surface enhanced Raman scattering of benzotriazole: A molecular orentational study. Spectrochim. Acta Part A 2004, 60, 25–29. [Google Scholar] [CrossRef]
- Aruchamy, A.; Fujishima, A.; Ibrahim, A.; Loo, B.H. A surface-enhanced Raman spectroscopic study of benzotriazole and 6-tolyltriazole corrosion inhibitors on copper electrodes in alkaline solutions. J. Electroanal. Chem. 1990, 281, 299–304. [Google Scholar] [CrossRef]
- Sockalingum, D.; Fleischmann, M.; Musiani, M.M. Near-infrared Fourier transform surface-enhanced Raman scattering of azole copper corrosion inhibitors in aqueous chloride media. Spectrochim. Acta Part A 1991, 47, 1475–1485. [Google Scholar] [CrossRef]
- Tangoulis, V.; Raptopoulou, C.P.; Terzis, A.; Bakalbassis, E.G.; Diamantopoulou, E.; Perlepes, S.P. Polynuclear Nickel(II) Complexes: Preparation, Characterization, Magnetic Properties, and Quantum-Chemical Study of [Ni5(OH)(Rbta)5(acac)4 (H2O)4] (RbtaH = Benzotriazole and 5,6-Dimethylbenzotriazole). Inorg. Chem. 1998, 37, 3142–3153. [Google Scholar] [CrossRef]
- Biswas, S.; Tonigold, M.; Volkmer, D. Homo- and Heteronuclear Coordination Compounds with Td Symmetry- the Solid State Structures of [MZn4(L)4(L’)6] (M = CoII or ZnII; L = chloride or acac; L’ = 1,2,3-benzotriazolate). Z. Anorg. Allg. Chem. 2008, 634, 2532–2538. [Google Scholar] [CrossRef]
- Biswas, S.; Tonigold, M.; Speldrich, M.; Kögerler, P.; Volkmer, D. Nonanuclear Coordination Compounds Featuring {M9L12}6+ Cores (M = NiII, CoII, or ZnII; L = 1,2,3-Benzotriazolate). Eur. J. Inorg. Chem. 2009, 2009, 3094–3101. [Google Scholar] [CrossRef]
- Biswas, S.; Tonigold, M.; Kelm, H.; Krüger, H.-J.; Volkmer, D. Thermal spin-crossover in the [M3Zn6Cl6L12] (M = Zn, FeII; L = 5,6-dimethoxy-1,2,3-benzotriazolate) system: Structural, electrochemical, Mössbauer, and UV-Vis spectroscopic studies. Dalton Trans. 2010, 39, 9851–9859. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Tonigold, M.; Speldrich, M.; Kögerler, P.; Weill, M.; Volkmer, D. Syntheses and Magnetostructural Investigations on Kuratowski-Type Homo- and Heteropentanuclear Coordination Compounds [MZn4Cl4(L)6] (MII = Zn, Fe, Co, Ni, or Cu; L = 5,6-Dimethyl-1,2,3-benzotriazolate) Represented by the Nonplanar K3,3 Graph. Inorg. Chem. 2010, 49, 7424–7434. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Li, G.-T.; Peng, Y.-H.; Wu, L.; Wu, B.-L. Two photoluminescent pentanuclear homo- and hetero-metal complexes based on benzotriazole bridge. J. Coord. Chem. 2011, 64, 1593–1962. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Grzywa, M.; Tonigold, M.; Sastre, G.; Schüttrigkeit, T.; Leeson, N.S.; Volkmer, D. Photophysical properties of Kuratowski-type coordination compounds [MIIZn4Cl4(Me2bta)6] (MII = Zn or Ru) featuring long-lived excited electronic states. Dalton Trans. 2011, 40, 5926–5938. [Google Scholar] [CrossRef]
- Werner, T.W.; Reschke, S.; Bunzen, H.; Krug von Nidda, H.-A.; Deisenhofer, J.; Loidl, A.; Volkmer, D. [Co5Tp4*(Me2bta)6]: A Highly Symmetrical Pentanuclear Kuratowski Complex Featuring Tris(pyrazolyl)borate and Benzotriazolate Ligands. Inorg. Chem. 2016, 55, 1053–1060. [Google Scholar] [CrossRef]
- Bunzen, H.; Grzywa, M.; Kalytta-Mewes, A.; Volkmer, D. One-pot synthesis of ultrastable pentanuclear alkylzinc complexes. Dalton Trans. 2017, 46, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-J.; Chen, W.-P.; Yu, Y.; Qin, L.; Han, T.; Zheng, Y.-Z. Filling the Missing Links of M3n Prototype 3d-4f and 4f Cyclic Coordination Cages: Syntheses, Structures and Magnetic Properties of the Ni10Ln5 and the Er3n Wheels. Inorg. Chem. 2017, 56, 12821–12829. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllopoulou, C.; Diamantopoulou, E.; Terzis, A.; Tangoulis, V.; Lalioti, N.; Perlepes, S.P. High-nuclearity nickel(II) clusters: Ni13 complexes from the use of 1-hydroxybenzotriazole. Polyhedron 2009, 28, 1903–1911. [Google Scholar] [CrossRef]
- Andrews, P.C.; Clegg, W.; Mulvey, R.E.; O’Neil, P.A.; Wilson, M.M. An Infinite Ladder Structure of Alternating, Fused K2N2 Rhomboids and KN2 Triangles: Synthesis and Crystallographic Characterization of Benzotriazolatopotassium-HPMA (HPMA = hexamethylphosphoric triamide). J. Chem. Soc. Chem. Commun. 1993, 1142–1144. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Mokaddem, Y. Rare Earth Benzotriazolates: Coordination Polymers Incorporating Decomposition Products from Ammonia to 1,2-Diaminobenzene in 1/∞ [Ln(Btz)3(BtzH)] (Ln = Ce, Pr), 1/∞ [Ln(Btz)3{Ph(NH2)2}] (Ln = Nd, Tb, Yb), and 1/∞ [Ho2(Btz)6(BtzH)(NH3)]. Eur. J. Inorg. Chem. 2006, 2006, 2000–2010. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Mokaddem, Y. MOFs by Transformation of 1D-Coordination Polymers: From 1/∞ [Ln(Btz)3BtzH] to the Homoleptic Rare Earth 3D-Benzotriazolate Frameworks 3/∞ [Ln(Btz)3], Ln = La, Ce. Z. Anorg. Allg. Chem. 2008, 634, 2360–2366. [Google Scholar] [CrossRef]
- Biswas, S.; Grzywa, M.; Nayek, H.P.; Dehnen, S.; Senkovska, I.; Kaskel, S.; Volkmer, D. A cubic coordination framework constructed from benzobistriazolate ligands and zinc ions having selective gas sorption properties. Dalton Trans. 2009, 33, 6487–6495. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, B.-Y.; Wei, G.; Huang, X.-C. Solvent Induced Diverse Dimensional Coordination Assemblies of Cupric Benzotriazole-5-carboxylate: Syntheses, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2011, 50, 11032–11038. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Xue, L.-P.; Zhao, B.-T.; Kan, J.; Su, W.-P. 2D lanthanide-organic frameworks constructed from lanthanide acetate skeletons and benzotriazole-5-carboxylic acid connectors: Synthesis, structure, luminescence and magnetic properties. Cryst. Eng. Commun. 2012, 14, 8485–8491. [Google Scholar] [CrossRef]
- Li, Z.-H.; Hong, D.-F.; Xue, L.-P.; Fu, W.-J.; Zhao, B.-T. Two lanthanide-bound 1H-benzotriazole polymers: New potential metal-organic scaffold for solid-phase organic chemistry. Inorg. Chim. Acta 2013, 400, 239–243. [Google Scholar] [CrossRef]
- Schmieder, P.; Denysenko, D.; Grzywa, M.; Baumgärtner, B.; Senkovska, I.; Kaskel, S.; van Wüllen, L.; Volkmer, D. CFA-1: The first chiral metal-organic framework containing Kuratowski-type secondary building units. Dalton Trans. 2013, 42, 10786–10797. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Zhou, D.; Li, Y.; Li, W.-J.; Kang, Z.-T. Syntheses, Structures, and Fluorescent Properties of Lanthanide Complexes Based on the Ligand Benzotriazole-5-carboxylic Acid. Z. Anorg. Allg. Chem. 2014, 640, 2498–2502. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.-B.; Tan, Y.-X.; Wang, F.; Kang, Y.; Zhang, J. Structural Diversity and Photoluminescent Properties of Zinc Benzotriazolate-5-carboxylate Coordination Polymers. Inorg. Chem. 2014, 53, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Bunzen, H.; Grzywa, M.; Hambach, M.; Spirkl, S.; Volkmer, D. From Micro to Nano: A Toolbox for Tuning Crystal Size and Morphology of Benzotriazolate-Based Metal-Organic Frameworks. Cryst. Growth Des. 2016, 16, 3190–3197. [Google Scholar] [CrossRef]
- Brede, F.A.; Mühlbach, F.; Sextl, G.; Müller-Buschbaum, K. Mechanochemical and thermal formation of 1H-benzotriazole coordination polymers and complexes of 3d-transition metals with intriguing dielectric properties. Dalton Trans. 2016, 45, 10609–10619. [Google Scholar] [CrossRef]
- Xie, W.; Qin, J.-S.; He, W.-W.; Shao, K.-Z.; Su, Z.-M.; Du, D.-Y.; Li, S.-L.; Lan, Y.-Q. Encapsulation of an iridium complex in a metal–organic framework to give a composite with efficient white light emission. Inorg. Chem. Front. 2017, 4, 547–552. [Google Scholar] [CrossRef]
- Xie, W.; Ning, S.; Zhang, Y.; Tang, Z.; Zhang, S.; Tang, R. A 3D supramolecular network as highly selective and sensitive luminescent sensor for PO43− and Cu2+ ions in aqueous media. Dyes Pigm. 2018, 150, 36–43. [Google Scholar] [CrossRef]
- Feng, X.; Guo, N.; Li, R.; Chen, H.; Ma, L.; Li, Z.; Wang, L. A facile route for tuning emission and magnetic properties by controlling lanthanide ions in coordination polymers incorporating mixed aromatic carboxylate ligands. J. Solid State Chem. 2018, 268, 22–29. [Google Scholar] [CrossRef]
- Bunzen, H.; Grzywa, M.; Aljohani, R.; von Nidda, H.-A.K.; Volkmer, D. Synthesis, Thermal Stability and Magnetic Properties of a Manganese(II) Coordination Framework Containing Bistriazolate Ligands. Eur. J. Inorg. Chem. 2019, 2019, 4471–4476. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; An, X.; Xu, L.; Yan, J.-H.; Zhang, S.; Xie, W.; Su, Z.-M. Syntheses, structure and properties of an especially stable Cd metal-organic framework driven by benzotriazole-5-carboxylic acid. Inorg. Chem. Commun. 2020, 112, 107726. [Google Scholar] [CrossRef]
- Tangoulis, V.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Skorda, K.; Perlepes, S.P.; Cador, O.; Kahn, O.; Bakalbassis, E.G. Ferromagnetism in an Extended Three-Dimensional, Diamond-like Copper(II) Network: A New Copper(II)/1-hydroxybenzotriazolate Complex Exhibiting Soft-Magnet Properties and two Transitions at 6.4 and 4.4 K. Inorg. Chem. 2000, 39, 2522–2529. [Google Scholar] [CrossRef]
- Palmer, M.H.; Findlay, R.H.; Kennedy, S.M.F.; McIntyre, P.S. Reactivity of Indazoles and Benzotriazole towards N-Methylation and Analysis of the 1H Nuclear Magnetic Resonance Spectra of Indazoles and Benzotriazoles. J. Chem. Soc. Perkin II 1975, 7, 1695–1700. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Kuzmierkiewicz, W.; Greenhill, J.V. An improved method for the N-alkylation of benzotriazole and 1,2,4-triazole. Recl. Trav. Chim. Pays-Bas 1991, 110, 369–373. [Google Scholar] [CrossRef]
- Dimitropoulos, A.; Stamou, C.; Perlepes, S.P.; Lada, Z.G.; Petsalakis, I.D.; Marinakis, S. A study of 1-Methylbenzotriazole (MEBTA) Using Quantum Mechanical Calculations and Vibrational, Electronic and Nuclear Magnetic Resonance Spectroscopies. J. Eng. Sci. Technol. Rev. 2023, 16, 77–84. [Google Scholar] [CrossRef]
- Mudzakir, A.; Liebing, P.; Haak, E.; Fischer, A.; Hilfert, L.; Goldhahn, R.; Edelmann, F.T. An unusual phosphide addition reaction of 1,3-dimethyl-1,2,3-benzotriazolium iodide. Inorg. Chem. Commun. 2024, 161, 111924. [Google Scholar] [CrossRef]
- Fang, B.-S.; Olson, C.G.; Lynch, D.W. A Photoemission Study of Benzotriazole on Clean Copper and Cuprous Oxide. Surf. Sci. 1986, 176, 476–490. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Girotto, M.; Enrichi, F.; Vomiero, A. Luminescent copper(I) coordination polymer with 1-methyl-1H-benzotriazole, iodide and acetonitrile as ligands. Inorg. Chem. Commun. 2019, 102, 141–146. [Google Scholar] [CrossRef]
- Jones, L.F.; O’Dea, L.; Offermann, D.A.; Jensen, P.; Moubaraki, B.; Murray, K.S. Benzotriazole based 1-D, 2-D and 3-D metal dicyanamide and tricyanomethanide coordination networks. Polyhedron 2006, 25, 360–372. [Google Scholar] [CrossRef]
- Plakatouras, J.C.; Bakas, T.; Huffman, C.J.; Huffman, J.C.; Papaefthymiou, V.; Perlepes, S.P. Two Different Terminal Nitrate Bonding Modes in [Fe2O(NO3)4(C7H7N3)4]. J. Chem. Dalton Trans. 1994, 2737–2738. [Google Scholar] [CrossRef]
- Anastasiadis, N.C.; Bilis, G.; Plakatouras, J.C.; Raptopoulou, C.P.; Psycharis, V.; Beavers, C.; Teat, S.J.; Louloudi, M.; Perlepes, S.P. Iron(III) chloride-benzotriazole adducts with trigonal bipyramidal geometry: Spectroscopic, structural and catalytic studies. Polyhedron 2013, 64, 189–202. [Google Scholar] [CrossRef]
- Plakatouras, J.C.; Perlepes, S.P.; Mentzafos, D.; Terzis, A.; Bakas, T.; Papaefthymiou, V. Coordination Chemistry of Corrosion Inhibitors of the Benzotriazole Type: Preparation and Characterization of Cobalt(II) Complexes with 1-methylbenzotriazole (Mebta) and the Crystal Structures of [CoCl2(Mebta)2], trans-[Co(NCS)2(Mebta)4], trans-[Co(NCS)2(MeOH)2(Mebta)2] and cis-[Co(NO3)2(Mebta)2]. Polyhedron 1992, 11, 2657–2672. [Google Scholar]
- Diamantopoulou, E.; Zafiropoulos, T.F.; Perlepes, S.P.; Raptopoulou, C.P.; Terzis, A. Synthetic and Structural Chemistry of Nickel(II)/1-methylbenzotriazole Complexes. Polyhedron 1994, 13, 1593–1608. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Perlepes, S.P. Coordination compounds of palladium(II) and platinum(II) with benzotriazoles. Transition Met. Chem. 1994, 19, 7–11. [Google Scholar] [CrossRef]
- Skorda, K.; Perlepes, S.P.; Raptopoulou, C.P.; Keuleers, R.; Terzis, A.; Plakatouras, J. A structural model for the copper(II) site of Cu-Zn superoxide dismutase: Preparation, crystal structure and properties of [Cu(Mebta)4(H2O)](ClO4)2∙0.4EtOH (Mebta = 1-methylbenzotriazole). Transition Met. Chem. 1999, 24, 541–545. [Google Scholar] [CrossRef]
- Skorda, K.; Bakalbassis, E.G.; Mrozinski, J.; Perlepes, S.P.; Raptopoulou, C.P.; Terzis, A. Copper(II) Chloride-1-Methylbenzotriazole Chemistry: Variation of Product as a Function of Metal-to-Ligand Reaction Ratio; Synthesis, Structure and Properties of a Dinuclear Complex and a Novel Chain Polymer with two Alternating Chromophores. J. Chem. Soc. Dalton Trans. 1995, 2317–2319. [Google Scholar] [CrossRef]
- Skorda, K.; Stamatatos, T.C.; Vafiadis, A.P.; Lithoxoidou, A.T.; Terzis, A.; Perlepes, S.P.; Mrozinski, J.; Raptopoulou, C.P.; Plakatouras, J.C.; Bakalbassis, E.G. Copper(II) chloride/1-methylbenzotriazole chemistry: Influence of various synthetic parameters on the product identity, structural and magnetic characterization, and quantum-chemical studies. Inorg. Chim. Acta 2005, 358, 562–582. [Google Scholar] [CrossRef]
- Skorda, K.; Keuleers, R.; Terzis, A.; Raptopoulou, C.P.; Perlepes, S.P.; Plakatouras, J.C. Copper(II) bromide/1-methylbenzotriazole chemistry. Variation of product as a function of solvent and ligand-to-metal reaction ratio. Polyhedron 1999, 18, 3067–3075. [Google Scholar] [CrossRef]
- Lazari, G.; Grammatikopoulos, S.; Perlepes, S.P.; Stamatatos, T.C. Combining benzotriazoles and azides in copper(II) chemistry: Synthesis, structural and spectroscopic characterization of a 1-D corrugated tape [Cu(N3)2(1-Mebta)]n coordination polymer (1-Mebta = 1-methylbenzotriazole). J. Coord. Chem. 2021, 74, 1823–1833. [Google Scholar] [CrossRef]
- Wu, T.; Li, M.; Li, D.; Huang, X.-C. Anions CunIn Cluster-Based Architectures Induced by In Situ Generated N-Alkylated Cationic Triazolium Salts. Cryst. Growth Des. 2008, 8, 568–574. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Sun, N.; Liu, Y. Gold-Catalyzed Cadiot-Chodkiewicz-Type Cross-Coupling of Terminal Alkynes with Alkynyl Hypervalent Iodine Reagents: Highly Selective Synthesis of Unsymmetrical 1,3-Diynes. Angew. Chem. Int. Ed. 2017, 56, 6994–6998. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Mouzakitis, M.; Savvidou, A.; Raptopoulou, C.P.; Psycharis, V.; Perlepes, S.P. The “periodic table” of benzotriazoles: Uranium(VI) complexes. Inorg. Chem. Commun. 2015, 59, 57–60. [Google Scholar] [CrossRef]
- Ward, J.S.; Frontera, A.; Rissanen, K. Utility of Three-Coordinate Silver Complexes Toward the Formation of Iodonium Ions. Inorg. Chem. 2021, 60, 5383–5390. [Google Scholar] [CrossRef]
- Yu, S.; Kumar, P.; Ward, J.S.; Frontera, A.; Rissanen, K. A “nucleophilic” iodine in a halogen-bonded iodonium complex manifests an unprecedented I+⋯Ag+ interaction. Chem 2021, 7, 948–958. [Google Scholar] [CrossRef]






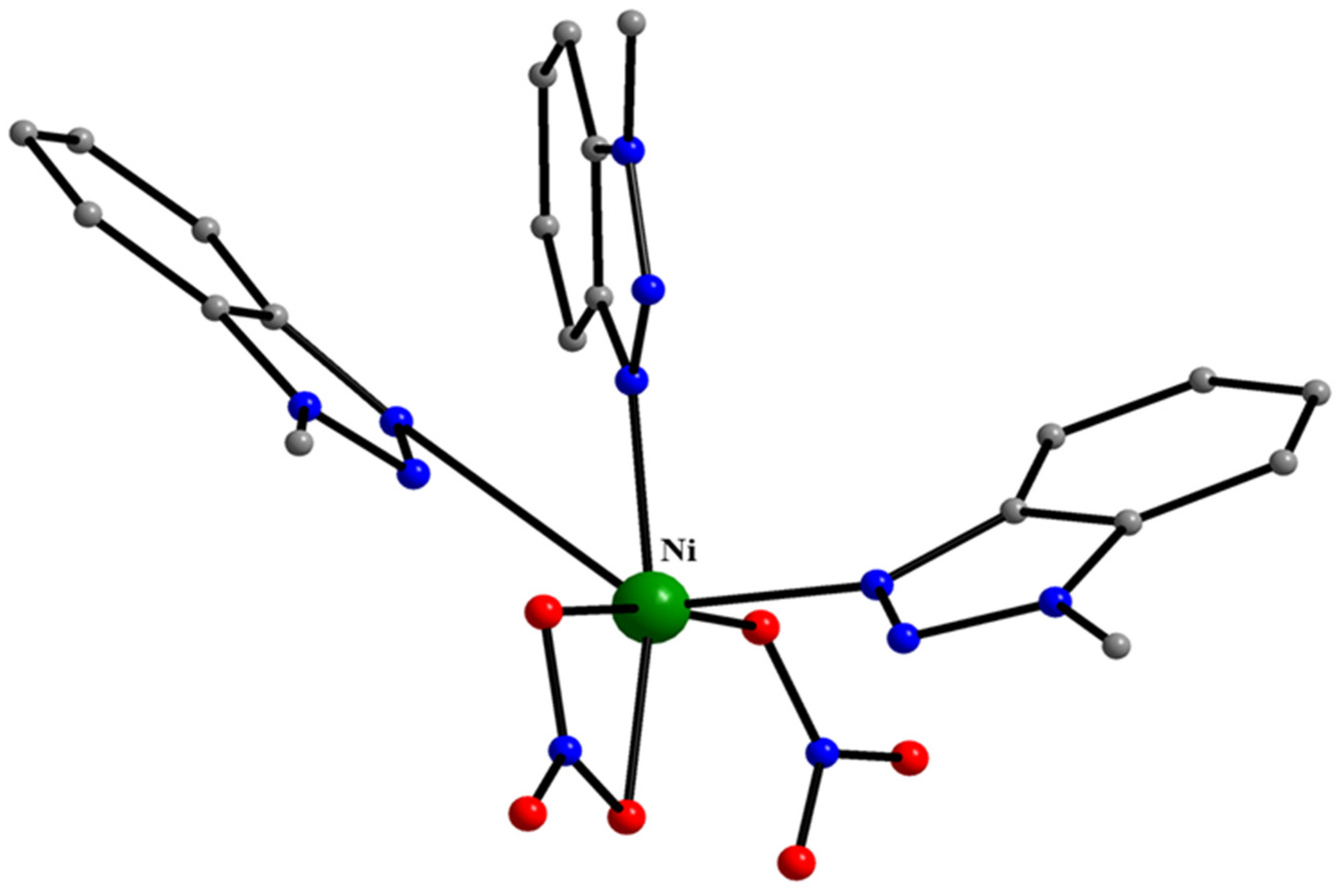
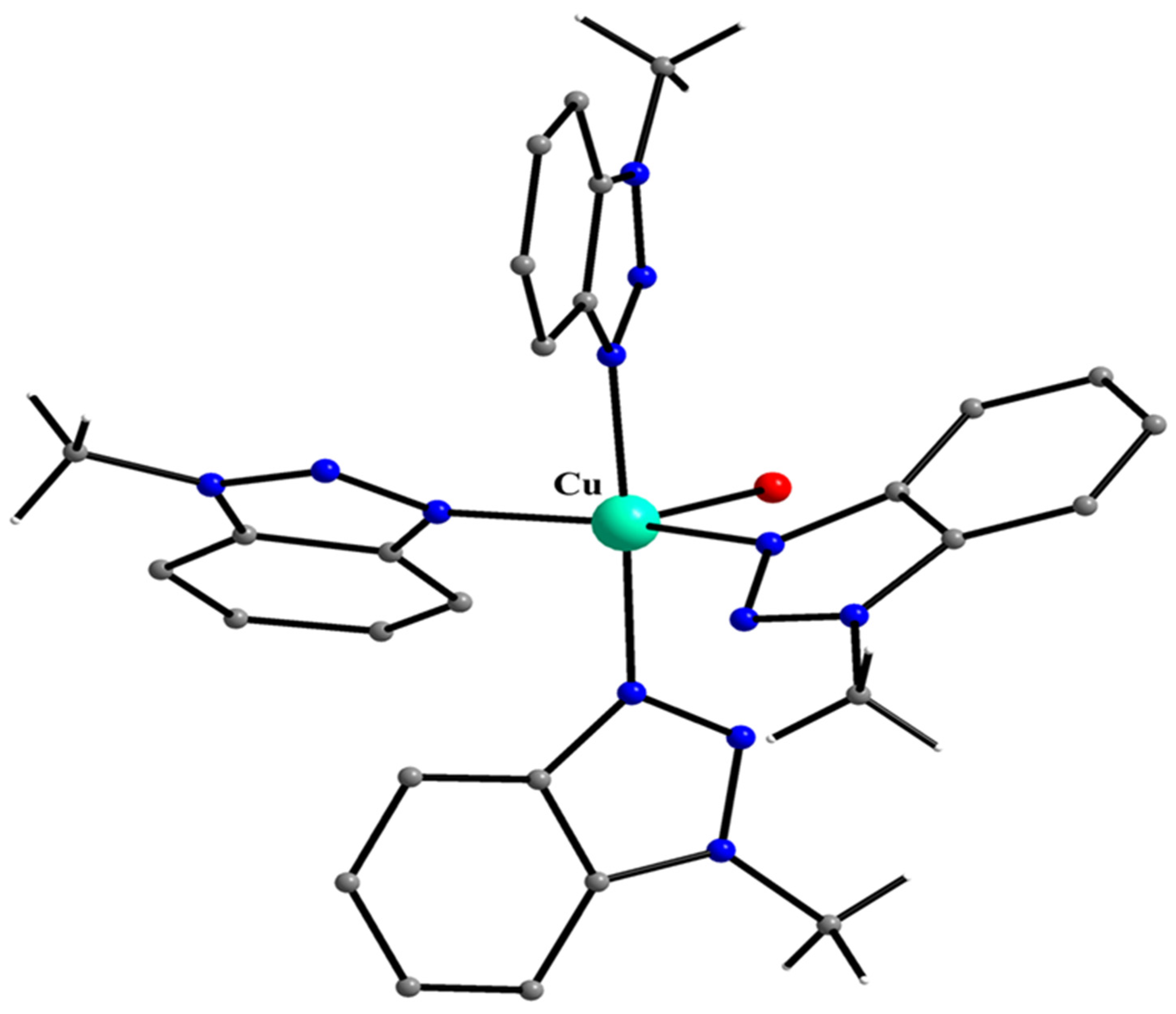
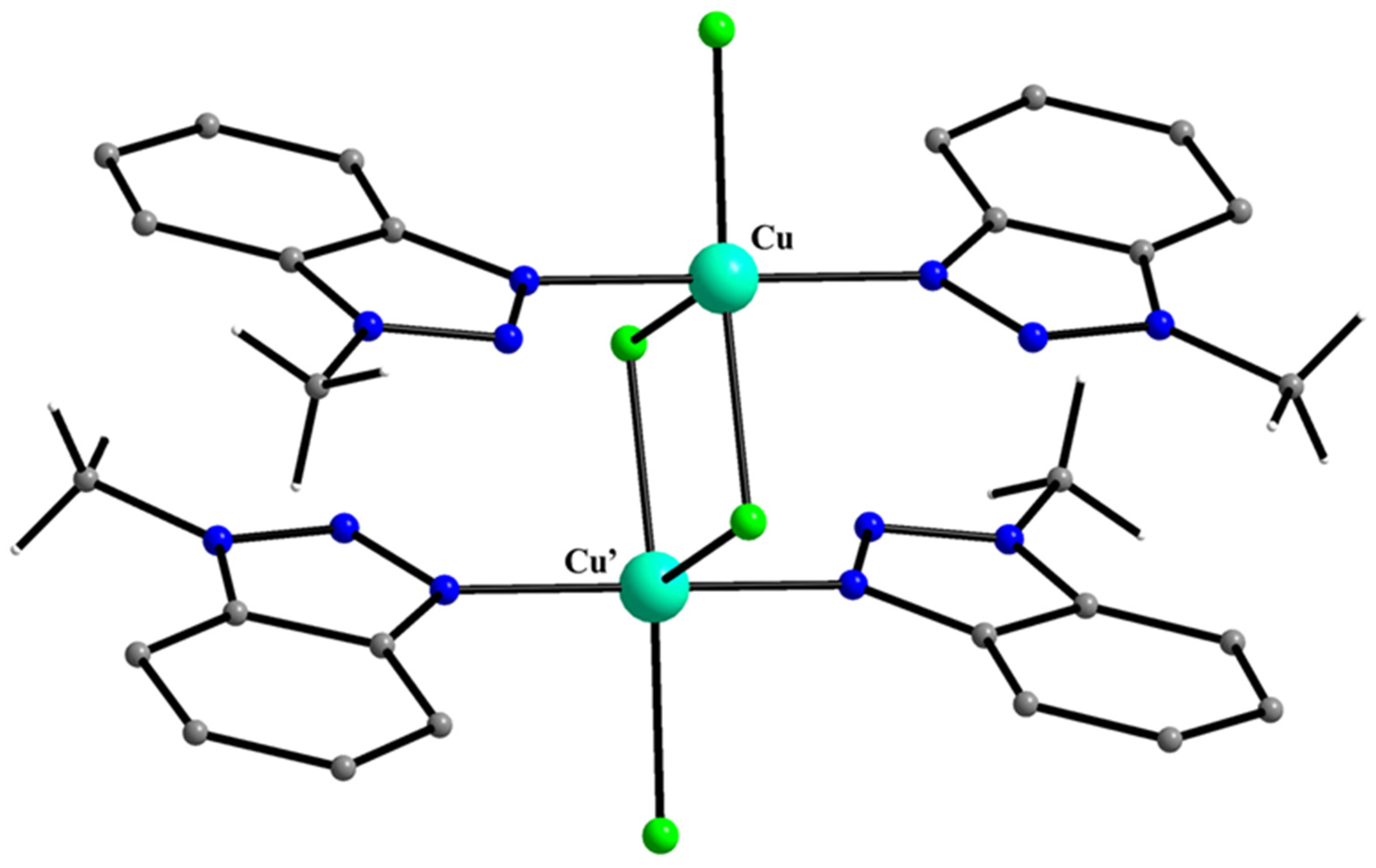
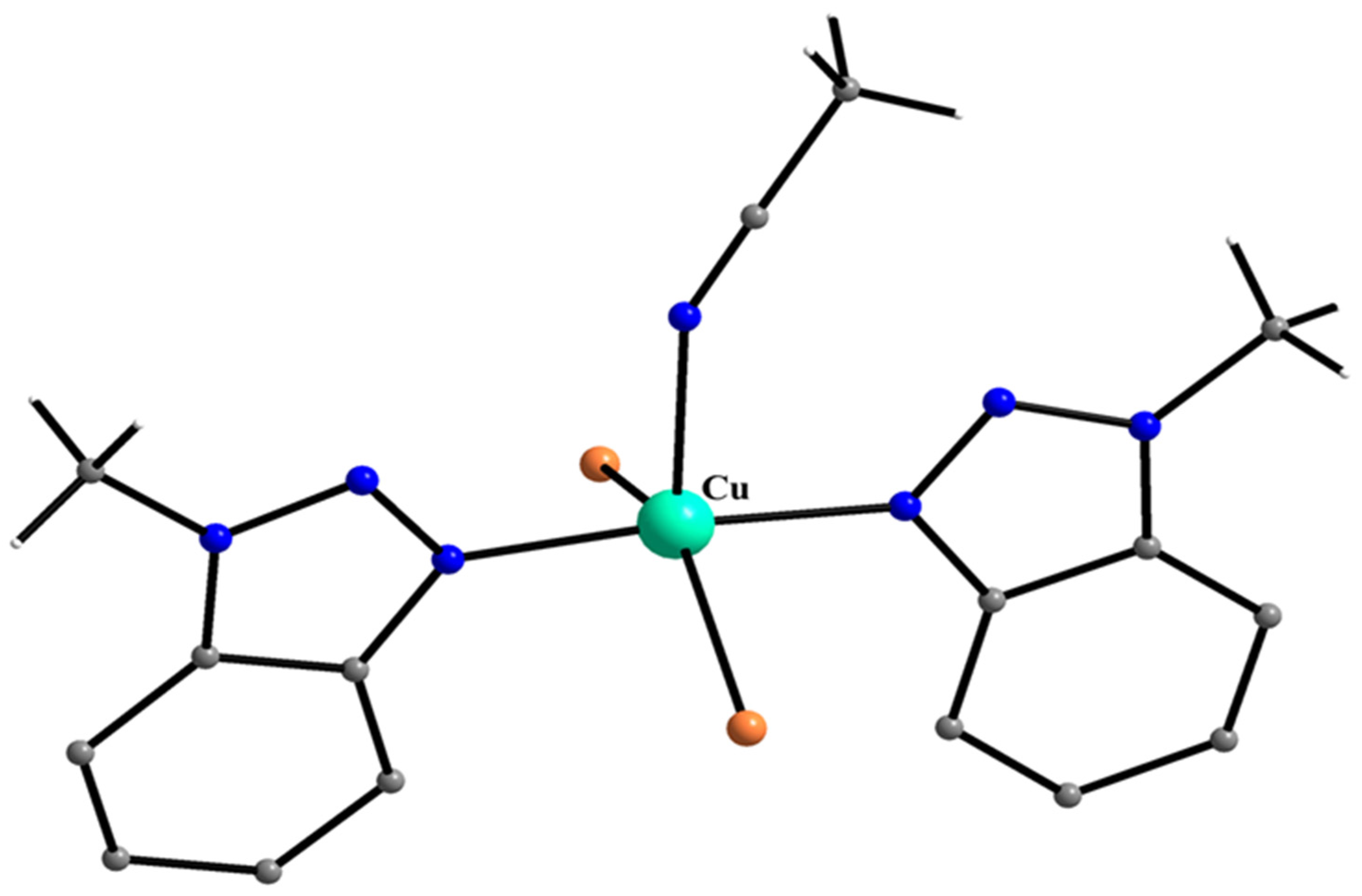
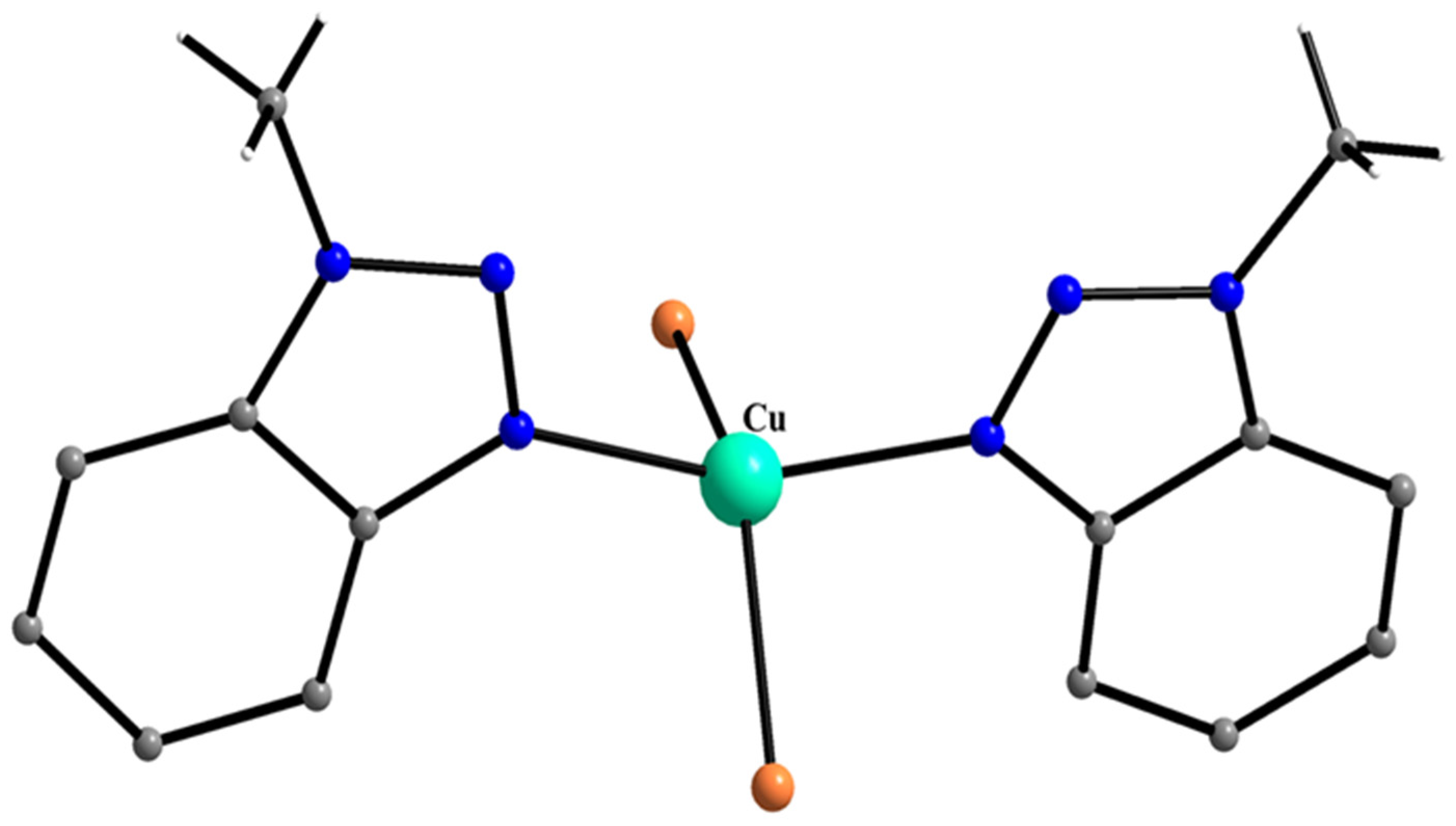
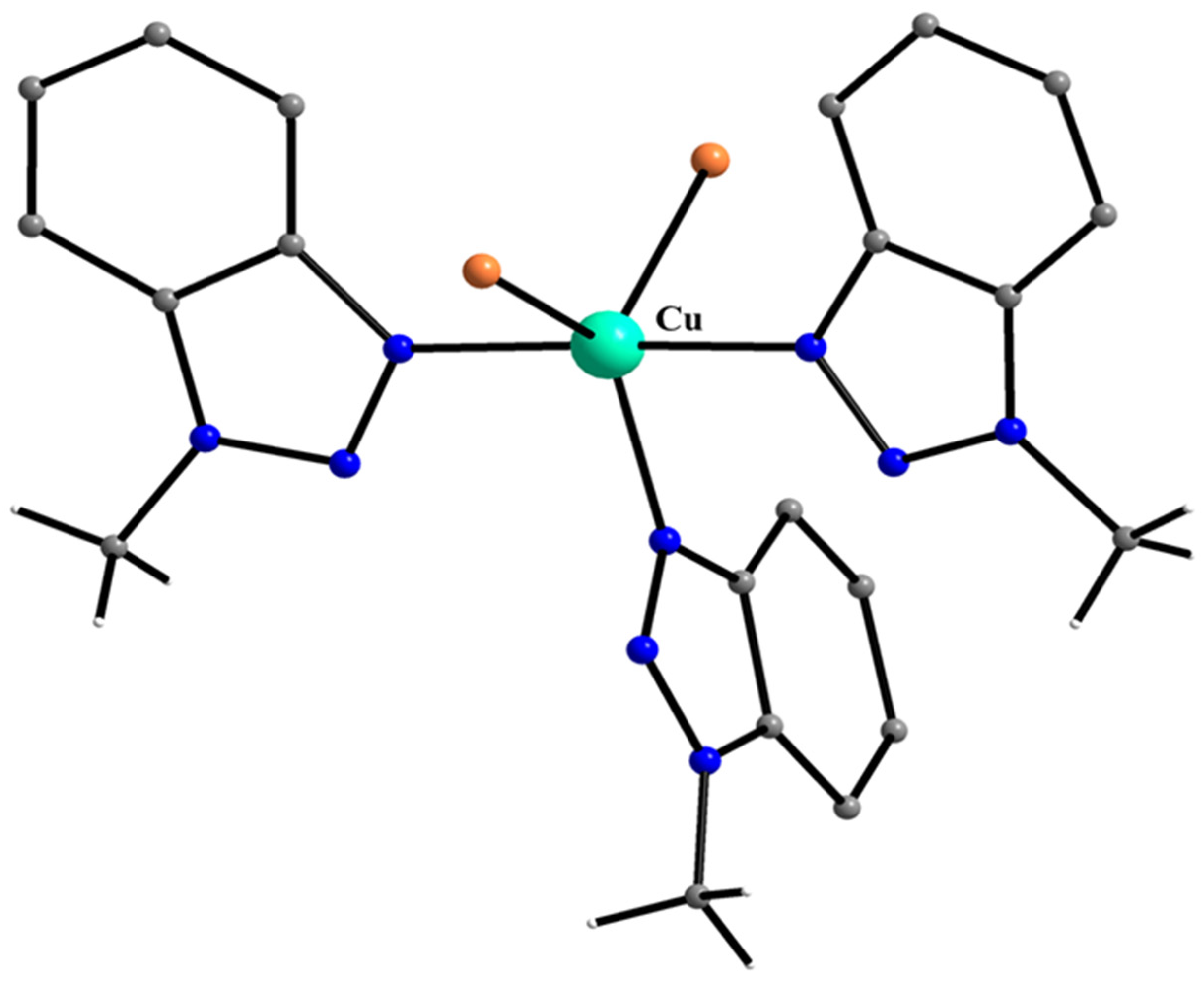
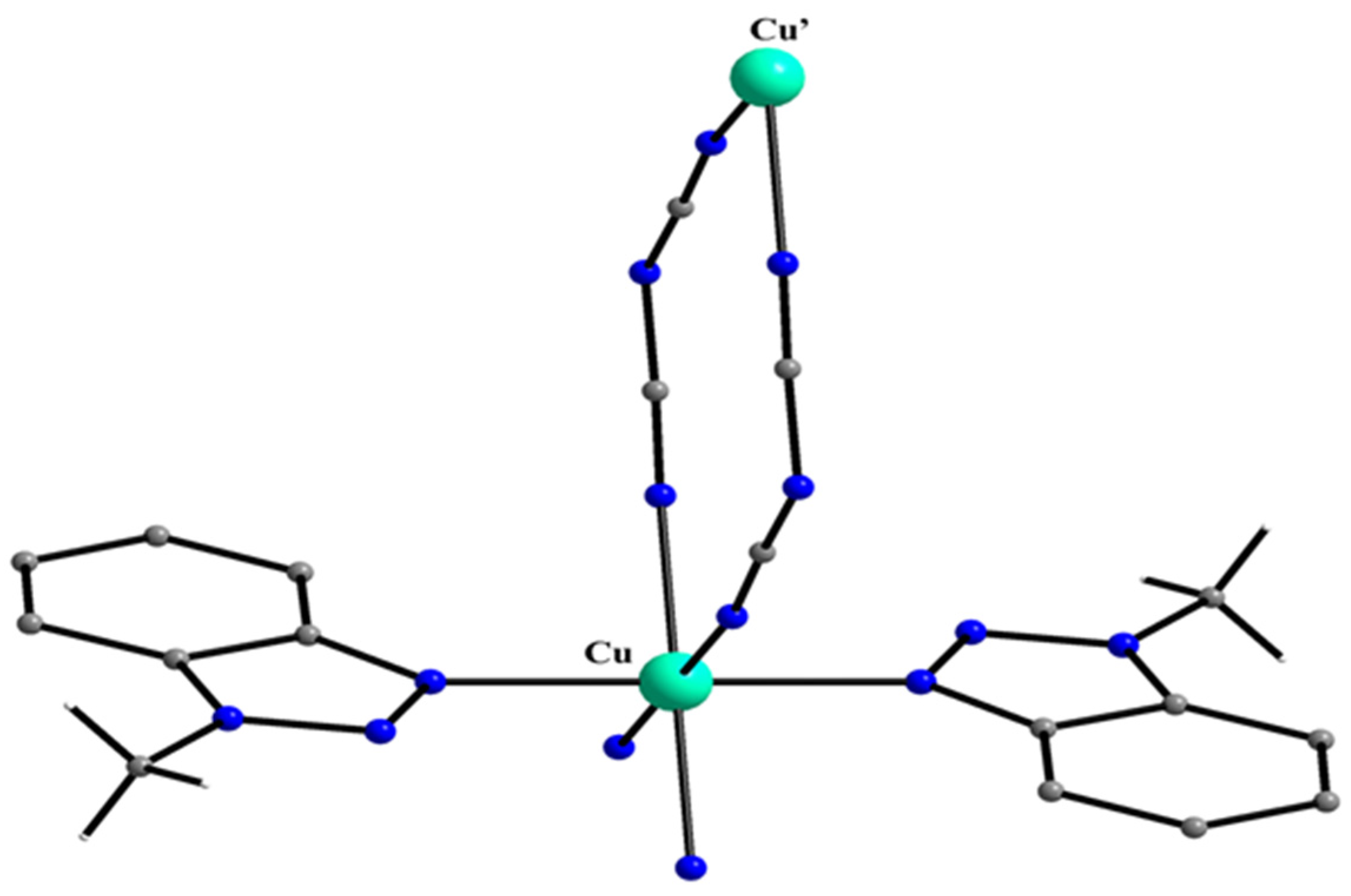





| A Ni(II) Complex |
|---|
| trans-[Ni(SCN)2(MeOH)2(Mebta)2] (37) a |
| A Trinuclear Cu(II) Cluster |
| [Cu3(OH)2(Mebta)10](BF4)4 (38) |
| Polymeric Dicyanoamido Co(II) and Zn(II) Complexes |
| {[Co{N(CN)2}2(Mebta)2]}n b (39) |
| {[Zn{N(CN)2}2(Mebta)2]}n b (40) |
| A Trinuclear Carboxylato-Bridged Zn(II) Cluster |
| [Zn3(O2CPh)6(Mebta)2] (41) |
| Three-Coordinate Silver(I) Complexes |
| [Ag(NO3)(Mebta)2] (42) |
| [Ag(CF3SO3)(Mebta)2] (43) |
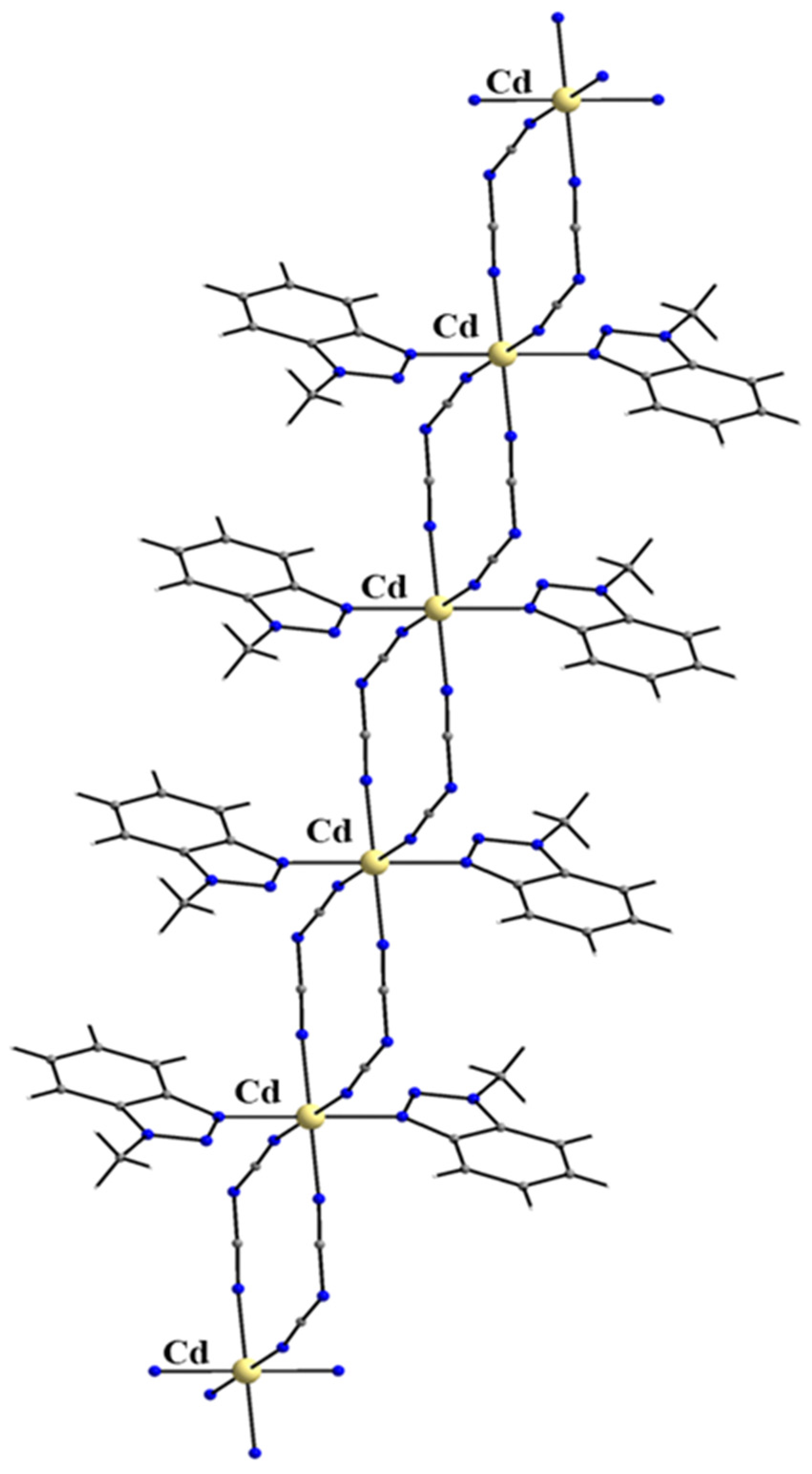
| Cd(II) Complexes |
| [CdBr2(Mebta)2] (44) |
| [CdI2(Mebta)2] (45) |
| trans-[Cd(NO3)2(H2O)2(Mebta)2] (46) |
| {[CdBr2(Mebta)]}n b (47) |
| {[Cd3(SCN)6(Mebta)(H2O)]}n b (48) |
| {[Cd2.5(SCN)5(Mebta)4(H2O)]}n b (49) |
| {[Cd{N(CN)2}2(Mebta)2]}n b (50) |
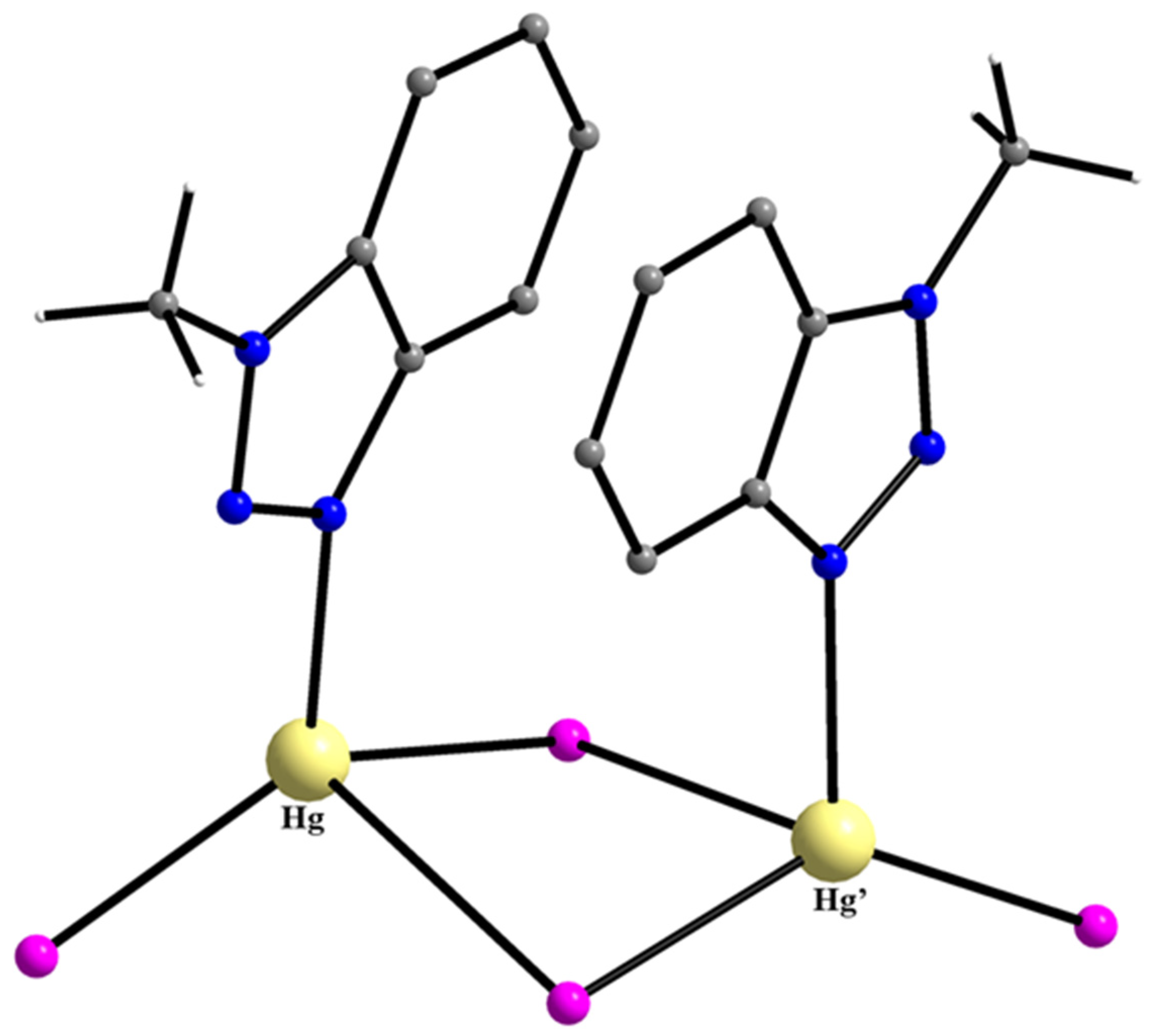
| Hg(II) Complexes |
| {[HgCl2(Mebta)]}n b (51) |
| {[HgBr2(Mebta)]}n b (52) |
| [Hg2I4(Mebta)2] (53) |
| {[Hg2(SCN)4(Mebta)3]}n b (54) |
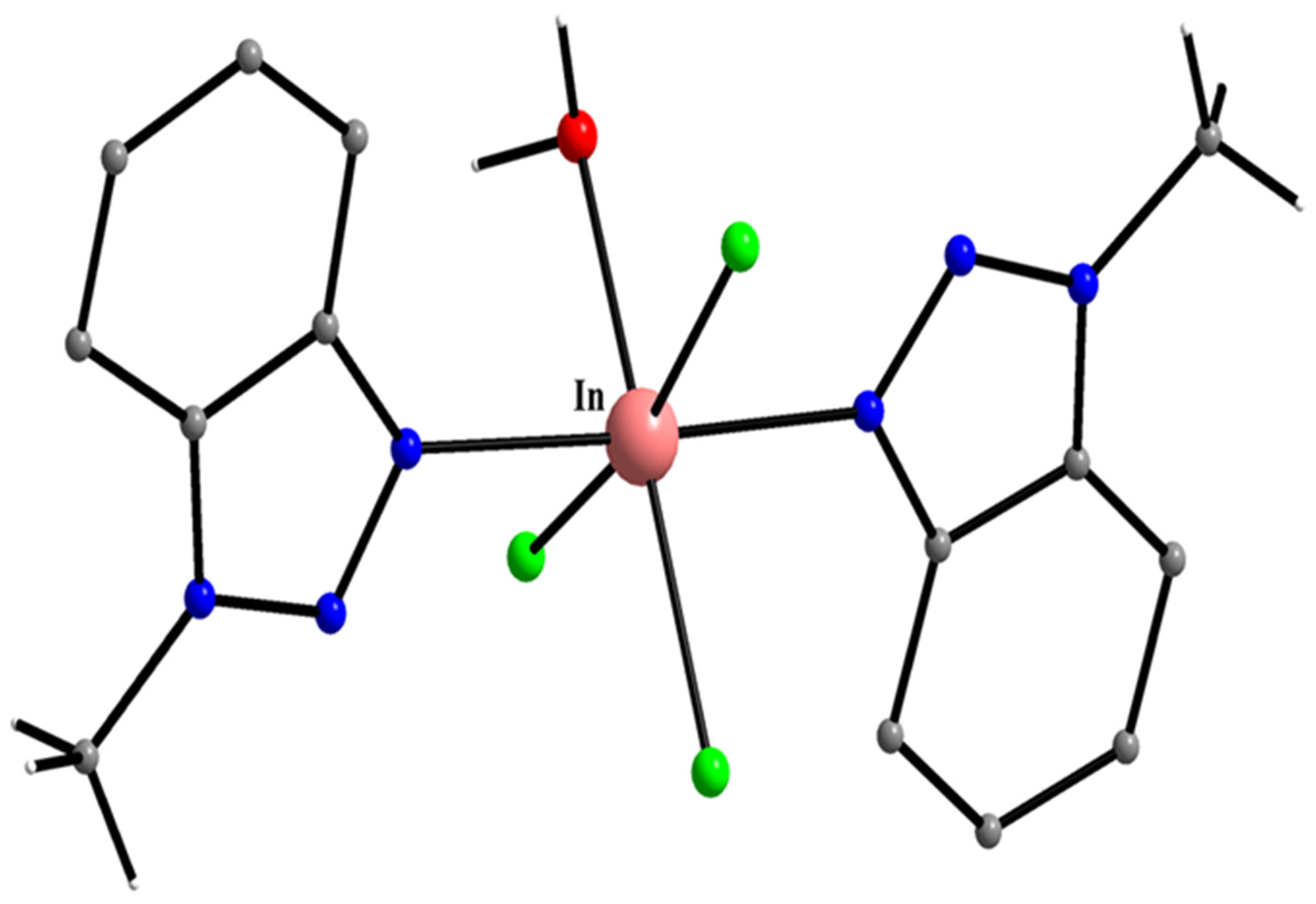
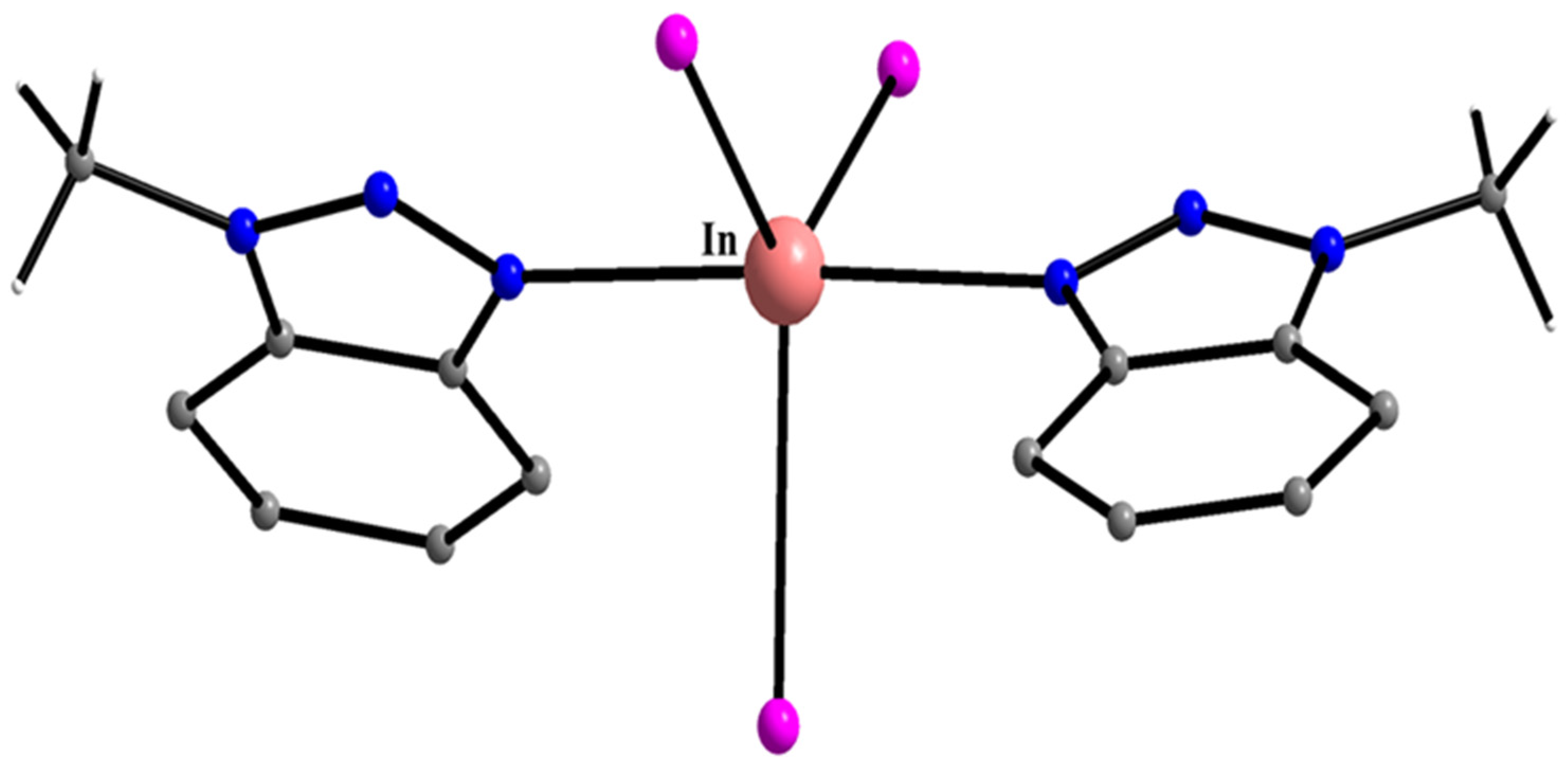
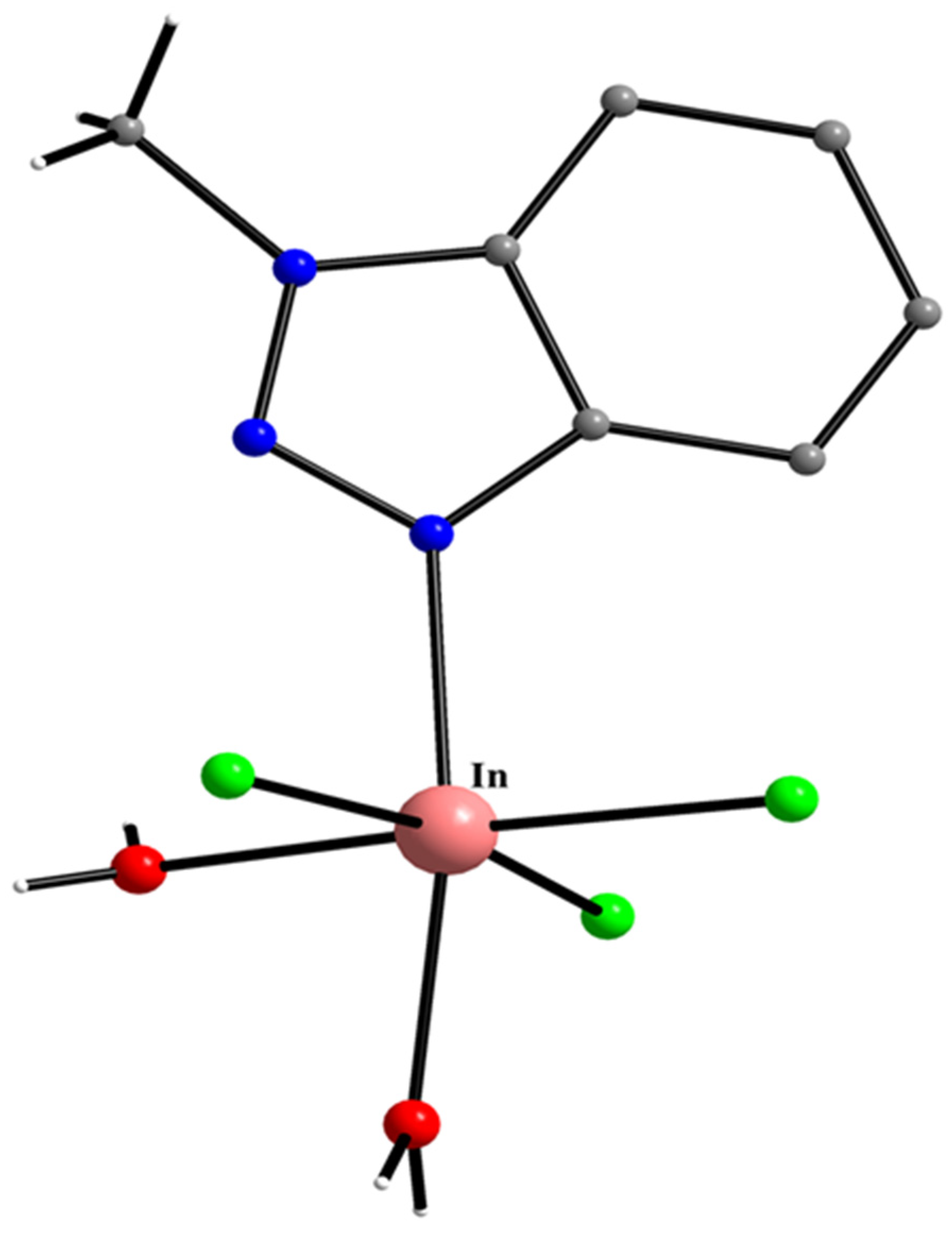
| In(III) Complexes |
| mer-[InCl3(H2O)(Mebta)2] (55) |
| mer-[InBr3(H2O)(Mebta)2] (56) |
| mer-[InCl3(H2O)2(Mebta)] (57) |
| mer-[In(Cl/Br)3(H2O)2(Mebta)] (58) |
| [InI3(Mebta)2] (59) |
| Sn(IV) Complexes |
| [(Me)2SnCl2(Mebta)2] (60) |
| (MebtaH)2[SnCl6] c (61) |
| (MebtaH)2[Sn(Cl0.55Br0.45)6] c (62) |
| Heterometallic Coordination Polymers |
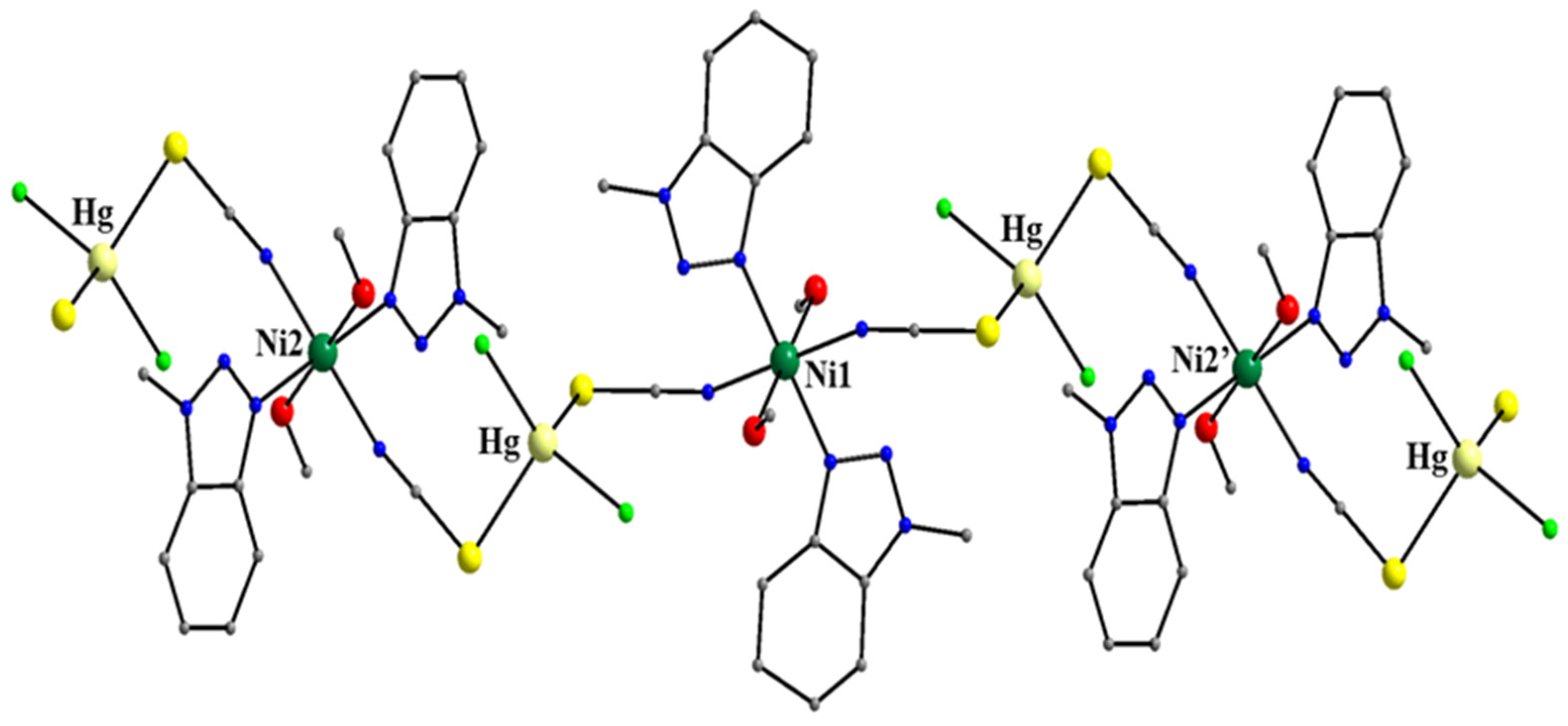
| {[HgCo(SCN)4(Mebta)2]}n d (63) |
| {[HgCo(SCN)2Cl2(H2O)2(Mebta)2]}n b (64) |
| {[HgCo(SCN)2Br2(DMF)2(Mebta)2]}n b (65) |
| {[HgNi(SCN)2Cl2(MeOH)2(Mebta)2]}n b (66) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamou, C.; Lada, Z.G.; Paschalidou, S.; Chasapis, C.T.; Perlepes, S.P. Towards Construction of the “Periodic Table” of 1-Methylbenzotriazole. Inorganics 2024, 12, 208. https://doi.org/10.3390/inorganics12080208
Stamou C, Lada ZG, Paschalidou S, Chasapis CT, Perlepes SP. Towards Construction of the “Periodic Table” of 1-Methylbenzotriazole. Inorganics. 2024; 12(8):208. https://doi.org/10.3390/inorganics12080208
Chicago/Turabian StyleStamou, Christina, Zoi G. Lada, Sophia Paschalidou, Christos T. Chasapis, and Spyros P. Perlepes. 2024. "Towards Construction of the “Periodic Table” of 1-Methylbenzotriazole" Inorganics 12, no. 8: 208. https://doi.org/10.3390/inorganics12080208






