Physical Vapor Deposition of Indium-Doped GeTe: Analyzing the Evaporation Process and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bulk Sample Preparation
2.2. Thin Film Preparation
2.3. Theoretical Foundations of Evaporation Processes
3. Results and Discussion
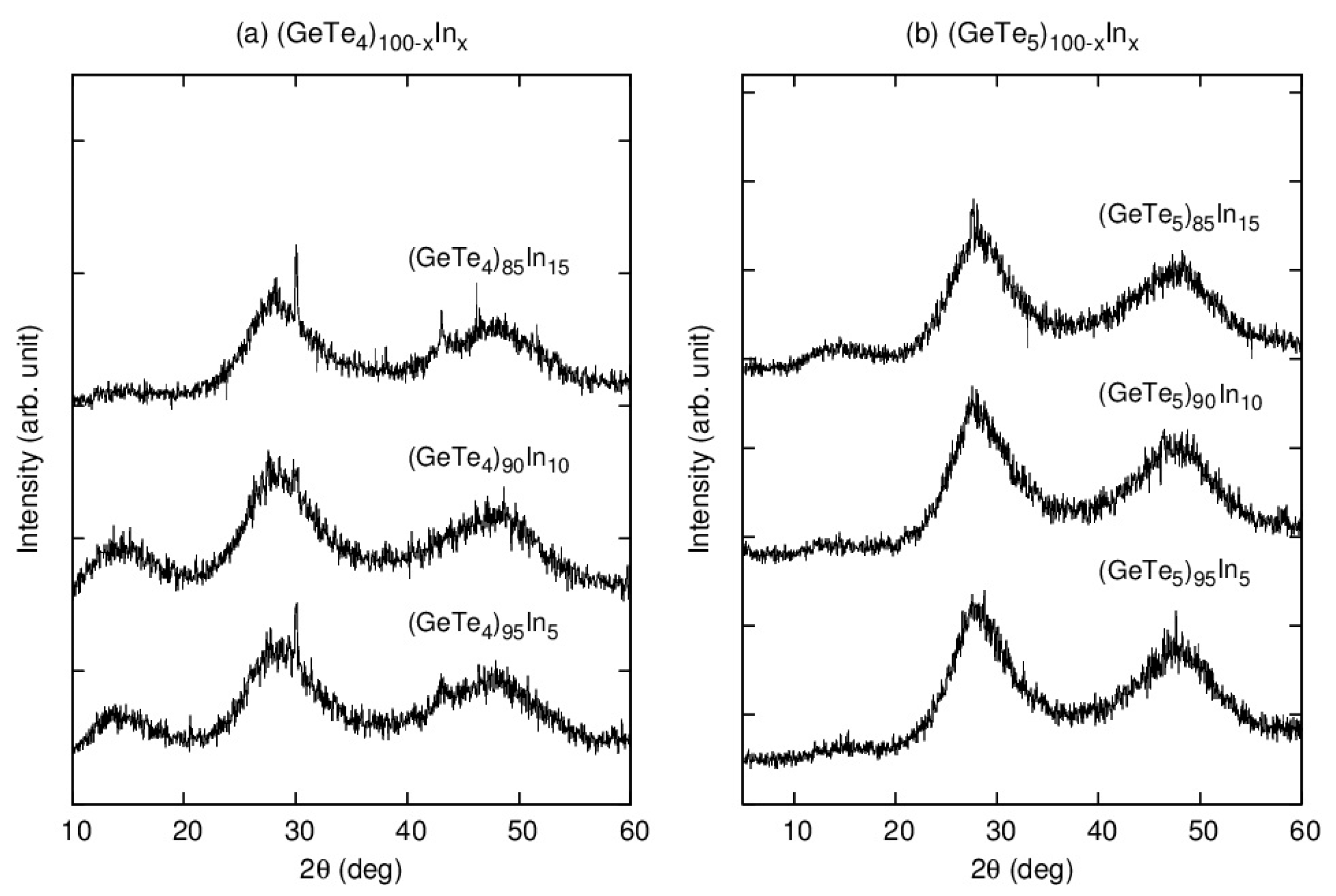
3.1. Structure and Thermal Properties of Bulk Samples
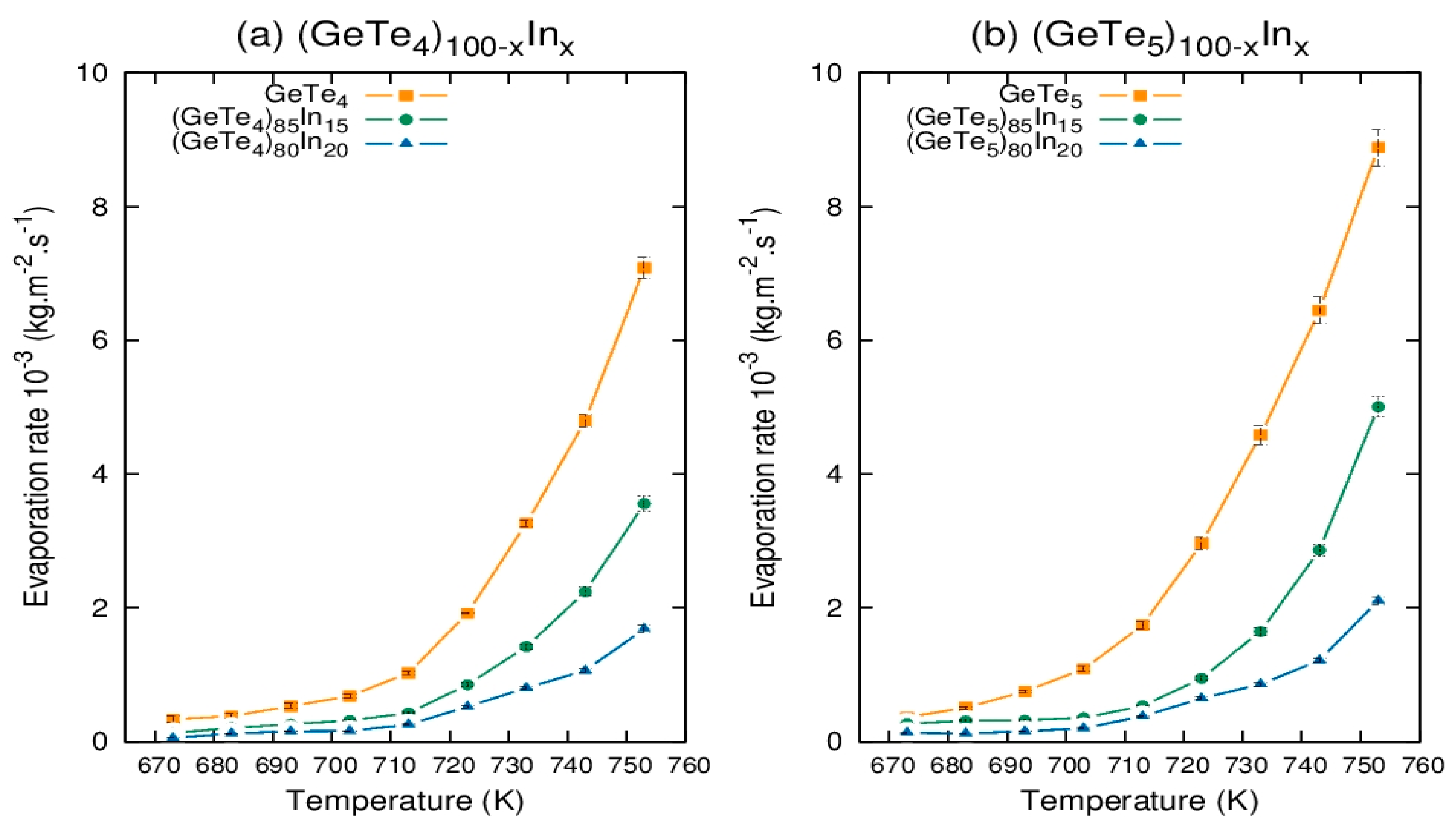
3.2. Evaporation Rate of Chalcogenide Ge-Te-In Glasses
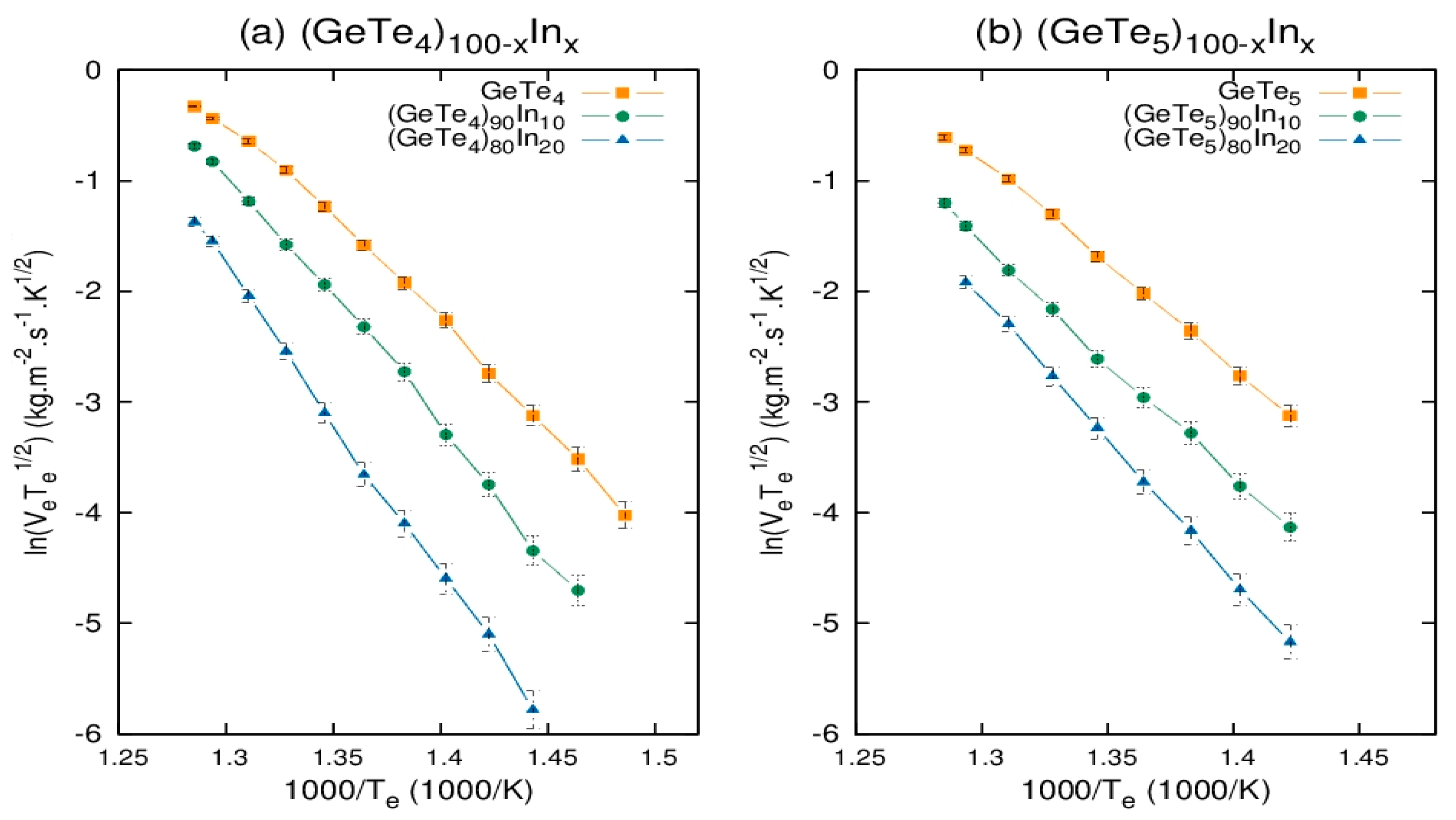
3.3. Evaporation Energy of Chalcogenide Ge-Te-In Glasses
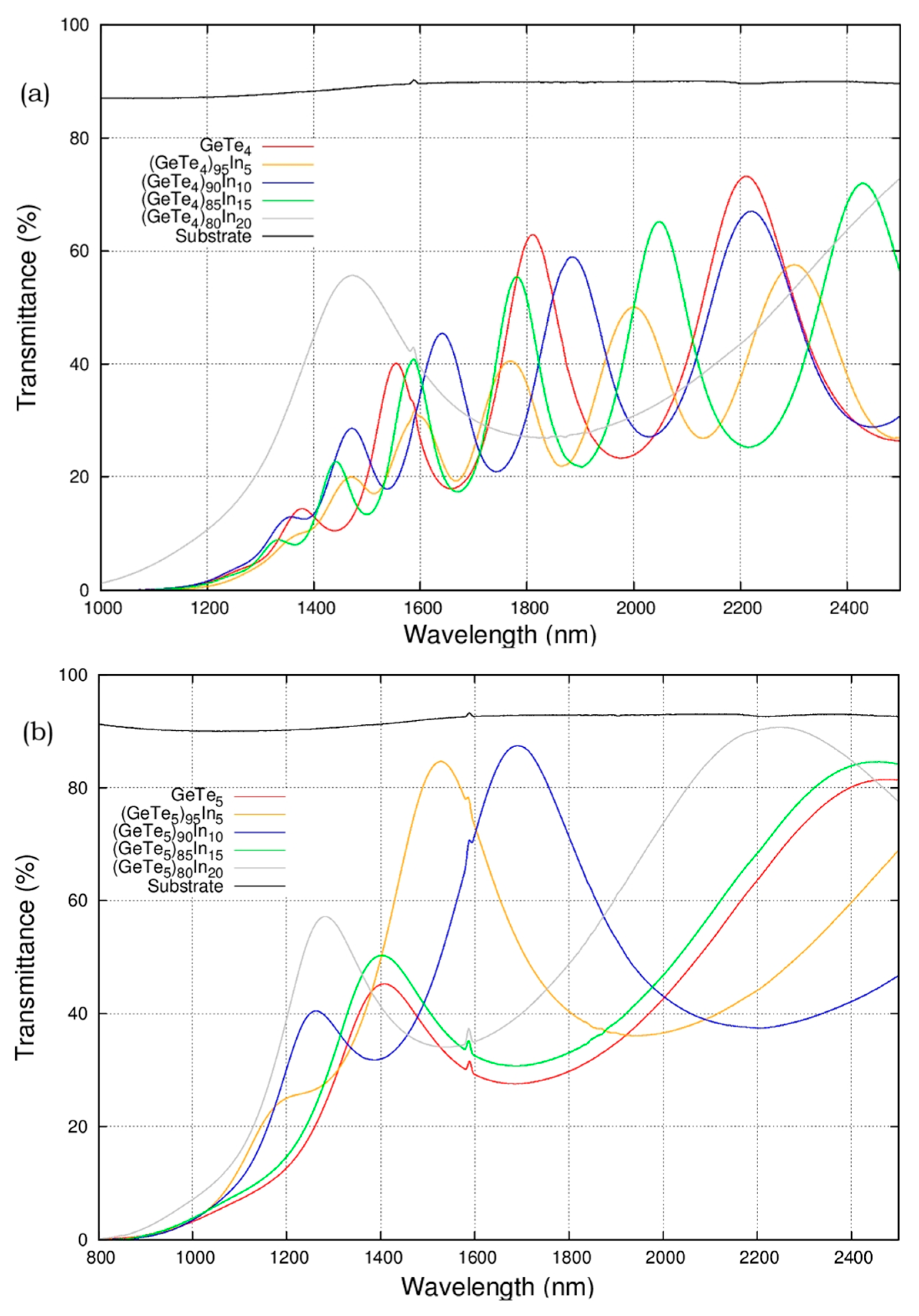
3.4. Thickness and Non-Equilibrium Nature of Deposition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Chen, W.; Wang, P.; Dai, S.; Li, J.; Li, Y.; Fu, Q.; Dai, T.; Yu, H.; Yang, J. Ultra-high-power-confinement-factor integrated mid-infrared gas sensor based on the suspended slot chalcogenide glass waveguide. Sens. Actuators B Chem. 2021, 347, 130466. [Google Scholar] [CrossRef]
- Oke, J.A.; Olotu, O.O.; Jen, T.-C. Atomic layer deposition of chalcogenide thin films: Processes film properties, applications, and bibliometric prospect. J. Mater. Res. Technol. 2022, 20, 991–1019. [Google Scholar] [CrossRef]
- Halenkovič, T.; Baillieul, M.; Gutwirth, J.; Němec, P.; Nazabal, V. Amorphous Ge-Sb-Se-Te chalcogenide films fabrication for potential environmental sensing and nonlinear photonics. J. Mater. 2022, 8, 1009–1019. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, K.; Tao, P.; Dai, S.; Wang, X.; Zhang, W.; Xu, T.; Wang, Y.; Lin, T.-J.; Zhang, P. Mid-infrared evanescent wave sensor based on side-polished chalcogenide fiber. Ceram. Int. 2023, 49, 1291–1297. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, C.; Qi, S.; Guo, W.; Yang, Z.; Yang, A.; Gai, X.; Yu, Y.; Wang, R.; Tang, D.; et al. High-resolution chalcogenide fiber bundles for infrared imaging. Opt. Lett. 2015, 40, 4384. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.E.; Kleine, T.S.; Zhang, Y.; Phan, D.D.; Namnabat, S.; LaVilla, E.A.; Konopka, K.M.; Diaz, L.R.; Manchester, M.S.; Schwiegerling, J.; et al. Chalcogenide Hybrid Inorganic/Organic Polymers: Ultrahigh Refractive Index Polymers for Infrared Imaging. ACS Macro Lett. 2017, 6, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Feng, S.; Yu, Y.; Shen, J.; Wei, X.; Shi, H. Electrochemical epitaxial (200) PbSe submicron-plates on single-layer graphene for an ultrafast infrared response. J. Mater. Chem. C 2021, 9, 6536–6543. [Google Scholar] [CrossRef]
- Nakotte, T.; Luo, H.; Pietryga, J. PbE (E = S Se) Colloidal Quantum Dot-Layered 2D Material Hybrid Photodetectors. Nanomaterials 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Cen, M. Fundamentals and Applications of Chalcogenide Phase-Change Material Photonics. Adv. Theory Simul. 2019, 2, 1900094. [Google Scholar] [CrossRef]
- Wuttig, M.; Bhaskaran, H.; Taubner, T. Phase-change materials for nonvolatile photonic applications. Nat. Photonics 2017, 11, 465–476. [Google Scholar] [CrossRef]
- Abdollahramezani, S.; Hemmatyar, O.; Taghinejad, H.; Krasnok, A.; Kiarashinejad, Y.; Zandehshahvar, M.; Alù, A.; Adibi, A. Tunable nanophotonics enabled by chalcogenide phase-change materials. Nanophotonics 2020, 9, 1189–1241. [Google Scholar] [CrossRef]
- Ríos, C.; Stegmaier, M.; Hosseini, P.; Wang, D.; Scherer, T.; Wright, C.D.; Bhaskaran, H.; Pernice, W.H.P. Integrated all-photonic nonvolatile multi-level memory. Nat. Photonic 2015, 9, 725–732. [Google Scholar] [CrossRef]
- Farmakidis, N.; Youngblood, N.; Li, X.; Tan, J.; Swett, J.L.; Cheng, Z.; Wright, C.D.; Pernice, W.H.P.; Bhaskaran, H. Plasmonic nanogap enhanced phase-change devices with dual electrical-optical functionality. Sci. Adv. 2019, 5, eaaw2687. [Google Scholar] [CrossRef] [PubMed]
- Gemo, E.; Faneca, J.; Carrillo, S.G.-C.; Baldycheva, A.; Pernice, W.H.P.; Bhaskaran, H.; Wright, C.D. A plasmonically enhanced route to faster and more energy-efficient phase-change integrated photonic memory and computing devices. J. Appl. Phys. 2021, 129, 110902. [Google Scholar] [CrossRef]
- Wong, P.H.-S.; Raoux, S.; Kim, S.; Liang, J.; Reifenberg, J.P.; Rajendran, B.; Asheghi, M.; Goodson, K.E. Phase change memory. Proc. IEEE 2010, 98, 2201–2227. [Google Scholar] [CrossRef]
- Rudé, M.; Pello, J.; Simpson, R.E.; Osmond, J.; Roelkens, G.; van der Tol, J.J.G.M.; Pruneri, V. Optical switching at 1.55 µm in silicon racetrack resonators using phase change materials. Appl. Phys. Lett. 2013, 103, 141119. [Google Scholar] [CrossRef]
- Rios, C.; Stegmaier, M.; Cheng, Z.; Youngblood, N.; Wright, C.D.; Pernice, W.H.P.; Bhaskaran, H. Controlled switching of phase-change materials by evanescent-field coupling in integrated photonics [Invited]. Opt. Mater. Express 2018, 8, 2455. [Google Scholar] [CrossRef]
- Stegmaier, M.; Ríos, C.; Bhaskaran, H.; Wright, C.D.; Pernice, W.H.P. Nonvolatile, All-Optical 1 2 Switch for Chipscale Photonic Networks. Adv. Opt. Mater. 2016, 5, 1600346. [Google Scholar] [CrossRef]
- Bendiab, A.T.; Bathily, M.; Vigreux, C.; Escalier, R.; Pradel, A.; Kribich, R.K.; Bendoula, R. Chalcogenide rib waveguides for the characterization of spray deposits. Opt. Mater. 2018, 86, 298–303. [Google Scholar] [CrossRef]
- Tewari, A.; Kumar, A.; Saini, T.S.; Sinha, R.K. Design of As2Se3 based chalcogenide ridge waveguide for generation of slow light. Optik 2016, 127, 11816–11822. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, P.; Yu, Z.; Shen, X.; Nie, Q. Rib chalcogenide glass waveguide with simultaneous dispersion flatting for both transverse electric and magnetic modes. Optik 2017, 138, 433–439. [Google Scholar] [CrossRef]
- Byrnes, A.; Pant, R.; Li, E.; Choi, D.-Y.; Poulton, C.G.; Fan, S.; Madden, S.; Luther-Davies, B.; Eggleton, B.J. Photonic chip based tunable and reconfigurable narrowband microwave photonic filter using stimulated Brillouin scattering. Opt. Express 2012, 20, 18836. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, B.J.; Vo, T.D.; Pant, R.; Schr, J.; Pelusi, M.D.; Choi, D.Y.; Madden, S.J.; Luther-Davies, B. Photonic chip based ultrafast optical processing based on high nonlinearity dispersion engineered chalcogenide waveguides. Laser Photonics Rev. 2011, 6, 97–114. [Google Scholar] [CrossRef]
- Hu, L.; Huang, S.; Patterson, R.; Halpert, J.E. Enhanced mobility in PbS quantum dot films via PbSe quantum dot mixing for optoelectronic applications. J. Mater. Chem. C 2019, 7, 4497–4502. [Google Scholar] [CrossRef]
- Heenkenda, R.; Sarangan, A. Design principles of tunable optical filters using phase change materials. In Proceedings of the International Workshop on Thin Films for Electronics, Electro-Optics, Energy and Sensors, Boston, MA, USA, 10–12 August 2022; SPIE: Bellingham, DC, USA, 2023; Volume 12477. [Google Scholar] [CrossRef]
- Tripathi, D.; Vyas, H.; Kumar, S.; Panda, S.; Hegde, R. Recent developments in Chalcogenide phase change material-based nanophotonics. Nanotechnology 2023, 34, 502001. [Google Scholar] [CrossRef] [PubMed]
- Burrow, J.; Toussaint, K.; Sarangan, A.; Agha, I. Reconfigurable opto-chirality enabled by chalcogenide phase change nanomaterials. In Proceedings of the International Workshop on Thin Films for Electronics, Electro-Optics, Energy and Sensors, Boston, MA, USA, 10–12 August 2022; SPIE: Bellingham, DC, USA, 2023; Volume 12477. [Google Scholar] [CrossRef]
- Joshi, S. Peculiarities of electrical switching and phase transition dynamics in bismuth-infused Se–Te chalcogenide glasses: From bulk to thin film devices. Acs Appl. Electron. Mater. 2024, 6, 3574–3588. [Google Scholar] [CrossRef]
- Kim, H.J.; Julian, M.; Williams, C.; Bombara, D.; Hu, J.; Gu, T.; Aryana, K.; Sauti, G.; Humphreys, W. Versatile spaceborne photonics with chalcogenide phase-change materials. NPJ Microgravity 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Attar, R.; Sawant, R.; Pandey, S.; Joglekar, P.; Mondal, S.; Kulkarni, S.; Behera, J. Design of tunable far-infrared plasmonic absorber based on chalcogenide phase change materials. J. Phys. Conf. Ser. 2023, 2426, 012068. [Google Scholar] [CrossRef]
- Prabhathan, P.; Sreekanth, K.; Teng, J.; Ko, J.; Yoo, Y.; Jeong, H.; Zhang, S.; Cao, T.; Popescu, C.; Mills, B.; et al. Roadmap for phase change materials in photonics and beyond. iScience 2023, 26, 107946. [Google Scholar] [CrossRef]
- Yan, A.; Wang, C.; Yan, J.; Wang, Z.; Zhang, E.; Dong, Y.; Yan, Z.-Y.; Lu, T.; Cui, T.; Li, D.; et al. Thin-film transistors for integrated circuits: Fundamentals and recent progress. Adv. Funct. Mater. 2024, 34, 2304409. [Google Scholar] [CrossRef]
- Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214–1222. [Google Scholar] [CrossRef]

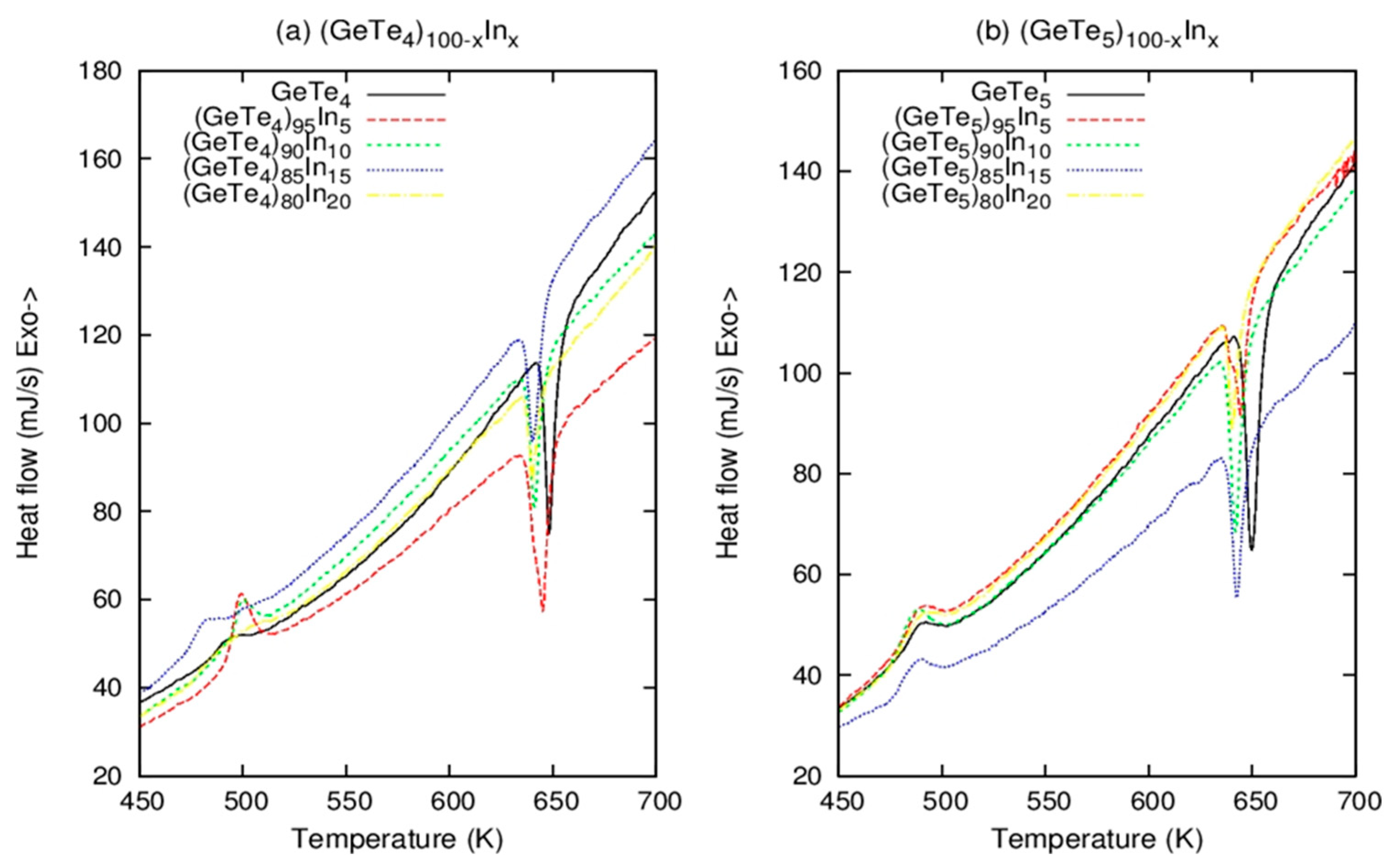
| Composition | (K) | (K) | (K) | |
|---|---|---|---|---|
| 2.40 | 481.8 | 487.7 | 648.7 | |
| 2.43 | 492.7 | 499.7 | 645.6 | |
| 2.46 | 476.6 | 500.7 | 641.6 | |
| 2.49 | 469.7 | 483.6 | 640.7 | |
| 2.52 | 477.5 | 500.4 | 640.6 | |
| 2.34 | 478.6 | 497.7 | 649.6 | |
| 2.37 | 480.7 | 498.6 | 644.7 | |
| 2.40 | 478.7 | 488.6 | 642.7 | |
| 2.43 | 476.6 | 490.7 | 642.6 | |
| 2.47 | 478.7 | 497.6 | 640.7 |
| Composition | Slope | |
|---|---|---|
| 20.184 | 167.819 0.622 | |
| 21.749 | 180.831 0.816 | |
| 22.627 | 188.131 0.709 | |
| 23.615 | 196.346 0.708 | |
| 24.291 | 201.967 0.735 | |
| 18.634 | 154.932 0.683 | |
| 19.682 | 163.645 0.659 | |
| 20.935 | 174.063 0.640 | |
| 22.102 | 183.733 0.733 | |
| 23.581 | 196.063 0.769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidan, A.; Ivanova, V.; Petkov, P. Physical Vapor Deposition of Indium-Doped GeTe: Analyzing the Evaporation Process and Kinetics. Inorganics 2024, 12, 209. https://doi.org/10.3390/inorganics12080209
Zaidan A, Ivanova V, Petkov P. Physical Vapor Deposition of Indium-Doped GeTe: Analyzing the Evaporation Process and Kinetics. Inorganics. 2024; 12(8):209. https://doi.org/10.3390/inorganics12080209
Chicago/Turabian StyleZaidan, Andi, Vladislava Ivanova, and Plamen Petkov. 2024. "Physical Vapor Deposition of Indium-Doped GeTe: Analyzing the Evaporation Process and Kinetics" Inorganics 12, no. 8: 209. https://doi.org/10.3390/inorganics12080209





