Improving Charge Transport in Perovskite Solar Cells Using Solvent Additive Technique
Abstract
1. Introduction
2. Results
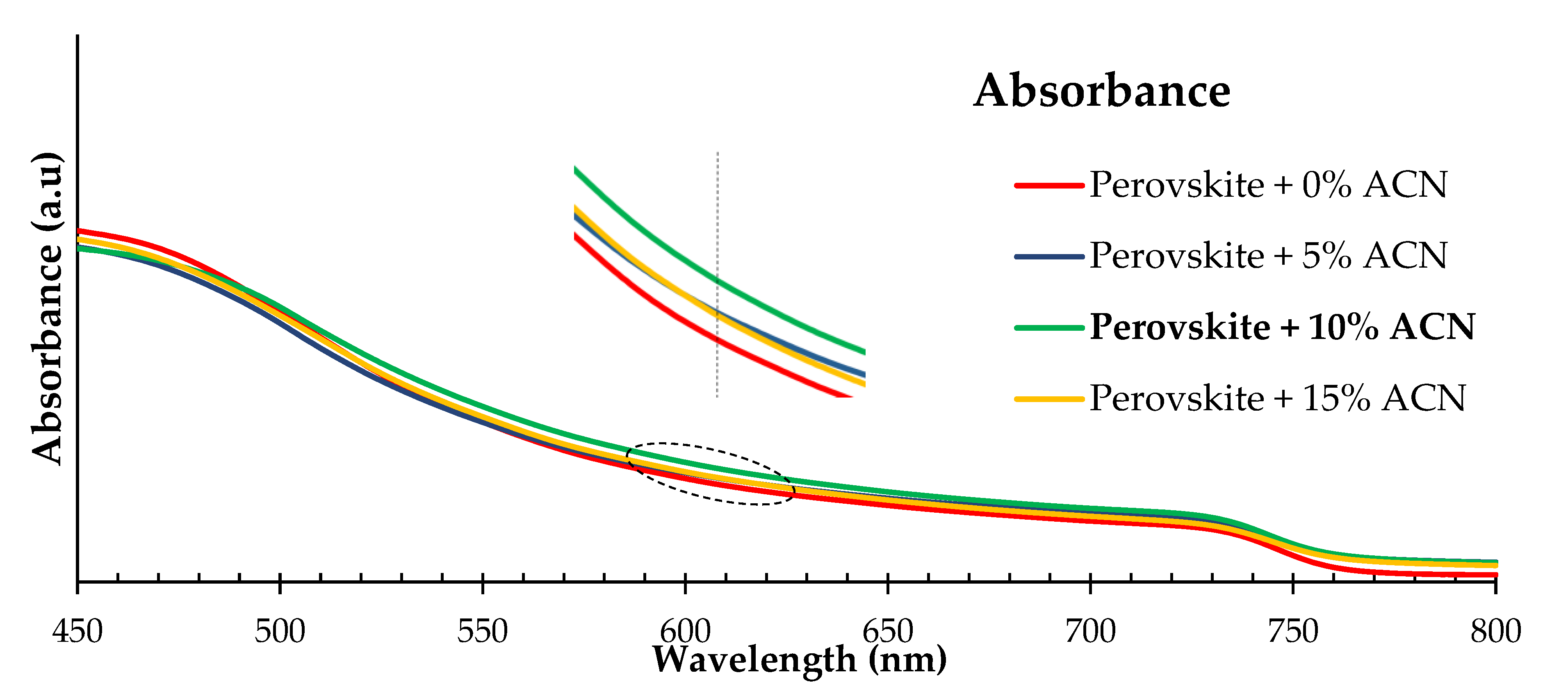
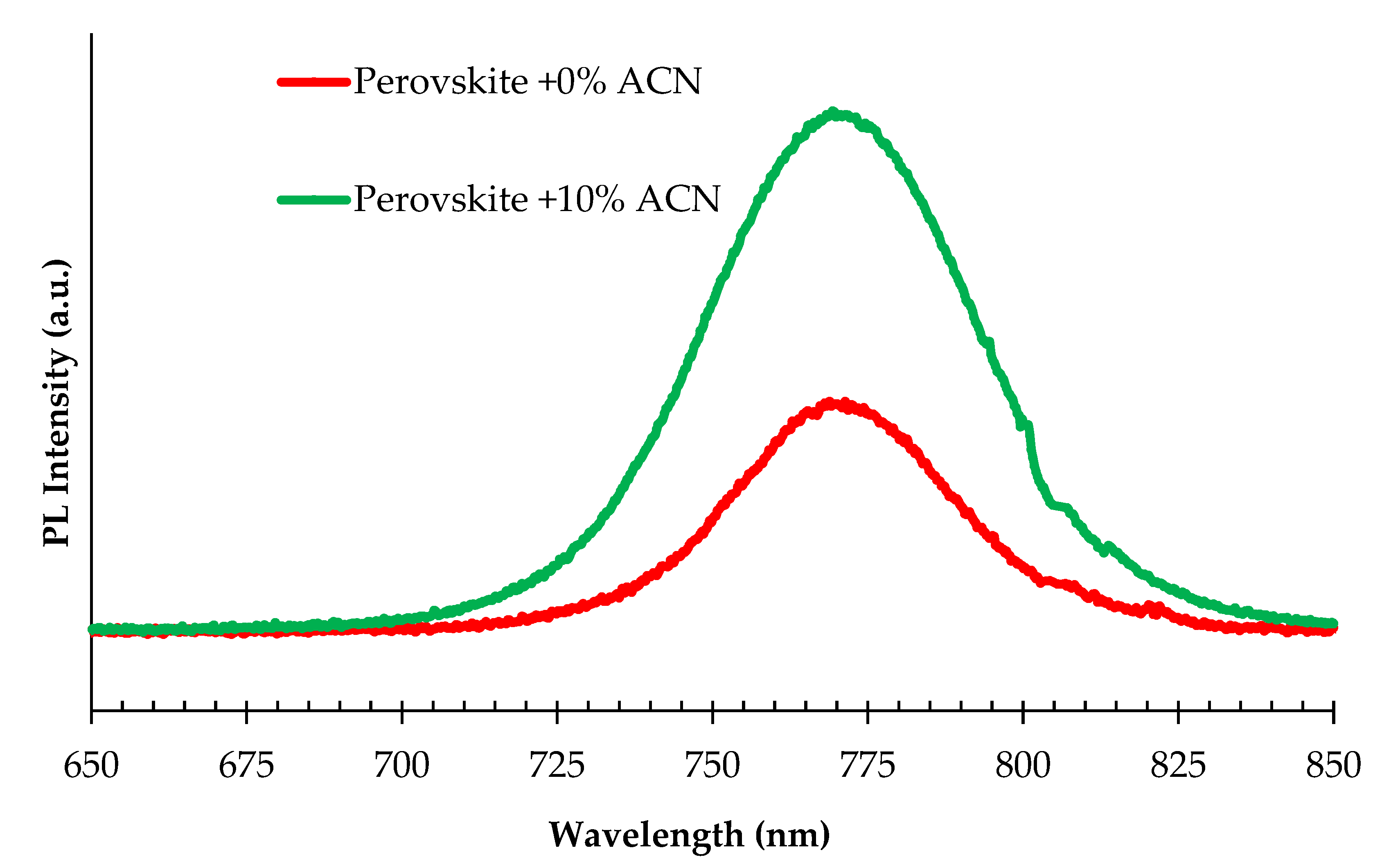
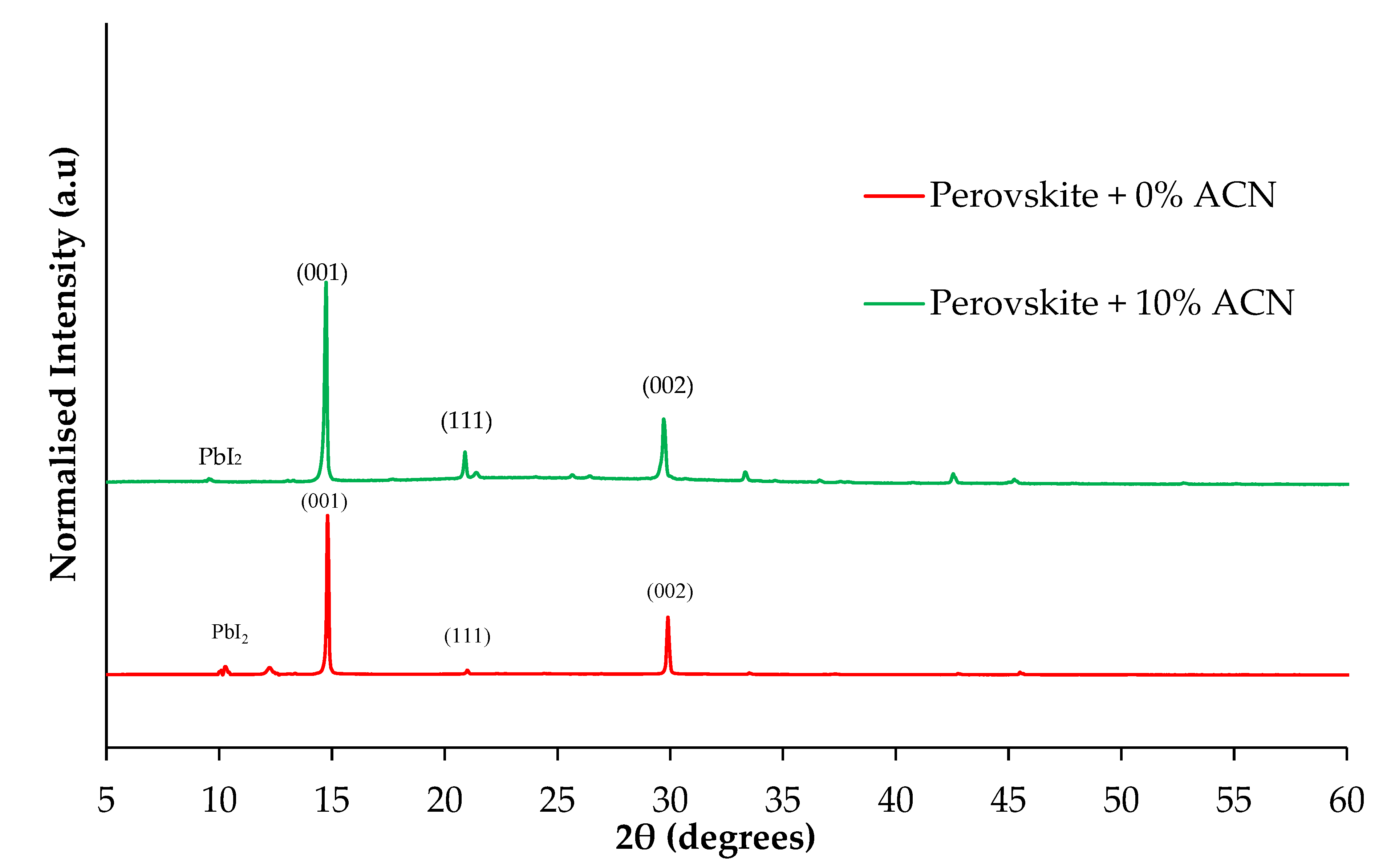
2.1. Structural and Optical Properties of Perovskite Films Fabricated under Ambient Air Laboratory Conditions
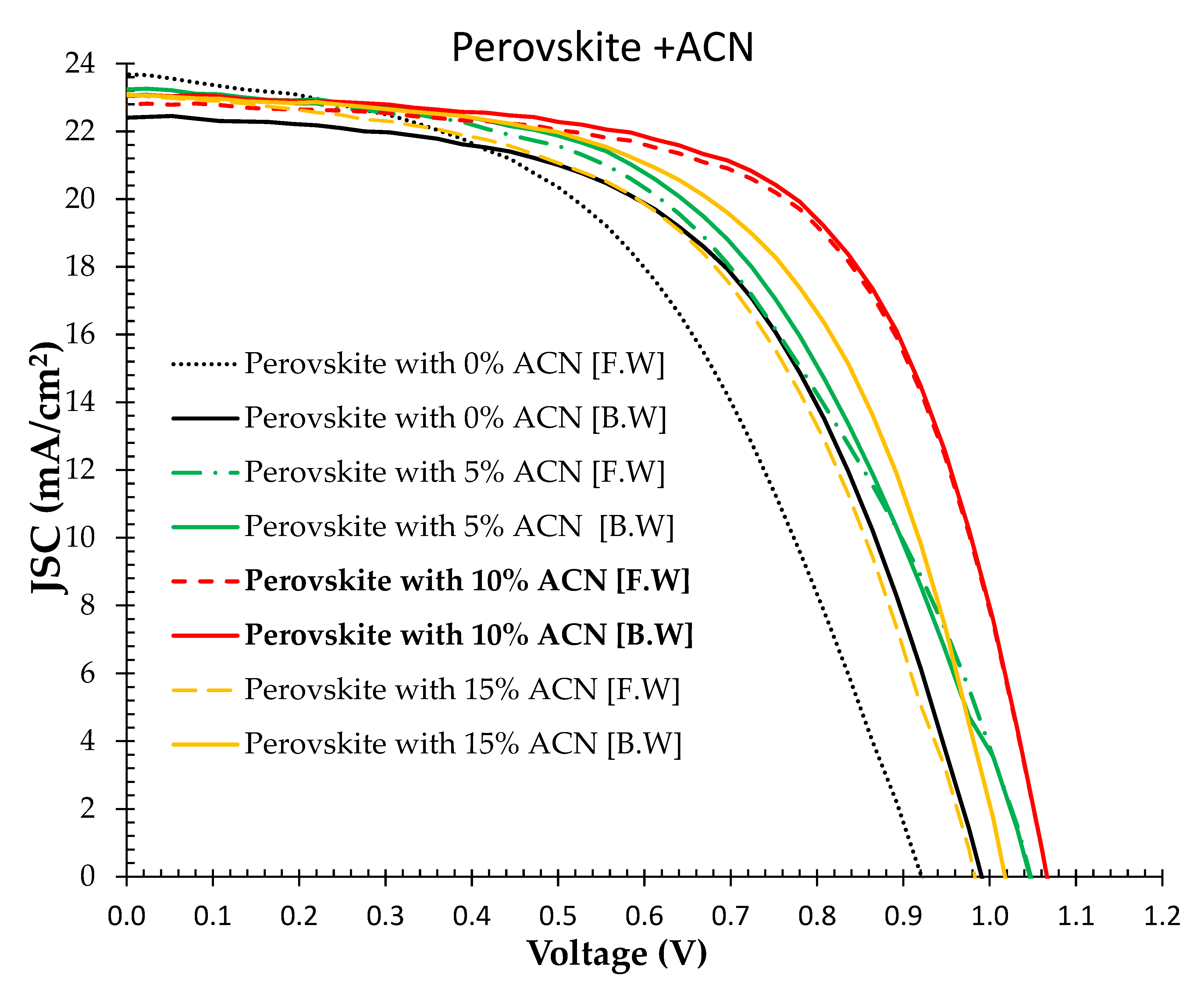
2.2. (J-V) Characteristics of PSC Devices Prepared under Ambient Air Laboratory Conditions Using Solvent Additive Technique
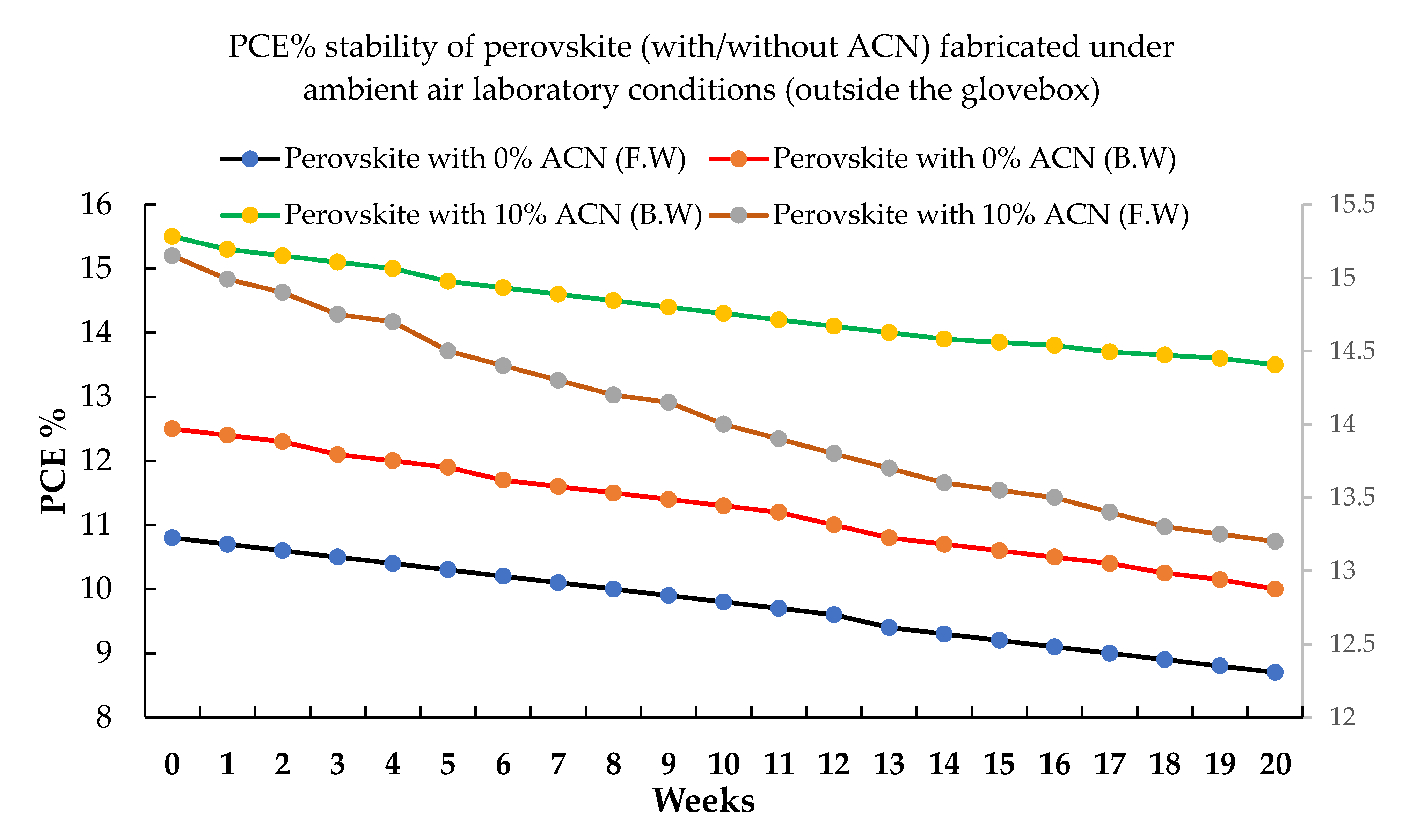
2.3. Stability of Perovskite Films with Solvent Additive Fabricated under Ambient Air Laboratory Conditions
3. Materials and Methods
3.1. Materials
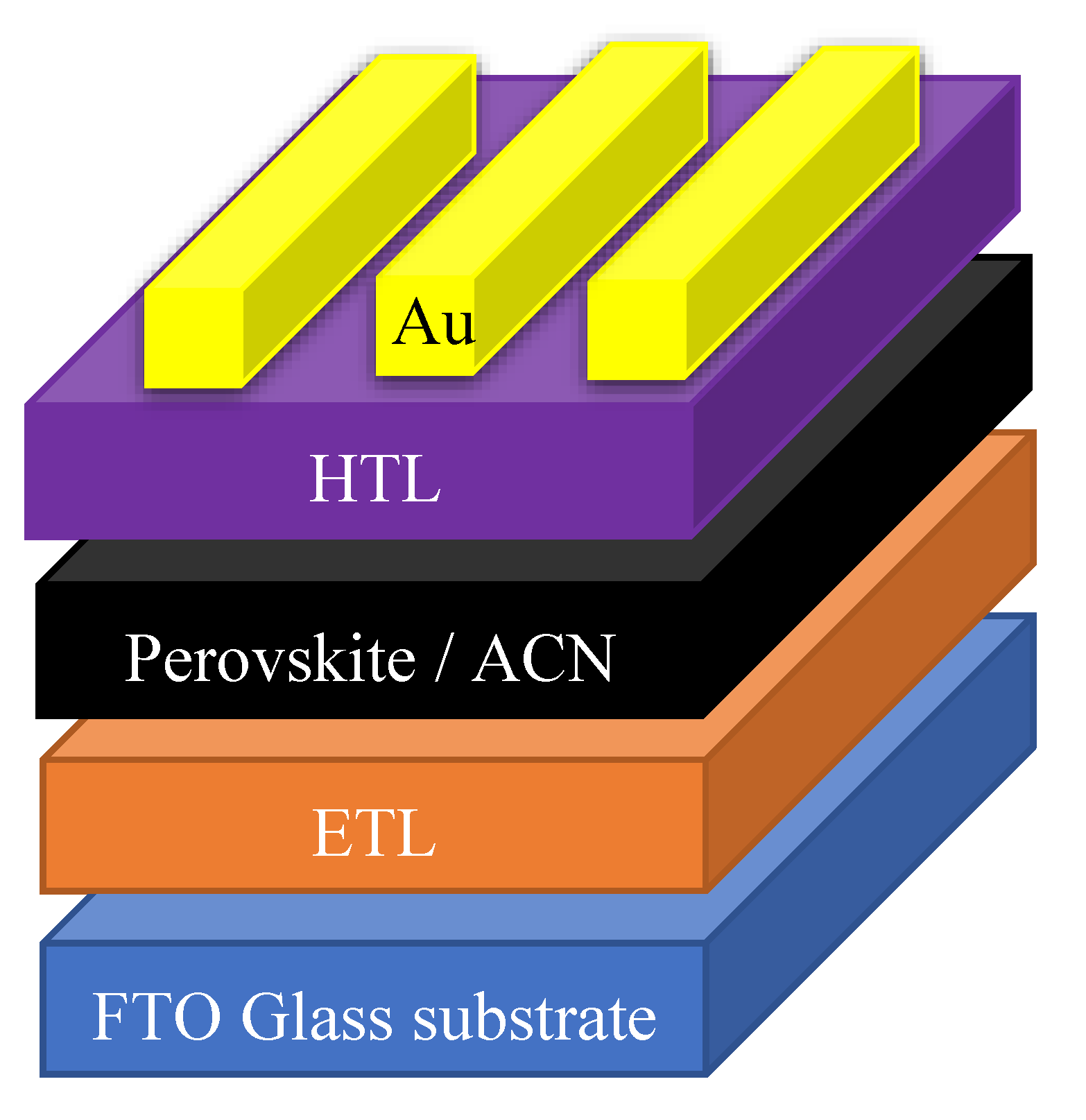
3.2. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Zhuang, R.; Wu, X.; Tian, C.; Sun, A.; Chen, C.; Guo, Y.; Hua, Y.; Meng, K.; et al. Towards 26% Efficiency in Inverted Perovskite Solar Cells via Interfacial Flipped Band Bending and Suppressed Deep-Level Traps. Energy Environ. Sci. 2023, 17, 1153–1162. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Gu, Z.; Ye, T.; FU, W.; Wu, G.; Li, H.; Chen, H. Enhanced Photovoltaic Performance of CH3NH3PbI3 Perovskite Solar Cells through Interfacial Engineering Using Self-Assembling Monolayer. J. Am. Chem. Soc. 2015, 137, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A Review of Aspects of Additive Engineering in Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent Engineering for High-Performance Inorganic-Organic Hybrid Perovskite Solar Cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Lin, F.; Cheng, Y. Structural Modulation and Assembling of Metal Halide Perovskites for Solar Cells and Light-emitting Diodes. InfoMat 2021, 3, 1218–1250. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, M.; Liu, X.; Cheung, S.H.; Chandran, H.T.; Li, H.W.; Xu, X.; Xie, Y.M.; So, S.K.; Yip, H.L.; et al. Impact of Surface Dipole in NiOx on the Crystallization and Photovoltaic Performance of Organometal Halide Perovskite Solar Cells. Nano Energy 2019, 61, 496–504. [Google Scholar] [CrossRef]
- Li, M.; Yue, Z.; Ye, Z.; Li, H.; Luo, H.; Yang, Q.-D.; Zhou, Y.; Huo, Y.; Cheng, Y. Improving the Efficiency and Stability of MAPbI3 Perovskite Solar Cells by Dipeptide Molecules. Small 2024, 20, 2311400. [Google Scholar] [CrossRef]
- Tai, Q.; You, P.; Sang, H.; Liu, Z.; Hu, C.; Chan, H.L.W.; Yan, F. Efficient and Stable Perovskite Solar Cells Prepared in Ambient Air Irrespective of the Humidity. Nat. Commun. 2016, 7, 11105. [Google Scholar] [CrossRef]
- Mesquita, I.; Andrade, L.; Mendes, A. Effect of Relative Humidity during the Preparation of Perovskite Solar Cells: Performance and Stability. Sol. Energy 2020, 199, 474–483. [Google Scholar] [CrossRef]
- Hayali, A.; Alkaisi, M.M. High Efficiency Perovskite Solar Cells Using DC Sputtered Compact TiO2electron Transport Layer. EPJ Photovolt. 2021, 12, 8. [Google Scholar] [CrossRef]
- Hussein, H.T.; Zamel, R.S.; Mohamed, M.S.; Mohammed, M.K.A. High-Performance Fully-Ambient Air Processed Perovskite Solar Cells Using Solvent Additive. J. Phys. Chem. Solids 2021, 149, 109792. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, X.; Xie, Y.; Li, H.W.; Qing, J.; Ma, C.; Lee, C.S.; So, F.; Tsang, S.W. 18% High-Efficiency Air-Processed Perovskite Solar Cells Made in a Humid Atmosphere of 70% RH. Sol. RRL 2017, 1, 1700097. [Google Scholar] [CrossRef]
- Hayali, A.; Reeves, R.J.; Alkaisi, M.M. Wavelength Selective Solar Cells Using Triple Cation Perovskite. Nanomaterials 2022, 12, 3299. [Google Scholar] [CrossRef]
- Gao, L.; Yang, G. Organic-Inorganic Halide Perovskites: From Crystallization of Polycrystalline Films to Solar Cell Applications. Sol. RRL 2020, 4, 1900200. [Google Scholar] [CrossRef]
- Bu, T.; Liu, X.; Zhou, Y.; Yi, J.; Huang, X.; Luo, L.; Xiao, J.; Ku, Z.; Peng, Y.; Huang, F.; et al. A Novel Quadruple-Cation Absorber for Universal Hysteresis Elimination for High Efficiency and Stable Perovskite Solar Cells. Energy Environ. Sci. 2017, 10, 2509–2515. [Google Scholar] [CrossRef]
- Moore, D.T.; Sai, H.; Tan, K.W.; Smilgies, D.M.; Zhang, W.; Snaith, H.J.; Wiesner, U.; Estroff, L.A. Crystallization Kinetics of Organic-Inorganic Trihalide Perovskites and the Role of the Lead Anion in Crystal Growth. J. Am. Chem. Soc. 2015, 137, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Wu, T.; Xu, Z.; Liu, X.; Guo, Q.; He, X. An Efficient Solvent Additive for the Preparation of Anion-Cation-Mixed Hybrid and the High Performance Perovskite Solar Cells. J. Colloid Interface Sci. 2018, 531, 602–608. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; Wang, H. High-Efficiency Solution-Processed Perovskite Solar Cells with Millimeter-Scale Grains. Science 2015, 347, 522–526. [Google Scholar] [CrossRef]
- Yang, B.; Han, K. Charge-Carrier Dynamics of Lead-Free Halide Perovskite Nanocrystals. Acc. Chem. Res. 2019, 52, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Tavakoli, M.M.; Wolska-Pietkiewicz, M.; Jędrzejewska, M.; Trivedi, S.; Kumar, M.; Zakeeruddin, S.M.; Lewiński, J.; Graetzel, M.; Yadav, P. Suppressing Recombination in Perovskite Solar Cells via Surface Engineering of TiO2 ETL. Sol. Energy 2020, 197, 50–57. [Google Scholar] [CrossRef]
- Park, B.W.; Kedem, N.; Kulbak, M.; Lee, D.Y.; Yang, W.S.; Jeon, N.J.; Seo, J.; Kim, G.; Kim, K.J.; Shin, T.J.; et al. Understanding How Excess Lead Iodide Precursor Improves Halide Perovskite Solar Cell Performance. Nat. Commun. 2018, 9, 3301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced Electron Extraction Using SnO2 for High-Efficiency Planar-Structure HC(NH2)2PbI3-Based Perovskite Solar Cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Wolff, C.M.; Caprioglio, P.; Stolterfoht, M.; Neher, D. Nonradiative Recombination in Perovskite Solar Cells: The Role of Interfaces. Adv. Mater. 2019, 31, 1902762. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Liu, Z.; Chen, Q.; Wang, X.; Zhou, H. The Additive Coordination Effect on Hybrids Perovskite Crystallization and High-Performance Solar Cell. Adv. Mater. 2016, 28, 9862–9868. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Wang, C.; Wang, G.; Chen, Y.; Guo, L.; Luo, D.; Wen, S. High-Efficiency and Stable Perovskite Solar Cells Prepared Using Chlorobenzene/Acetonitrile Antisolvent. ACS Appl. Mater. Interfaces 2019, 11, 34989–34996. [Google Scholar] [CrossRef]
- Tao, H.; Li, Y.; Zhang, C.; Wang, K.; Tan, B.; Wang, J.; Tao, J. Efficiency Enhancement of Perovskite Solar Cells by Forming a Tighter Interface Contact of C/CH3NH3PbI3. J. Phys. Chem. Solids 2018, 123, 25–31. [Google Scholar] [CrossRef]
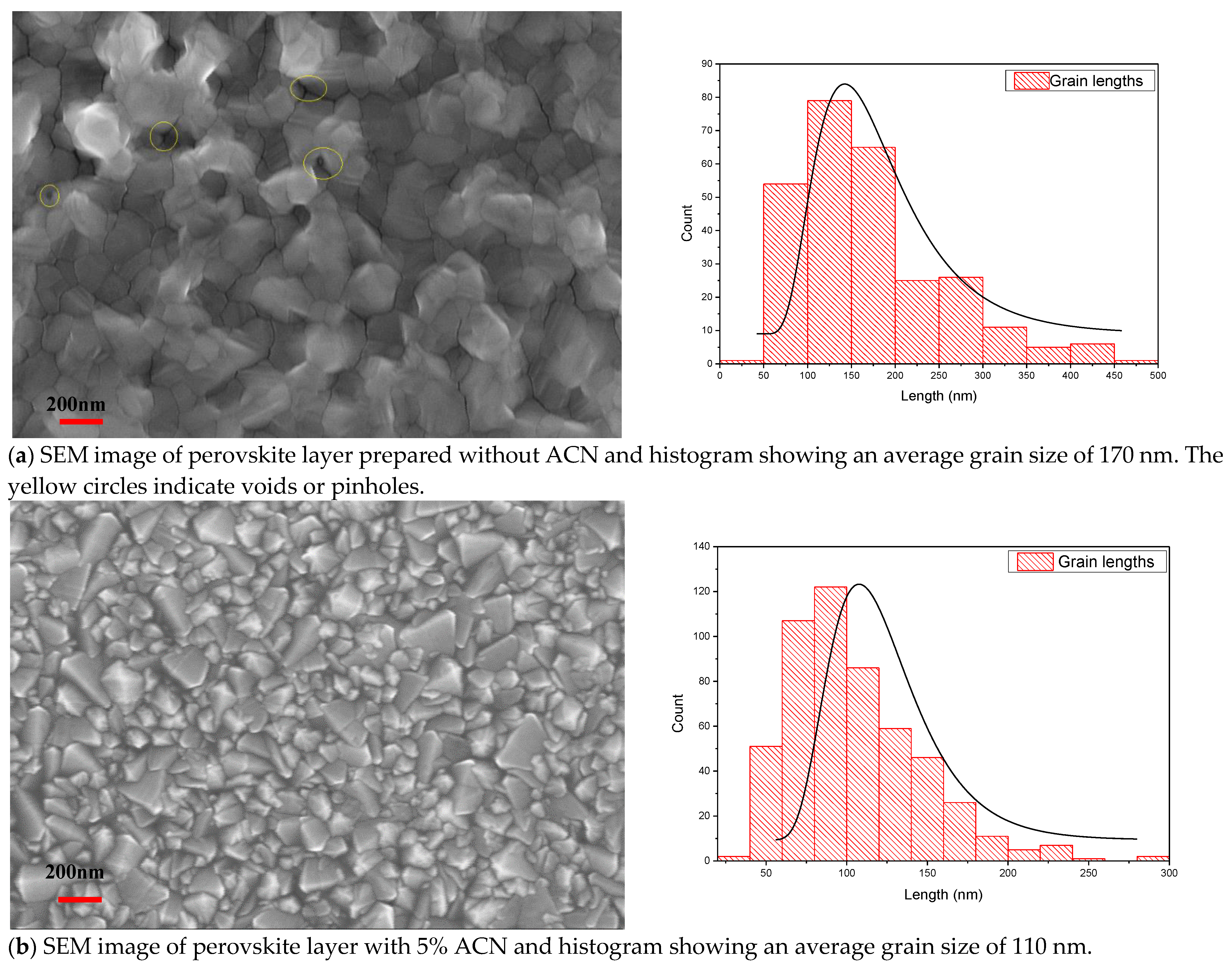
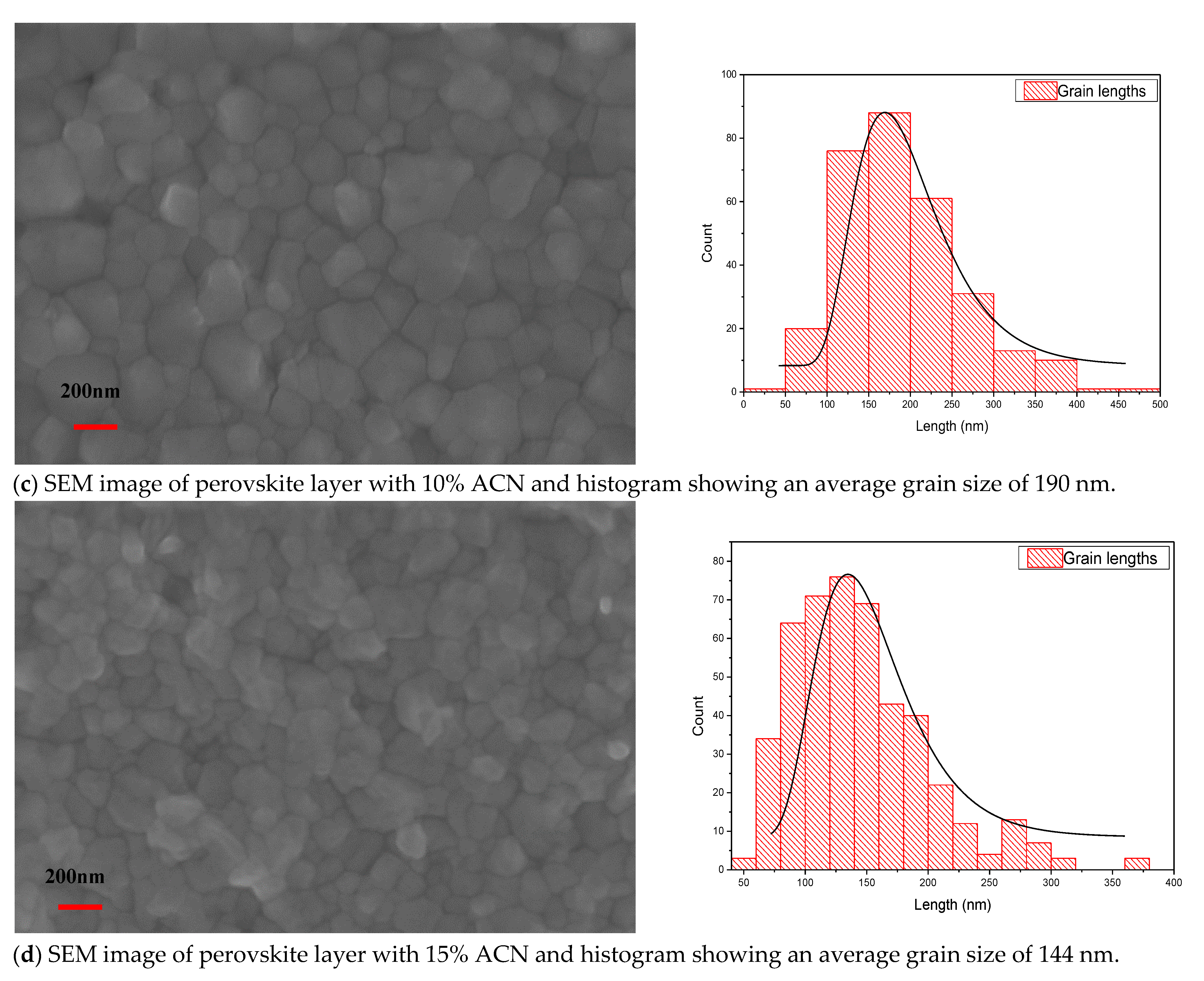

| Sample Description | Sweep Direction | EFF% | FF% | Voc [mV] | Jsc [mA/cm2] | Vmax [mV] | Jmax [mA/cm2] | Isc [mA] | Rshunt [Ω.cm2] | Rseries [Ω.cm2] |
|---|---|---|---|---|---|---|---|---|---|---|
| Perovskite with 0% ACN (control) was fabricated under laboratory ambient air | FW | 10.8 | 49.5 | 921 | 23.7 | 584 | 18.5 | 8.4 | 962 | 13 |
| BW | 12.5 | 56 | 990 | 22.4 | 696 | 18 | 8.1 | 1403 | 12 | |
| Avg. | 11.6 | 52.7 | 955 | 23 | 640 | 18.2 | 8.2 | 1182 | 12.5 | |
| Perovskite with 5% ACN was fabricated under laboratory ambient air | FW | 12.6 | 52 | 1047 | 23 | 668 | 18.9 | 8.3 | 701 | 12 |
| BW | 13.1 | 53.8 | 1046 | 23.2 | 696 | 18.8 | 8.3 | 7004 | 11 | |
| Avg. | 12.8 | 52.9 | 1046 | 23.1 | 682 | 18.8 | 8.3 | 3852 | 11.5 | |
| Perovskite with 10% ACN was fabricated under laboratory ambient air | FW | 15.2 | 61 | 1044 | 22.4 | 764 | 19.5 | 8.06 | 7562 | 7.8 |
| BW | 15.5 | 63 | 1066 | 23 | 780 | 19.9 | 8.3 | 7716 | 7.9 | |
| Avg. | 15.35 | 62 | 1055 | 22.7 | 772 | 19.7 | 8.18 | 7639 | 7.85 | |
| Perovskite with 15% ACN was fabricated under laboratory ambient air | FW | 12.3 | 54 | 983 | 23 | 668 | 18.35 | 8.2 | 420 | 9.4 |
| BW | 13.7 | 58.5 | 1017 | 23 | 724 | 18.9 | 8.3 | 955 | 8.6 | |
| Avg. | 13 | 56.2 | 1000 | 23 | 696 | 18.6 | 8.2 | 687 | 9 |
| References | Fabrication Atmosphere | Technique of Fabrication | Using ACN as Additive Technique | Composition of Perovskite | Active Area cm2 | Efficiency Improvement |
|---|---|---|---|---|---|---|
| [13] | Laboratory ambient air | Sequential deposition process | Cation perovskite precursor + CAN | (CsMAFA)Pb (IBr)3 | - | 20% |
| [19] | Dry atmosphere in a glovebox | One-step process | Cation perovskite precursor + ACN | (CsMAFA)Pb (IBr)3 | 0.12 | 9.6% |
| [26] | N2 | Sequential deposition process | PbI2 precursor + ACN | CH3NH3PbI3 | - | 18.5% |
| [27] | Glovebox | One-step process | ACN + CBZ anti-solvent | CH3NH3PbI3 | - | 16.6% |
| [28] | Not mentioned | Two-step process | Carbon slurry + ACN | CH3NH3PbI3 | 0.10 | 11.5% |
| Our work | Not mentioned | Two-steps spin-coating | Triple cation perovskite precursor + ACN | CsI0.05[(FAPbI3)0.85(MAPbBr3)0.15]0.95 | 0.36 | 25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayali, A.; Alkaisi, M.M. Improving Charge Transport in Perovskite Solar Cells Using Solvent Additive Technique. Inorganics 2024, 12, 214. https://doi.org/10.3390/inorganics12080214
Hayali A, Alkaisi MM. Improving Charge Transport in Perovskite Solar Cells Using Solvent Additive Technique. Inorganics. 2024; 12(8):214. https://doi.org/10.3390/inorganics12080214
Chicago/Turabian StyleHayali, Ahmed, and Maan M. Alkaisi. 2024. "Improving Charge Transport in Perovskite Solar Cells Using Solvent Additive Technique" Inorganics 12, no. 8: 214. https://doi.org/10.3390/inorganics12080214
APA StyleHayali, A., & Alkaisi, M. M. (2024). Improving Charge Transport in Perovskite Solar Cells Using Solvent Additive Technique. Inorganics, 12(8), 214. https://doi.org/10.3390/inorganics12080214








