Abstract
Chromium nitride is an important transition metal nitride for studying fundamental properties and for advanced technological applications. It is considered a model system for exploring structural, electronic, and magnetic transitions. These transitions occur at 275 ± 10 K and appear to be coupled; however, many discrepant studies on these transitions can be found in the published literature. The underlying reasons for these controversies are suspected to be the CrN nanoparticles preparation methods, strains, impurities, stoichiometry, nanoparticle size, characterization methods, and ambient conditions for characterizing them. This article is focused on the review of the nanoparticle synthesis methods and the use of these nanoparticles for studying structural, electronic, and magnetic transitions. The focus is mainly on the experimental methods, while theoretical simulations are briefly reviewed at the end of the article.
1. Introduction
Transition metal nitrides (TMNs) exhibit interesting structural, electronic, magnetic, and noble-metal-like characteristics [1,2,3,4,5,6]. These unique characteristics of TMNs arise from the combination of the d-orbitals of transition metals and the p-orbital of nitrogen; the d-orbital contributes to both magnetic and electronic properties, whereas the p-orbital contributes mainly to the electronic properties of materials. TMNs are found at room temperature and normal pressure in face-centered cubic (W2N) [7], hexagonal close-packed (-Ti2N) [8], and hexagonal crystal (BN) [9] structures. The crystal structures of some TMNs transform into other crystal structures with temperature [10] or pressure [11,12] variations. These crystal structures can be understood as metal lattices in which nitrogen occupies the interstitial sites. The nature of the bond between a metal and nitrogen is mostly covalent with an overtone of the ionic bonding nature. The bond between the metal and nitrogen leads to the expansion of the metal lattice but causes constriction in the d-bonds between the metals [13]. Therefore, the d-orbital constriction and high density of states at the Fermi level produce noble-metal-like properties in electrolysis, making them ideal candidates for electrochemical conversion and energy storage applications [14,15,16,17,18,19,20]. Their chemical inertness enables TMNs to function across a broad pH range, increasing their versatility for use in various electrolytes. Due to these characteristics, they could solve the persistent issues of capacitor and battery electrode erosion or collapse while reacting with lithium, zinc, or other electrolytes [21,22,23,24,25,26]. Many TMNs such as molybdenum nitride (MoN), vanadium nitride (VN), titanium nitride (TiN), niobium nitride (NbN), cobalt nitride (CoN), nickel nitride (NiN), Boron nitride (BN), iron nitride (FeN), chromium nitride (CrN), etc. have been successfully synthesized and studied as electrode materials for water splitting, electrolysis, hydrogen production, supercapacitors, and batteries [27,28,29,30,31,32,33,34].
Among TMNs, CrN is considered an important model system both for understanding the fundamental science behind the interesting properties of TMNs [35,36] and a candidate for advanced technological applications. Some of the interesting applications that CrN is studied for include photovoltaics, thermoelectrics [37], supercapacitor electrodes [30,38,39], battery electrode [40,41,42,43], dilute magnetic semiconductors [44,45,46], and spintronics [47,48,49]. In a recent study published by Liu et al. [50], they used a novel method for suppressing Cr2N phases and enhancing the thermoelectric performance of CrN to a maximum power factor of 1002 μW·m−1·K−2, a thermal conductivity of 4.78 W·m−1·K−1, and a figure of merit (zT) value of up to 0.20 at 973 K. A similar study by Yuan et al. [51] reported a power factor of 1230 μW·m−1·K−2, a thermal conductivity of 6.8 W·m−1·K−1, and a zT value of up to 0.18 at 973 K. These interesting studies indicate that CrN is a potential candidate for thermoelectric applications. Furthermore, Gubert et al. [52] reported a mixture of dichromium nitride (Cr2N) and CrN nanoparticles by using pulsed laser irradiation on a chromium target submerged in liquid nitrogen. The Cr ions removed from the target reacted with the readily available nitrogen and formed cylindrically shaped, chemically stable nanoparticles. The particles exhibited surface plasmon resonance, which can be used for plasmonics. Another interesting study reported by Das et al. [53] showed the Li-cycling performance using CrN nanoparticles as an active material in a battery’s anode. They achieved a first-cycle reversible capacity of up to 635 ± 10 mA·h/g, which stabilized at around 500 ± 10 mA·h/g up to 80 cycles, which is greater than the graphite specific capacity of 372 mA·h/g [54]. Shuang et al. [55] reported a Soret-effect-induced phase transition in CrN. Upon the application of repeated electrical pulses, they observed a nonvolatile and reversible crystalline-to-crystalline phase transition. This transition was facilitated by nitrogen diffusion from the hotter region to a relatively colder region, thereby stabilizing CrN2 in the hexagonal phase. Their resistive switching device offers a programming window of 105, low operating energy of about 100 pJ, and a fast speed of about 30 ns. A study by Guo et al. [52] investigated the stability of lattice nitrogen and deactivation mechanisms in cubic CrN nanoparticles during the electrochemical nitrogen reduction reaction. Their findings reveal that cubic CrN nanoparticles undergo degradation under operational conditions due to the loss of lattice nitrogen and metal dissolution caused by ammonia poisoning from surface-accumulated ammonia. These observations are crucial for understanding the long-term performance and durability of CrN nanoparticles as electrocatalysts in nitrogen reduction reactions.
Materials with a face-centered cubic crystal structure can have magnetization in any of five types of spin arrangements: type I, type II, type III, type IA, and type IV [56]. The magnetic structure of CrN is type IV, where two ferromagnetically coupled atomic layers along [110] are antiferromagnetically coupled to the next two adjacent layers. Thus, the net magnetic moment of all four atomic layers is zero [56]. The magnetization in CrN is mostly contributed by Cr, as Cr is a transition metal with a partially filled 3d-orbital, and nitrogen has an unfilled 2p-orbital. In a paramagnetic state, the net magnetization is zero because the unpaired spins are in a high energy state; they move randomly with no correlation to each other. The number of spins pointed in any arbitrary direction is equal to the number of spins pointed in the opposite direction. By decreasing the energy of the spins by lowering the temperature of the paramagnet, at a characteristic temperature, the spins can form an order. When the low-temperature state is antiferromagnetic, its magnetic susceptibility produces a cusp at the transition in the susceptibility-versus-temperature plot. If the low-temperature state is ferromagnetic, then the susceptibility diverges at the transition temperature. By cooling down a paramagnet below the Néel temperature, it transitions to an antiferromagnetic state, where the spins are in a low energy state and have a long-range correlation.
The magnetic and structural transitions from a paramagnetic (cubic) to an antiferromagnetic (orthorhombic) phase in chromium nitride (CrN) nanoparticles were reported by Corliss et al. in 1960 [10]. Mrozinska et al. [57] and Filippetti et al. [58] studied the magnetic transition using their analytical and computational models, respectively. However, many researchers found contradictory results to the initial observations. The proposed reasons included the crystallinity of CrN, epitaxial constraints for thin films grown on crystalline substrates [59,60], nitrogen vacancies and chromium-rich phases such as Cr2N [61], and lattice strains [62]. Similarly, Jin et al. suggested epitaxial-dependent phase transition in CrN thin films [63]. Their films grown by pulsed laser deposition on (010), (001), and (110) planes of NdGaO3 showed electronic transitions from semiconductor to semiconductor, metallic to semiconductor, and metallic to semiconductor, respectively [63].
This controversy is also evident in the high-pressure study of CrN nanoparticles. For example, Rivadulla et al. observed a 25% drop in the bulk modulus of CrN at high pressures, accompanied by a structural transition from cubic to orthorhombic at 1 GPa [64], whereas Yan et al. [65] observed the transition at 5 GPa. The calculations of Alling et al. [66] did not show the 25% reduction in the bulk modulus associated with the structural transition.
This article provides a comprehensive review of various methods for synthesizing nanoparticles, with a particular emphasis on their application in studying phase transitions. The primary focus is on experimental techniques, exploring how different synthesis methods can influence the structural, electronic, and magnetic phase transitions. The synthesis methods can induce different levels of stress, size variations, nitrogen vacancies, defects, and impurities. These transitions appear to be coupled, with the onset of one transition triggering the other. The transitions have been reported over a wide temperature range from 240 to 285 K, but most studies report them at 275 ± 10 K. In addition to experimental methods, the article briefly reviews theoretical simulations towards the end.
2. Chromium Nitride Nanoparticles
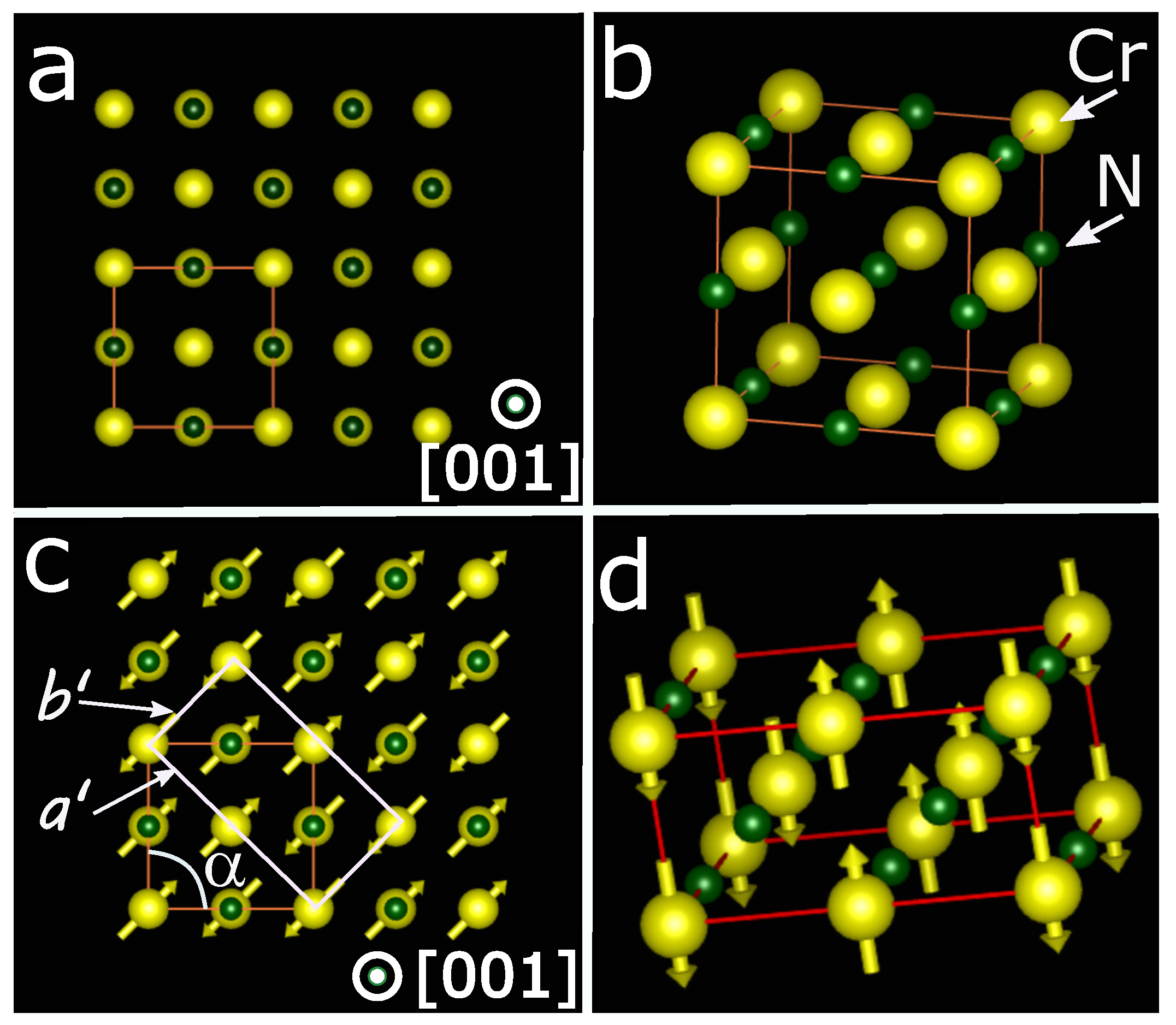
Corliss et al. [10] prepared chromium nitride nanoparticles by passing ammonia (NH3) over chromium nanoparticles at 1100 °C, followed by grinding and nitriding to minimize nitrogen-deficient phases. They studied the structural transition using variable-temperature X-ray diffraction and the magnetic transition using variable-temperature neutron diffraction. They were the first group to report that CrN is paramagnetic at room temperature and undergoes a magnetic transition to an antiferromagnetic phase around 290 K, consistent with later reports [3,67,68]. At the transition temperature, their neutron diffraction data revealed a change in pattern associated with the ordered magnetic state (antiferromagnetic phase). Concurrently, the variable-temperature X-ray diffraction showed that the crystal structure changes from FCC to orthorhombic. CrN is paramagnetic at room temperature with a face-centered cubic (FCC) crystal structure and two-atom basis (rock salt) crystal structure (i.e., the nitrogen occupies interstitial lattice sites in the FCC lattice structure). Its lattice parameters are a = b = c = 4.15 Å and [68,69,70]. Figure 1a,b shows the 2D and 3D crystal structure of CrN at room temperature, in which the large ball (yellow color) represents the Cr atom and small ball (dark green color) represents the N atom. Corliss et al. revealed that at the Neel temperature, it makes a structural transition to an orthorhombic crystal structure with two atom bases upon cooling, and its lattice parameters are , , and , ° [10]. Figure 1c,d show the 2D and 3D crystal structure of CrN at low temperatures. The model shows that the antiferromagnetic phase with the orthorhombic crystal structure consists of alternating double magnetic sheets parallel to a [110] direction, known as a type IV antiferromagnet. This magnetic transition induces stress in the crystal structure, as two adjacent parallel spins repel each other and two adjacent antiparallel spins attract each other [71,72,73]. The stresses thus produced are released by shear deformation, which results in ° instead of 90° [58,74].
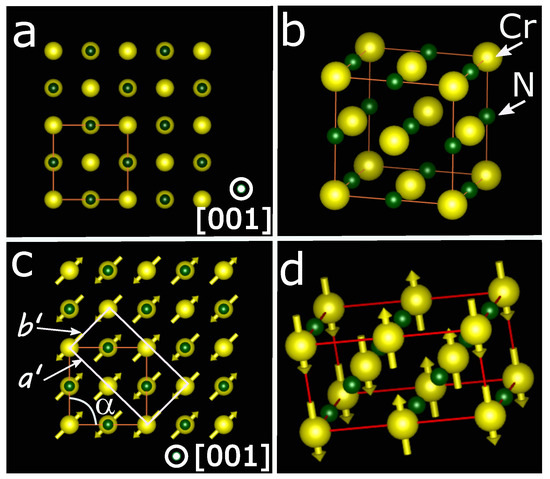
Figure 1.
(a) A view of the fcc lattice of CrN along the [001] direction. (b) A 3D model representation of the fcc lattice. (c) A 2D view along [001] of the orthorhombic model for CrN at a low temperature. A shear distortion of the cubic crystal structure leading to . The double ferromagnetic layers alternate along [110] and spins choose an orthorhombic unit cell arrangement. (d) A 3D view of the orthorhombic unit cell.
Nasr-Eddine et al. studied CrN nanoparticles using variable temperature X-ray diffraction (VT-XRD) and neutron diffraction [75]. The sample preparation method was not provided. Their VT-XRD and neutron diffraction data were acquired over a temperature range from 5 K to 360 K. The VT-XRD data revealed a first-order transition from a rock salt crystal structure to an orthorhombic crystal structure at 285 K, which is in agreement with the results of Corliss et al. [10]. The neutron diffraction showed a transition from a paramagnetic to an antiferromagnetic state at TN = 285 K upon cooling, with a hysteresis of about 5 K during the transition between heating and cooling cycles.
A study by Browne et al. [76] showed structural, electronic, and magnetic transitions between 278 and 287 K. Their samples were prepared by enclosing Cr powder in a tube where high-purity nitrogen gas was passed at 1 atm pressure and 1123 K for about 100 h. During the preparation process, sample oxidation was prevented by inserting titanium pieces at the entrance port of the tube. The structural transition was determined by measuring the length of the specimen loaded in a silica dilatometer as a function of temperature. In the dilatometer, a differential transformer measured the relative change in the length of the outer diameter of the silica tube, in which the specimen was placed, and a spring-loaded silica rod held in contact with the specimen. The resistivity versus temperature data showed a transition from one metallic phase to another metallic phase between 284 and 287 K, which is different from what Browne et al. reported. The magnetic transition was determined by studying magnetic susceptibility as a function of temperature. Using the dilatometer, they observed a volume contraction of −0.59% at 284 K, which corresponds to the reported difference between the volumes of the cubic and orthorhombic crystals of CrN [10,75]. The data were recorded by a modified Sucksmith ring balance over a temperature range from 85 to 500 K for both cooling and warming parts of a cycle in an applied magnetic field of up to 20 kG. Magnetic susceptibility in these samples showed a sharp, first-order phase transition at 287 K. The marginal temperature difference between the two observed transitions indicates that one transition triggers the other.
Interesting thermometric properties were reported in polycrystalline CrN-powered samples [3,77] as well as in a thin film form [78]. Quintela et al. synthesized CrN by ammonolysis of Cr3S4 at 800 °C for 10 h, with an iteration of grinding. They produced samples with 0.3 GPa pressure and sintered them for high-density and transport measurements [77]. The electrical resistivity of their stoichiometric CrN samples at room temperature was measured to be 30 × 10−3 Ω·cm. The sample showed electronic transition from one semiconducting phase to another at 286 K. This observation is different from the report by Wang et al. [79] of metallic (room temperature) and metallic (low temperature) phase transitions, which they observed in nanoparticles produced by a high-pressure synthesis method of Na2CrO4 (99.5% purity) and hexagonal-BN (99.9% purity). It also contradicts the semiconductor (room temperature) to metallic (low temperature) transition observed in CrN thin films [70,80]. Quintela et al. [77] measured the activation energy to be 75 meV, which matches the value reported by Constantine et al. [70].
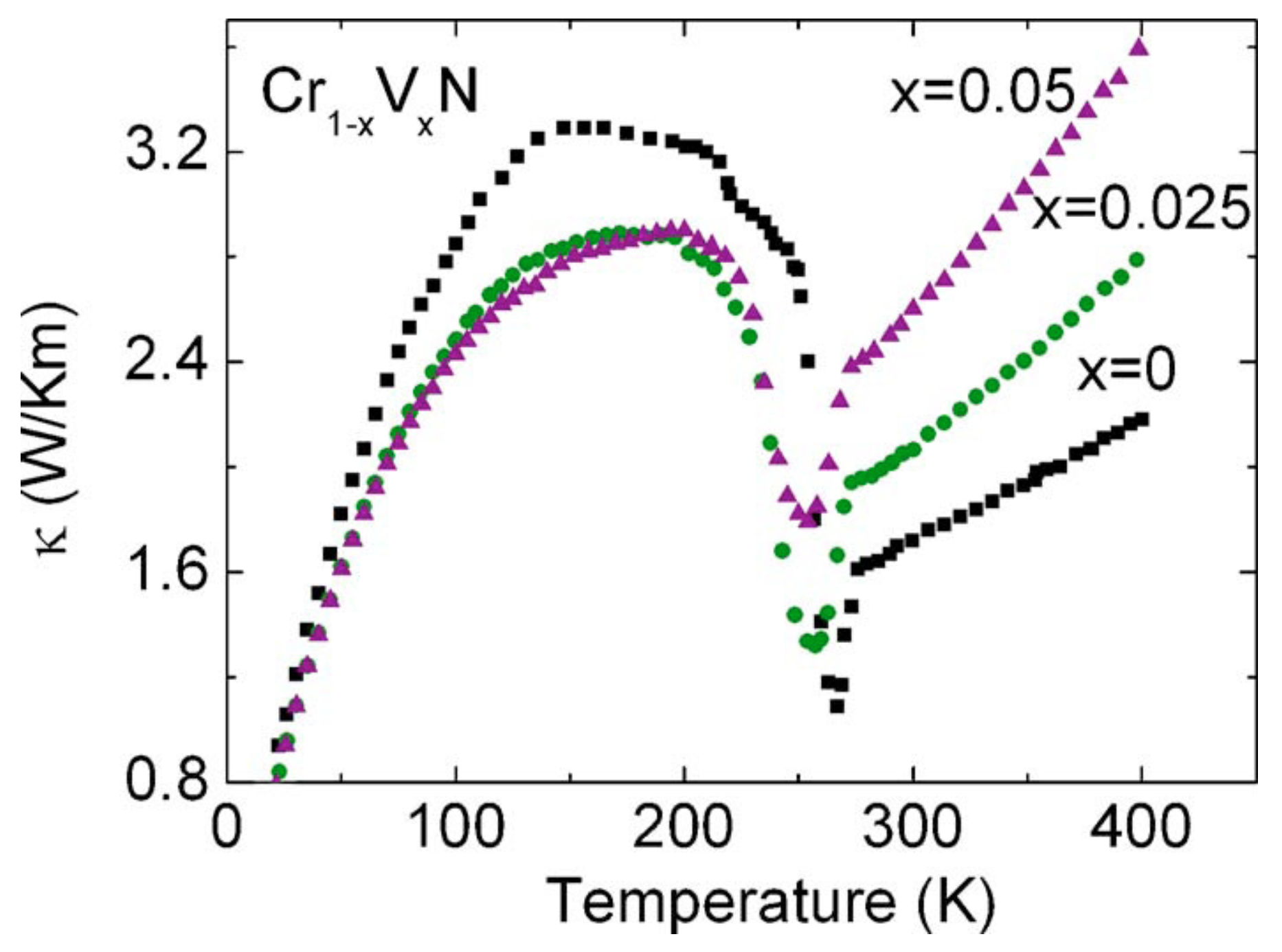
The sample shows a kink at the transition temperature in Seebeck versus temperature, thermoelectric power factor versus temperature, and thermal conductivity versus temperature data [78]. The thermal conductivity versus temperature data of Cr1−xVxN are shown in Figure 2. Regarding the change in the heat conduction mechanism due to the latent heat involvement in the phase transition, the thermal conductivity shows a cusp at 286 K. In the scope of this paper, the interest is in the x = 0 curve, which represents the stoichiometric CrN sample. The other vanadium doped CrN samples also show the cusp, which indicates that the structural transition strongly alters the heat transport mechanism even in Cr1−xVxN samples. The broad maximum observed at around 150 K, which is related to phonons at a low-temperature propagation of phonons, is limited by the grain size. A more detailed discussion of the phonon peaks is provided by Ebad-Allah et al. [81]. Vanadium incorporation in CrN reduces the thermal conductivity at around 150 K, but the cusp temperature remains almost unchanged.
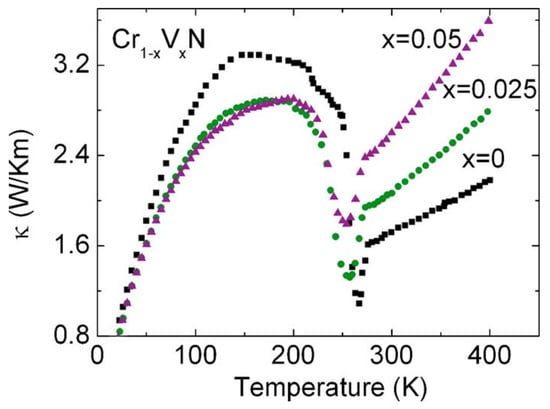
Figure 2.
Thermal conductivity of Cr1−xVxN. The dip around 286 K corresponds to the temperature of the orthorhombic-to-cubic structural transition [77].
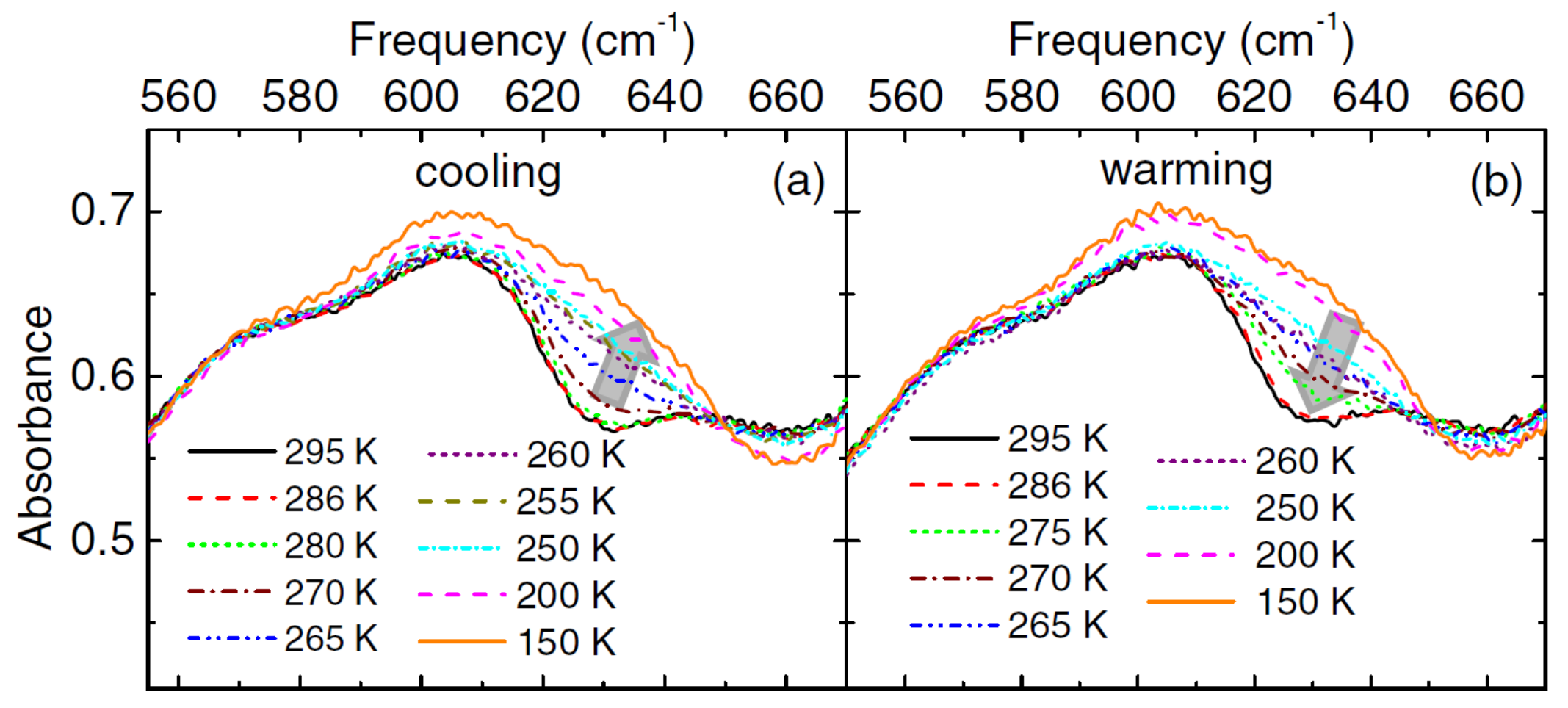
The polycrystalline CrN samples prepared by ammonolysis of Cr3S4 at 800 °C for 10 h was studied by Ebad-Allah et al. [81]. They studied optical transmittance as a function of temperature in a frequency range of 100 to 20,000 cm−1. They mixed the polycrystalline CrN powered with CsI to prepare pellets for the study. Figure 3 shows that the major changes in the absorbance spectra due to the phonon mode are between 550 cm−1 and 670 cm−1. The phonon mode is excited at around 270 K; the peak first broadens and then splits during further cooling of the samples. The change is particularly noticeable at 630 cm−1: the absorbance increases by cooling the sample and decreases by warming the sample. The onset of the new phonon mode at 270 K matches is related to the lowering symmetry of crystal structure from cubic to orthorhombic. The measure bandgap at room temperature from the data is between 100 and 150 meV [59,70,77,78,82]. They also studied optical absorption as a function of applied pressure on the sample at room temperature. The samples show an onset of phonon mode related to the lowering in crystal symmetry at a 0.6 GPa pressure. It confirms the structural transition from a cubic to an orthorhombic crystal structure. The pressure induces a metallic phase at 0.6 GPa as the optical reflectance increases with an increase in the pressure. The data reveal that at room temperature and atmospheric pressure, CrN is a charge-transfer insulator than a Mott insulator [81,83]. A report by Yan et al. [65], which focused on a pressure-driven phase transition in CrN nanoparticles, contradicts the reports by Ebad-Allah et al. [81] and Rivadullah et al. [64] on the structural phase transitions. Yan et al. [65] observed a cubic-to-orthorhombic phase transition at ∼5 GPa, which is about 8 and 5 times larger than what Ebad-Allah et al. [81] and Rivadullah et al. [64] reported.
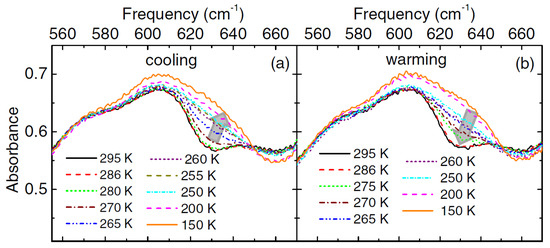
Figure 3.
Absorbance of a CrN pellet as a function of temperature for (a) cooling and (b) warming parts of cycles. The arrows highlight changes in the data acquired while cooling and warming the samples [81].
A study on the correlated paramagnetic cubic-to-antiferromagnetic metal transition published by Bhobe et al. investigated the magnetostructural transition in polycrystalline CrN samples [84]. Their CrN powdered samples were prepared by heating 3N pure CrCl3 in the continuous flow of 5N NH3 at a 1173 K temperature for 20 h. For further characterization, it was pressed into a pellet and sintered at 1323 K for 30 h under N2 gas. The resistivity-versus-temperature data showed as , which indicates that the sample was metallic below TN [70,80,81]. Resonant photoemission spectroscopy (Res-PES) revealed a bandgap in the Cr 3d-orbital density of states near the Fermi level, and bulk-sensitive laser photoemission spectroscopy showed a Fermi edge associated with the antiferromagnetic metal phase below TN. Moreover, Bhobe et al. determined the Coulomb energy term U = 4.5 eV. It is consistent with the value used by Alam et al. [68] for their computational modeling of local density approximation with Hubbard correction (LDA + U)-based calculations [85].
Zieschang et al. prepared CrN nanoparticles by a chemical reaction of 9.9 mg Na and CrCl3 with 50 mL liquid ammonia at 195 K [74]. The solution was kept at a low temperature for 1.5 h. Intermittently, the flask was taken out for stirring for 1 min after a duration of 20 min. Once the reaction was completed, the mixture was allowed to warm to room temperature. After evaporating the ammonia, the powders were further dried in vacuum and annealed at 873 K. The powders were then washed with methanol. They studied the structural and magnetic transitions within their samples with variable temperature X-ray diffraction and SQUID magnetometer, respectively. Their as-prepared CrN nanoparticles were amorphous and did not produce any peaks in XRD spectra. The crystal quality was improved after annealing and washing the samples. According to their report, they observed only CrN peaks in the XRD data and Cr-rich phases such as Cr2N. They studied two samples, one annealed at 773 K and another at 873 K. The sample annealed at a higher temperature had a larger grain size and better crystal quality.
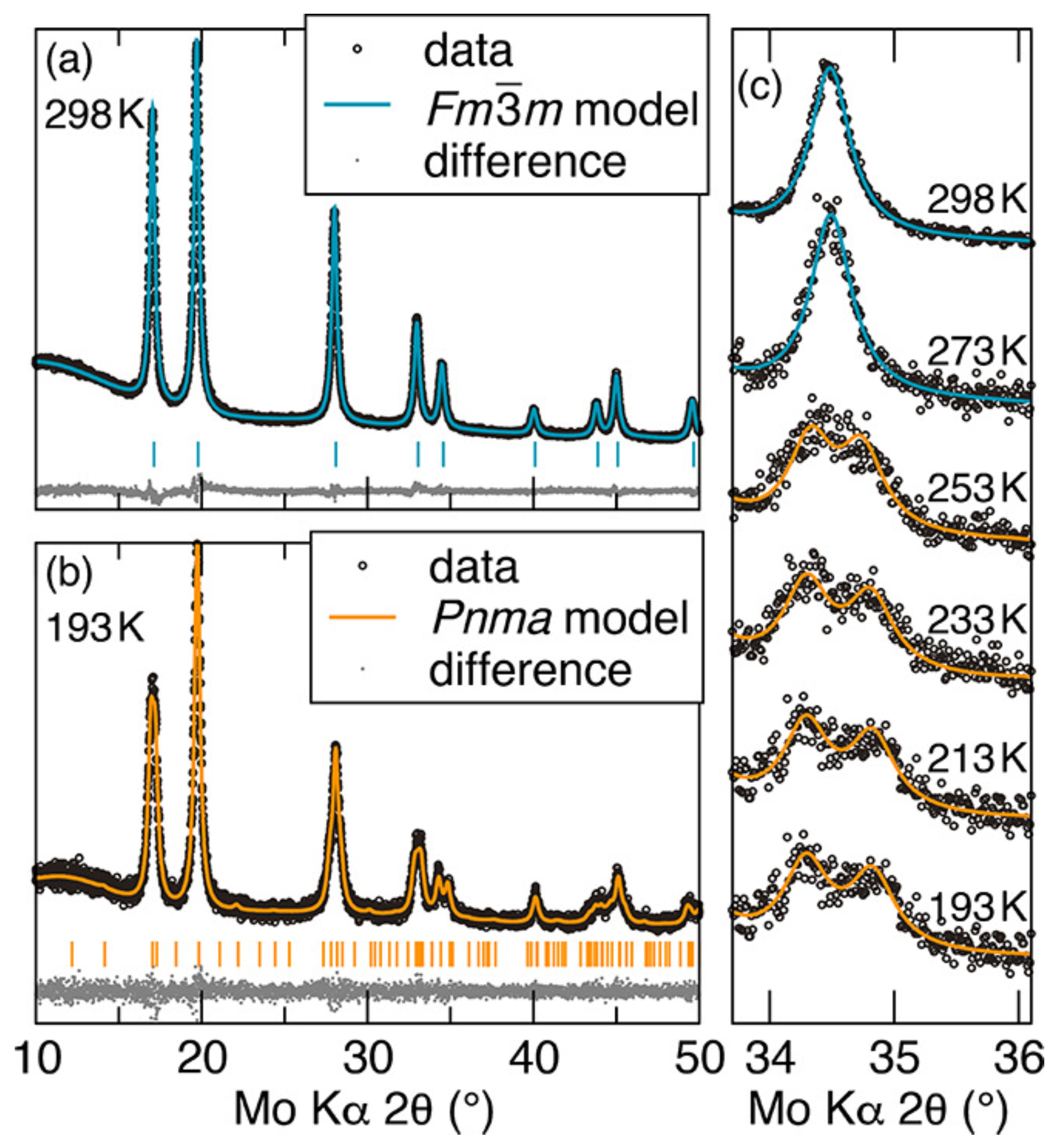
The VT-XRD data of the nanoparticles annealed at 873 K and Rietveld refinement simulated data are shown in Figure 4. The data shown in Figure 4a were acquired at 298 K and show a cubic crystal structure of the space group Fmm; and the data in Figure 4b were acquired at 193 K and show an orthorhombic crystal structure of the space group Pnma. These indicate that the samples went through a structural transition between 298 K and 193 K. The main difference is that the 222 peak (2θ = 34.49° produced by the cubic symmetry was split into three peaks—022, 402, and 122—related to the orthorhombic symmetry. Scanning the 022, 402, and 122 peaks in steps of 20 K while warming the sample from 193 K to 298 K revealed the transition temperature between 253 and 273 K.
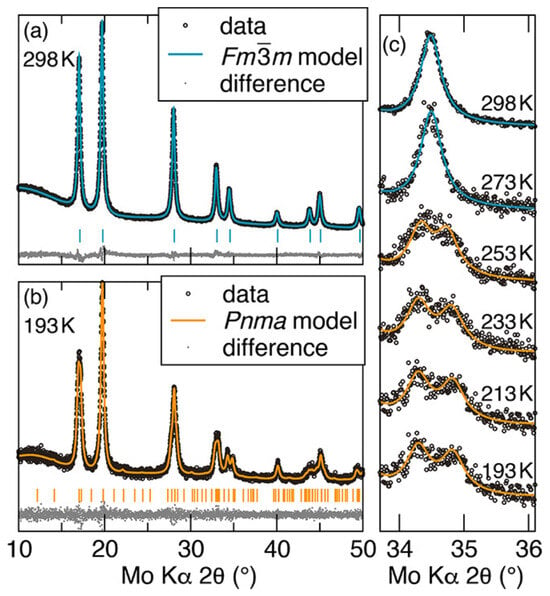
Figure 4.
Variable temperature X-ray diffraction spectra of CrN nanoparticles (a) at 298 K and (b) at 193 K. The theoretical data are the Rietveld refinement of (a) cubic CrN (Fmm) and (b) orthorhombic CrN (Pnma). (c) The 222 peak related to the cubic crystal structure is split into three peaks, which are the 022, 402, and 122 peaks, related to the orthorhombic crystal structure [74].
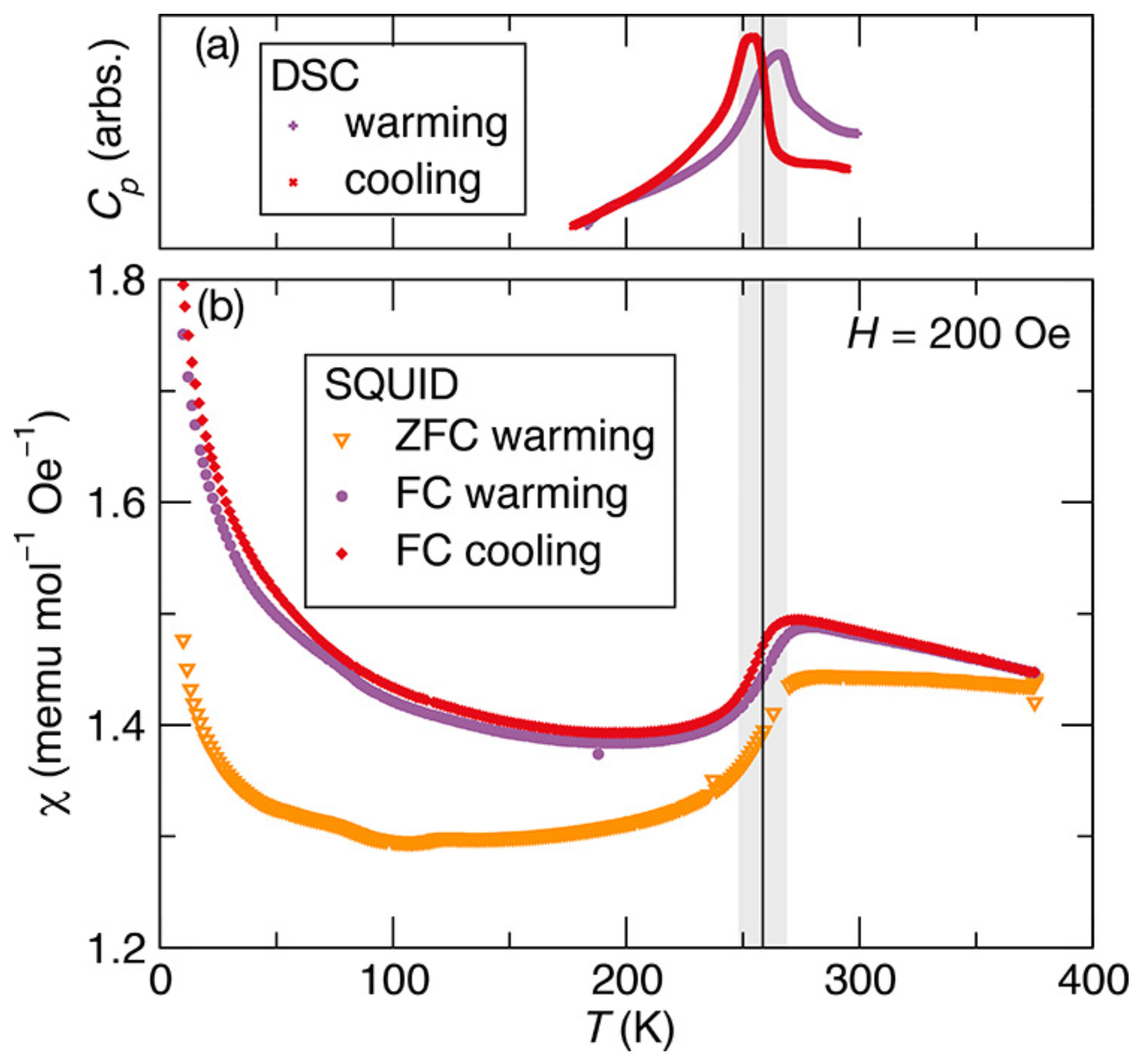
Furthermore, Zieschang et al. studied transition in heat capacity and magnetic transition as a function of temperature by differential scanning calorimetry and magnetic susceptibility by SQUID as shown in Figure 5. In Figure 5a, the data recorded for the cooling and warming cycles show a transition in the heat capacity at around 259 K, which indicates that the transition is reversible and reproducible. Similarly, the magnetic susceptibility-versus-temperature data in Figure 5b reveal magnetic phase transition from the paramagnetic to the antiferromagnetic phase between 248 and 269 K, which matches the structural and heat capacity transition. The observation of all these transitions in CrN particles at about the same temperature strengthens the earlier theoretical and experimental claims that these transitions are coupled [58,68].
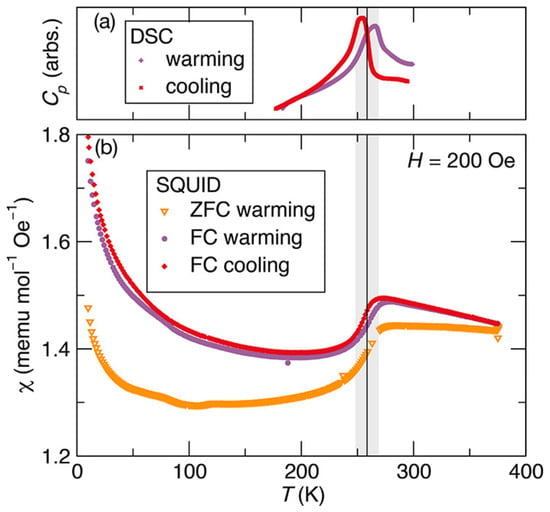
Figure 5.
(a) Heat capacity versus temperature and (b) magnetic susceptibility versus temperature of CrN nanoparticles. The heat capacity and susceptibility data were recorded for cooling as well as for warming cycle [74].
Jankovsky et al. [86] synthesized CrN nanoparticles by ammonolysis of anhydrous CrCl3. The CrCl3 was first dried in thionylchloride under reflux for 36 h in a control ambient in a glove box. The ammonolysis of anhydrous CrCl3 was conducted in a continuous flow of 100 cm3/min NH3 and 50 cm3/min N2 at 1073 K for 72 h. After cooling the sample to room temperature, one part of the powder sample was sintered at 1073 K under the same flow of NH3 and N2, and other parts were processed with spark plasma sintering at 1023 K, 1073 K, and 1123 K. All samples were kept under an 80 MPa pressure during sintering. The samples showed a peak in the heat capacity-versus-temperature data at 291 K and exhibited a magnetic transition from the paramagnetic state to the antiferromagnetic state at around the same temperature. All the samples prepared by different methods as described above showed metallic behavior above and below the Neel temperature, which agrees with Gui et al. [87]. The common part of synthesizing the nanoparticles was the use of high pressure either during the synthesis step or during the sintering process. Further investigation is needed to check whether the pressure induces any permanent changes in the nanoparticles.
CrN nanoparticles were synthesized by a chemical reaction of 1 g of nonahydrated chromium nitrate [Cr(NO3)39H2O] with 6 g urea (NH2CONH2) at 623 K for 2 h by Singh et al. [88]. Ammonia [NH3] was used for the nitridation reaction of the prepared precursor in a combustion quartz tube furnace. The ammonia was flowing at 180–200 mL/min at a temperature of 1073 K for 6 h. The nanoparticles thus prepared were 22.92 nm in diameter. They used a superconducting quantum interference device (SQUID) magnetometer for studying magnetization as a function of temperature. Both field cold and zero field cold magnetization data of the samples were obtained. The data revealed that the magnetization of the sample increased by warming up the samples from the liquid helium temperature to 265 K, which is a typical antiferromagnetic behavior. At 265 K, the magnetization quickly saturated and showed no appreciable increase when the temperature was further increased beyond 275 K. The Neel temperature of 265 K is slightly lower than most of the reported values in the literature [68,69,76], which could be attributed to the unintentional oxygen or carbon doping of their samples as both elements exist in their precursor or due to the finite size of the nanoparticles [89,90]. In a paper published in 2021, Jin et al. demonstrated that by decreasing the size of the thin film, the Neel temperature decreased following TN = 14t0.46, where t represents the thickness of the film [91].
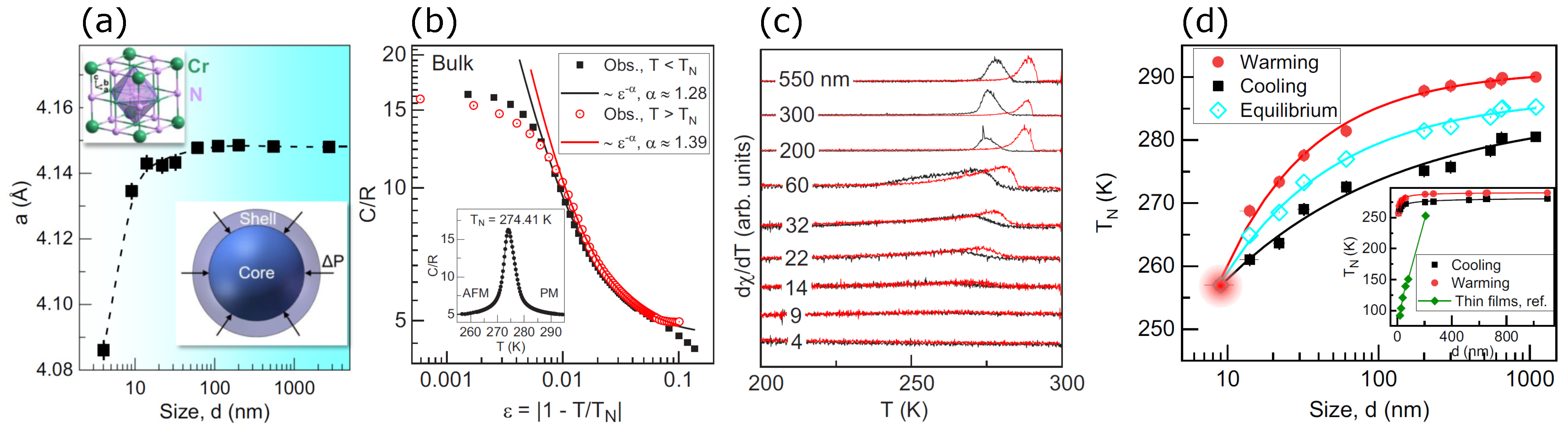
A meticulous study of the finite-size effect on the magnetic and structural transitions in CrN nanoparticles was reported by Wang et al. [92]. They synthesized CrN nanoparticles by mixing anhydrous CrCl3 of 99.5% purity and NaNH2 of 99.5% purity in a 1:4 ratio. The mixture was compressed into a cylindrical shape 10 mm in diameter and height in argon ambient. The samples were then encapsulated into a molybdenum capsule and loaded into a high-pressure cell. For controlling the nanoparticles’ size, the cell was first exposed to a different target pressure between 1 and 5 GPa, followed by heating to a different temperature between 573 and 1773 K for 20 min, before quenching and decompressing the cell to atmospheric pressure. The nanoparticles thus prepared were washed with distilled water. The average diameter of the nanoparticles synthesized under different conditions was in a range of 4 nm to 2750 nm. The lattice constant determined by XRD is provided in Figure 6a. The lattice sharply increases from 4.086 Å to 4.418 Å for nanoparticle diameters of 4 nm and 100 nm, respectively, and it shows a plateau for all nanoparticle diameters larger than 100 nm, which matches the reported values in [68,69,80,93]. It shows that smaller particles have a larger stress compared to larger ones. Figure 6b shows heat capacity versus reduced temperature , where T is the samples’ temperature. Heat capacity manifests discontinuity across TN and shows a peak at 274.41 K for the bulk samples, which is related to the spin reordering in the magnetic phase transition from the paramagnetic to the antiferromagnetic phase. The critical behaviors of heat capacity can be explored by fitting the data by a power law of , with values of 1.39 and 1.28 for the paramagnetic and antiferromagnetic phases, which are larger than any conventional universality classes with values of less than 0.25, which leads to a conclusion that the spins were reordered by the lattice due to its spin–lattice coupling [58,94]. In Figure 6c, the temperature derivative of dc susceptibility (dχ/dT) as a function of temperature shows peaks associated with the magnetic transition in both the cooling and warming data. The peaks can be observed in particles of size 550 nm to 9 nm, but below 9 nm, the transition vanishes due to a small size effect [92]. The Neel temperature as a function of the particle size is illustrated in Figure 6d, where TN for 9 nm particles is about 256 K and for the 1000 nm particle, it is 285 K, which matches the values reported by [68,70].
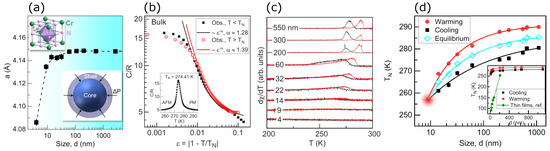
Figure 6.
(a) Lattice parameter of CrN versus the grain size. It shows cubic CrN, and the model shows the core and shell of the CrN nanoparticle with surface compression . (b) Specific heat (C/R) as a function of temperature is shown for the bulk sample. The inset shows the specific heat against temperature in a linear scale. (c) Temperature derivative of susceptibility of CrN nanoparticles recorded for cooling (black colored data) and warming (red colored data) processes. (d) TN versus particle size and its linear plot provided in the inset. The inset also shows the thickness-dependent TN Ref. [92].
Stoichiometric CrN nanoparticles synthesized by Wang et al. [95] using NaCrO2 and hexagonal-BN precursors at 5 GPa and heated to 1573 K for 20 min. The solid-state ion-exchange reaction produced CrN along with NaBO2; the byproduct NaBO2 can be removed by washing the product with distilled water. The nanoparticles’ preparation method was reported in another study by the same group [79]. They studied the the structural transition by XRD as a function of pressure and temperature and observed cubic to orthorhombic at 5.46 GP while keeping the sample at 315 K. The pressure value at which the transition occurred is in agreement with 5 GPa as reported by Yan et al. [65], but it is more than 5 times higher than Revidulla et al.’s [64] value of ∼1 GPa and about 9 times higher than the value reported by Ebad-Allah et al. [81]. The electrical transition studied with resistivity-versus-temperature data occurs at 240 K, which is much smaller than most of the reported values [68,70]. The low transition temperature could be either due to the small size of their nanoparticles [92], impurities [80], or strain in the nanoparticles [91]. They [95] determined the bulk modulus of CrN cubic and orthorhombic crystal structures to be 257 GPa and 262 GPa, which indicates no huge reduction in the bulk modulus of CrN with phase change. The determined value of bulk modulus supports Alling et al. [66] and contradicts Revidulla et al.’s [64] reports.
Improper multiferroic-like transition in CrN nanoparticles was reported by Gui et al. [87]. The nanoparticles were prepared by chemical reaction at a high pressure as reported in [79,95]. Their neutron powder diffraction experiment revealed a structural transition from cubic to orthorhombic upon cooling. However, their electrical resistivity-versus-temperature data showed metal-to-metal transition around 240 K, which contradicts other reported observations of semiconducting to metallic [70], semiconducting to semiconducting [77], and impurities [80] and epitaxial constraints [63]-dependent transitions. The low-temperature metallic state appears only in a tetragonal/cubic crystal structure [68]; however, their neutron diffraction shows the prevailing orthorhombic structure at low temperatures. They used DFT calculations with a Hubbard correction of 0.5 eV to match the observed metallicity and magnetic moment in the orthographic phase; however, 0.5 eV is a much smaller value of U determined both experimentally [84] as well as adopted in theoretical models [48,68,96,97].
Gui et al. meticulously explained the lattice modulation produced by the frustrated chain of Cr spins in the orthorhombic phase [87]. Accompanied by the magnetostructural transition, CrN nanoparticles also showed an antiferroelectric-like transition, which is driven by the frustrated Cr t2g spins in the antiferromagnetic order. Metallicity, on the other hand, originates from the Cr eg and N p orbitals [87]. The magnetic and lattice modulations buckle up the lattice along the c-axis, which moves the negative and positive charge centers in CrN6 octahedra in opposite directions to electric dipole. These results agree with the reported buckled CrN (001)surface [68] observed with low-temperature high-energy electron diffraction. Alam et al. reported the doubling of the structural periodicity on the CrN (001) surface, which appeared at 273 K in an epitaxial CrN film grown on a MgO (001) substrate [68].
Table 1 shows the experimental results reviewed in this article. The sample synthesis methods, transition temperature, and structural, electronic, and magnetic transitions are summarized in the table. The temperature above the transition temperature is labeled as HT and that below the phase transition temperature is labeled as LT.

Table 1.
CrN nanoparticles’ preparation methods and structural, electronic, and magnetic transitions.
3. Chromium Nitride Computational Models
Although analytical and computational research is not the focus of this review, I provide a brief overview of the research carried out on CrN. Many groups have applied various theories to understand the structural and magnetic transitions in CrN [57,83,93,94,96,99,100,101]. The analytical methods include applying renormalization group theory and the Landau–Ginsburg–Wilson models to check critical behavior around the transition temperature [57], and other similar models [75]. Computational methods often use density functional theory (DFT) in combination with the projector augmented wave (PAW) method, typically applied in the Vienna ab-initio simulation package (VASP). The local spin density approximation (LDA) [102], the generalized gradient approximation (GGA) [103], and the LDA combined with a Hubbard Coulomb term (LDA + U) [104,105] are commonly used to understand electron exchange-correlation effects. The Hubbard term is used to understand the Cr 3d-orbital interactions [106] and the opening of the bandgap. Tawinan et al. [107] studied CrN using self-consistent GW methods. They started with the local spin density approximation with U and then removed U to let the GW energy define the bandgap. They found a bandgap of about 1 eV, which is in agreement with Botana et al. [108], who determined the bandgap using LDA + U.
Following the renormalization group theory method, Mrozinska et al. [57] constructed a 12-component order parameter system, which led to the formation of a relatively simple Landau–Ginsburg–Wilson Hamiltonian. They noted that the order parameter in CrN transforms with respect to the 12-dimensional irreducible representation of the Fm3m space group. They proved that the RG transformations have no stable fixed points, indicating that the transition should be first-order.
4. Conclusions and Prospects
Various methods for synthesizing CrN nanoparticles and their influence on structural, electronic, and magnetic phase transitions have been reviewed. It has been observed that the synthesis methods can induce different levels of stress, size variations, nitrogen vacancies, changes in stoichiometry, defects, and impurities. For instance, particle size and strain can alter the transition temperature or even eliminate the transition. Characterization methods and the ambient conditions for studying properties also affect the physical properties of the materials. It is observed that the onset of one transition is often concurrent with another, indicating that these transitions are correlated. Transitions have been reported over a wide temperature range, though most studies report them at 275 ± 10 K. In addition to experimental methods, the article briefly reviews theoretical simulations towards the end. This comprehensive review serves as a detailed reference for researchers and aims to stimulate further scientific interest in understanding the physical properties of CrN nanoparticles and their use in technological applications.
Despite the use of various synthesis methods, nanoparticles are seldom free from defects, stress, and impurities. Oxygen is one of the main impurities in CrN nanoparticles, as Cr reacts more readily with oxygen than with nitrogen. Many nanoparticles have been prepared either in air or in rough vacuum environments. The oxidation issue can be observed more easily when studying the CrN bandgap [109], which is extremely sensitive to oxygen doping. Many nanoparticle preparation methods require NH3, which is hazardous, expensive, and not environmentally friendly. Some methods can induce defects, such as encapsulating Cr with a nitrogen precursor and then quenching the nanoparticles from high temperature. The nanoparticles thus prepared will be polycrystalline and will have a range of strains. Although nanoparticle preparation by chemical reactions is well-established, it is not as clean and controlled as thin film preparations in ultrahigh vacuum. To converge all these divergent results, nanoparticles need to be prepared in a controlled environment in an ultrahigh vacuum chamber to ensure their quality and reproducibility.
To the best of my knowledge, no study has directly compared the impact of stress versus stoichiometry on the phase transitions of the films, or determined which one has a more dominant effect. However, by comparing different studies, one can conclude that the stoichiometry of the film plays a more dominant role than the stress within the film.
Another deficiency is that not all phase transitions have been studied with the same nanoparticles, making it difficult to understand the delicate correlation between different transitions. Comprehensive studies of all transitions using high-quality nanoparticles are needed.
It is computationally demanding to study phase transitions as a function of temperature or pressure variation; however, it is desirable and would provide the most accurate guide to experimental studies. Most models use only a fixed temperature and pressure and allow a system to relax from a predefined crystal structure. Such studies can sometimes be misleading as simulated models may become stuck in a local minimum rather than reaching to a global minimum. In the GGA+U or LDA+U methods, one has to define the value of U from other sources or by trial and error. The most complete study would begin from a cubic system of CrN and gradually cool down below the Neel temperature, where CrN adopts an antiferromagnetic order and an electronic phase with a certain crystal structure.
Combining experimental studies with theoretical modeling to understand the physical properties under different conditions and using the acquired knowledge to bridge the gap between science and engineering would be productive. Such collaboration would be beneficial for exploring CrN for phase change memory, temperature sensors, electrical sensors, and other advanced applications.
Funding
This work was supported by the Deanship of Research Oversight and Coordination of King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, under grant No. ISP23218.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cheng, Z.; Qi, W.; Pang, C.H.; Thomas, T.; Wu, T.; Liu, S.; Yang, M. Recent advances in transition metal nitride-based materials for photocatalytic applications. Adv. Funct. Mater. 2021, 31, 2100553. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.K. Synthesis of novel transition metal nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef]
- Eklund, P.; Kerdsongpanya, S.; Alling, B. Transition-metal-nitride-based thin films as novel energy harvesting materials. J. Mater. Chem. C 2016, 4, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, Y.; Liu, S.; Liu, H.; Hui, L.S.; Turak, A.; Wang, J.; Yang, M. Oxidized impurity in transition metal nitride for improving the hydrogen evolution efficiency of transition metal nitride-based catalyst. Appl. Mater. Today 2020, 18, 100476. [Google Scholar] [CrossRef]
- Juneja, S.; Shishodia, M.S. Surface plasmon amplification in refractory transition metal nitrides based nanoparticle dimers. Opt. Commun. 2019, 433, 89–96. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Smyrnova, K.; Sahul, M.; Haršáni, M.; Čaplovič, L.; Beresnev, V.; Čaplovičová, M.; Kusy, M.; Pogrebnjak, A. Effect of bias voltage on the structural properties of WN/NbN nanolayer coatings deposited by cathodic-arc evaporation. J. Phys. Conf. Ser. 2024, 2712, 012014. [Google Scholar] [CrossRef]
- Weinberger, C.R.; Yu, X.X.; Yu, H.; Thompson, G.B. Ab initio investigations of the phase stability in group IVB and VB transition metal nitrides. Comput. Mater. Sci. 2017, 138, 333–345. [Google Scholar] [CrossRef]
- Biswas, A.; Alvarez, G.A.; Tripathi, M.; Lee, J.; Pieshkov, T.S.; Li, C.; Gao, B.; Puthirath, A.B.; Zhang, X.; Gray, T.; et al. Cubic and hexagonal boron nitride phases and phase boundaries. J. Mater. Chem. C 2024, 12, 3053–3062. [Google Scholar] [CrossRef]
- Corliss, L.; Elliott, N.; Hastings, J. Antiferromagnetic structure of CrN. Phys. Rev. 1960, 117, 929. [Google Scholar] [CrossRef]
- Srivastava, A.; Chauhan, M.; Singh, R. Pressure induced phase transitions in transition metal nitrides: Ab initio study. Phys. Status Solidi B 2011, 248, 2793–2800. [Google Scholar] [CrossRef]
- Ojha, P.; Aynyas, M.; Sanyal, S.P. Pressure-induced structural phase transformation and elastic properties of transition metal mononitrides. J. Phys. Chem. Solids 2007, 68, 148–152. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Nagaosa, N. Orbital physics in transition-metal oxides. Science 2000, 288, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Börgel, J.; Campbell, M.G.; Ritter, T. Transition metal d-orbital splitting diagrams: An updated educational resource for square planar transition metal complexes. J. Chem. Educ. 2016, 93, 118–121. [Google Scholar] [CrossRef]
- Li, Y.H.; Hung, T.H.; Chen, C.W. A first-principles study of nitrogen-and boron-assisted platinum adsorption on carbon nanotubes. Carbon 2009, 47, 850–855. [Google Scholar] [CrossRef]
- Mananghaya, M.R.; Santos, G.N.; Yu, D. Nitrogen substitution and vacancy mediated scandium metal adsorption on carbon nanotubes. Adsorption 2017, 23, 789–797. [Google Scholar] [CrossRef]
- Ma, Y.; Foster, A.S.; Krasheninnikov, A.; Nieminen, R.M. Nitrogen in graphite and carbon nanotubes: Magnetism and mobility. Phys. Rev. B 2005, 72, 205416. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Qu, J.; Feng, X.; Seh, Z.W.; Wang, T.; Zhang, Q. Strain-controlled single Cr-embedded nitrogen-doped graphene achieves efficient nitrogen reduction. Mater. Adv. 2021, 2, 5704–5711. [Google Scholar] [CrossRef]
- Feng, H.; Ma, J.; Hu, Z. Nitrogen-doped carbon nanotubes functionalized by transition metal atoms: A density functional study. J. Mater. Chem. 2010, 20, 1702–1708. [Google Scholar] [CrossRef]
- Mateti, S.; Sultana, I.; Chen, Y.; Kota, M.; Rahman, M.M. Boron Nitride-Based Nanomaterials: Synthesis and Application in Rechargeable Batteries. Batteries 2023, 9, 344. [Google Scholar] [CrossRef]
- Thomas, S.A.; Pallavolu, M.R.; Khan, M.E.; Cherusseri, J. Graphitic carbon nitride (g-C3N4): Futuristic material for rechargeable batteries. J. Energy Storage 2023, 68, 107673. [Google Scholar] [CrossRef]
- Xiong, T.; Li, J.; Roy, J.C.; Koroma, M.; Zhu, Z.; Yang, H.; Zhang, L.; Ouyang, T.; Balogun, M.S.; Al-Mamun, M. Hetero-interfacial nickel nitride/vanadium oxynitride porous nanosheets as trifunctional electrodes for HER, OER and sodium ion batteries. J. Energy Chem. 2023, 81, 71–81. [Google Scholar] [CrossRef]
- Jin, C.; Huang, Y.; Li, L.; Wei, G.; Li, H.; Shang, Q.; Ju, Z.; Lu, G.; Zheng, J.; Sheng, O.; et al. A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries. Nat. Commun. 2023, 14, 8269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Zhang, X.Q.; Zhou, M.Y.; Huang, J.Q. Mechanism, quantitative characterization, and inhibition of corrosion in lithium batteries. Nano Res. Energy 2023, 2, e9120046. [Google Scholar] [CrossRef]
- Ren, L.; Hu, Z.; Peng, C.; Zhang, L.; Wang, N.; Wang, F.; Xia, Y.; Zhang, S.; Hu, E.; Luo, J. Suppressing metal corrosion through identification of optimal crystallographic plane for Zn batteries. Proc. Natl. Acad. Sci. USA 2024, 121, e2309981121. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Shu, D.; He, C.; Nan, J. Study on the electrochemical behavior of vanadium nitride as a promising supercapacitor material. J. Phys. Chem. Solids 2009, 70, 495–500. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Du, H. Electrochemical capacitance performance of titanium nitride nanoarray. Mater. Sci. Eng. B 2013, 178, 1443–1451. [Google Scholar] [CrossRef]
- Shen, H.; Wei, B.; Zhang, D.; Qi, Z.; Wang, Z. Magnetron sputtered NbN thin film electrodes for supercapacitors. Mater. Lett. 2018, 229, 17–20. [Google Scholar] [CrossRef]
- Arif, M.; Sanger, A.; Singh, A. Sputter deposited chromium nitride thin electrodes for supercapacitor applications. Mater. Lett. 2018, 220, 213–217. [Google Scholar] [CrossRef]
- Ting, Y.J.B.; Lian, K.; Kherani, N. Fabrication of titanium nitride and molybdenum nitride for supercapacitor electrode application. ECS Trans. 2011, 35, 133. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Cai, J.; Zheng, X.; Han, D.; Wu, Y.; Zang, Y.; Niu, S.; Liu, Y.; Zhu, J.; et al. Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis. Angew. Chem. Int. Ed. 2018, 57, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xing, Y.; Yang, R.; Wen, T.; Jiang, D.; Shi, W.; Yuan, S. Holey cobalt–iron nitride nanosheet arrays as high-performance bifunctional electrocatalysts for overall water splitting. ACS Appl. Mater. Interfaces 2020, 12, 29253–29263. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.; Surendran, S.; Kim, J.Y.; Janani, G.; Lee, D.K.; Kim, T.H.; Kim, J.K.; Sim, U. Syntheses and electronic structure engineering of transition metal nitrides for supercapacitor applications. J. Mater. Chem. A 2022, 10, 14655–14673. [Google Scholar] [CrossRef]
- Zhang, X.; Gall, D. CrN electronic structure and vibrational modes: An optical analysis. Phys. Rev. B 2010, 82, 045116. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, W.; Li, T. A review on transition metal nitrides as electrode materials for supercapacitors. Ceram. Int. 2019, 45, 21062–21076. [Google Scholar] [CrossRef]
- Gharavi, M.A.; Kerdsongpanya, S.; Schmidt, S.; Eriksson, F.; Nong, N.; Lu, J.; Balke, B.; Fournier, D.; Belliard, L.; Le Febvrier, A.; et al. Microstructure and thermoelectric properties of CrN and CrN/Cr2N thin films. J. Phys. D Appl. Phys. 2018, 51, 355302. [Google Scholar] [CrossRef]
- Wei, B.; Liang, H.; Zhang, D.; Wu, Z.; Qi, Z.; Wang, Z. CrN thin films prepared by reactive DC magnetron sputtering for symmetric supercapacitors. J. Mater. Chem. A 2017, 5, 2844–2851. [Google Scholar] [CrossRef]
- Gao, Z.; Wan, Z.; Wu, Z.; Huang, X.; Li, H.; Zhang, T.F.; Mayrhofer, P.H.; Wang, Q. Synthesis and electrochemical properties of nanoporous CrN thin film electrodes for supercapacitor applications. Mater. Des. 2021, 209, 109949. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, Z.W. An anode material of CrN for lithium-ion batteries. Electrochem. Solid-State Lett. 2007, 10, A189. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, P.; Shu, H.B.; Man, X.L.; Du, X.Q.; Chao, D.L.; Liu, Z.G.; Sun, Y.P.; Wan, H.Z.; Wang, H. Design rules of heteroatom-doped graphene to achieve high performance lithium–sulfur batteries: Both strong anchoring and catalysing based on first principles calculation. J. Colloid Interface Sci. 2018, 529, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, A.; Krott, M.; Streipert, B.; Uhlenbruck, S.; Winter, M.; Placke, T. Suppression of Aluminum Current Collector Dissolution by Protective Ceramic Coatings for Better High-Voltage Battery Performance. ChemPhysChem 2017, 18, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, Z.W. Cr1-xFexN (0 ≦x≦ 1) Ternary Transition-Metal Nitrides as Anode Materials for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2008, 11, A233. [Google Scholar] [CrossRef]
- Gu, L.; Wu, S.Y.; Liu, H.; Singh, R.; Newman, N.; Smith, D.J. Characterization of Al (Cr) N and Ga (Cr) N dilute magnetic semiconductors. J. Magn. Magn. Mater. 2005, 290, 1395–1397. [Google Scholar] [CrossRef]
- Ney, A.; Rajaram, R.; Parkin, S.; Kammermeier, T.; Dhar, S. Magnetic properties of epitaxial CrN films. Appl. Phys. Lett. 2006, 89, 112504. [Google Scholar] [CrossRef]
- Alsaad, A. Magnetic and structural properties of Cr-based diluted magnetic semiconductors and alloys. Phys. B Condens. Matter 2010, 405, 951–954. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.F.; Pan, J.; Du, S. Ultra-low Young’s modulus and high super-exchange interactions in monolayer CrN: A promising candidate for flexible spintronic applications. Chin. Phys. B 2021, 30, 047105. [Google Scholar] [CrossRef]
- Ponce-Pérez, R.; Cocoletzi, G.H.; Takeuchi, N. Antiferromagnetic coupling in the initial stages of the MnN epitaxial growth on the CrN (001) surface. Appl. Surf. Sci. 2022, 573, 151451. [Google Scholar]
- Feng, X.; Bao, K.; Huang, Y.; Ma, S.; Tao, Q.; Zhu, P.; Cui, T. Complete ligand reinforcing the structure of cubic-CrN. J. Alloys Compd. 2019, 783, 232–236. [Google Scholar] [CrossRef]
- Liu, L.; He, Z.; Peng, J.; Guo, D.; Xu, Z.; Wang, C. Enhancing thermoelectric performance of CrN ceramics by optimizing sintering temperature. J. Eur. Ceram. Soc. 2024, 44, 7660–7667. [Google Scholar] [CrossRef]
- Yuan, M.; Wan, X.; Meng, Q.; Lu, X.; Sun, L.; Wang, W.; Jiang, P.; Bao, X. Suppression of secondary phase in CrN matrix to boost the high-temperature thermoelectric performance. Mater. Today Phys. 2021, 19, 100420. [Google Scholar] [CrossRef]
- Guo, W.; Liang, Z.; Tang, Y.; Cai, K.; Qiu, T.; Wu, Y.; Zhang, K.; Gao, S.; Zou, R. Understanding the lattice nitrogen stability and deactivation pathways of cubic CrN nanoparticles in the electrochemical nitrogen reduction reaction. J. Mater. Chem. A 2021, 9, 8568–8575. [Google Scholar] [CrossRef]
- Das, B.; Reddy, M.; Rao, G.S.; Chowdari, B. Synthesis and Li-storage behavior of CrN nanoparticles. RSC Adv. 2012, 2, 9022–9028. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.Y.; Wang, Z.; Lv, W.; He, Y.B.; Yang, Q.H.; Kang, F. Revisiting the roles of natural graphite in ongoing lithium-ion batteries. Adv. Mater. 2022, 34, 2106704. [Google Scholar] [CrossRef] [PubMed]
- Shuang, Y.; Mori, S.; Yamamoto, T.; Hatayama, S.; Saito, Y.; Fons, P.J.; Song, Y.H.; Hong, J.P.; Ando, D.; Sutou, Y. Soret-Effect Induced Phase-Change in a Chromium Nitride Semiconductor Film. ACS Nano 2024, 18, 21135–21143. [Google Scholar] [CrossRef] [PubMed]
- Cox, D. Neutron-diffraction determination of magnetic structures. IEEE Trans. Magn. 1972, 8, 161–182. [Google Scholar] [CrossRef]
- Mrozińska, A.; Przystawa, J.; Sòlyom, J. First-order antiferromagnetic transition in CrN. Phys. Rev. B 1979, 19, 331. [Google Scholar] [CrossRef]
- Filippetti, A.; Hill, N.A. Magnetic stress as a driving force of structural distortions: The case of CrN. Phys. Rev. Lett. 2000, 85, 5166. [Google Scholar] [CrossRef]
- Gall, D.; Shin, C.; Haasch, R.; Petrov, I.; Greene, J. Band gap in epitaxial NaCl-structure CrN (001) layers. J. Appl. Phys. 2002, 91, 5882. [Google Scholar] [CrossRef]
- Zhang, X.; Chawla, J.; Deng, R.; Gall, D. Epitaxial suppression of the metal-insulator transition in CrN. Phys. Rev. B 2011, 84, 073101. [Google Scholar] [CrossRef]
- Biswas, B.; Chakraborty, S.; Joseph, A.; Acharya, S.; Pillai, A.I.K.; Narayana, C.; Bhatia, V.; Garbrecht, M.; Saha, B. Secondary phase limited metal-insulator phase transition in chromium nitride thin films. Acta Mater. 2022, 227, 117737. [Google Scholar] [CrossRef]
- Inumaru, K.; Koyama, K.; Imo-Oka, N.; Yamanaka, S. Controlling the structural transition at the Néel point of CrN epitaxial thin films using epitaxial growth. Phys. Rev. B 2007, 75, 054416. [Google Scholar] [CrossRef]
- Jin, Q.; Zhao, J.; Roldan, M.A.; Qi, W.; Lin, S.; Chen, S.; Hong, H.; Fan, Y.; Rong, D.; Guo, H.; et al. Anisotropic electronic phase transition in CrN epitaxial thin films. Appl. Phys. Lett. 2022, 120, 073103. [Google Scholar] [CrossRef]
- Rivadulla, F.; Bañobre-López, M.; Quintela, C.X.; Piñeiro, A.; Pardo, V.; Baldomir, D.; López-Quintela, M.A.; Rivas, J.; Ramos, C.A.; Salva, H.; et al. Reduction of the bulk modulus at high pressure in CrN. Nat. Mater. 2009, 8, 947–951. [Google Scholar] [CrossRef]
- Yan, M.; Zhou, X.; Cheng, H.; Wang, L.; Zhang, J.; Yu, X.; He, D.; Mei, J.W.; Zhao, Y.; Wang, S. Compressibility and thermoelasticity of CrN. High Press. Res. 2020, 40, 423–433. [Google Scholar] [CrossRef]
- Alling, B.; Marten, T.; Abrikosov, I.A. Questionable collapse of the bulk modulus in CrN. Nat. Mater. 2010, 9, 283–284. [Google Scholar] [CrossRef]
- Alam, K.; Ponce-Pérez, R.; Sun, K.; Foley, A.; Takeuchi, N.; Smith, A.R. Study of the structure, structural transition, interface model, and magnetic moments of CrN grown on MgO (001) by molecular beam epitaxy. J. Vac. Sci. Technol. A 2023, 41, 053411. [Google Scholar] [CrossRef]
- Alam, K.; Disseler, S.M.; Ratcliff, W.D.; Borchers, J.A.; Ponce-Pérez, R.; Cocoletzi, G.H.; Takeuchi, N.; Foley, A.; Richard, A.; Ingram, D.C.; et al. Structural and magnetic phase transitions in chromium nitride thin films grown by rf nitrogen plasma molecular beam epitaxy. Phys. Rev. B 2017, 96, 104433. [Google Scholar] [CrossRef]
- Alam, K.; Meng, K.Y.; Ponce-Pérez, R.; Cocoletzi, G.H.; Takeuchi, N.; Foley, A.; Yang, F.; Smith, A.R. Exchange bias and exchange spring effects in Fe/CrN bilayers. J. Phys. D Appl. Phys. 2020, 53, 125001. [Google Scholar] [CrossRef]
- Constantin, C.; Haider, M.B.; Ingram, D.; Smith, A.R. Metal/semiconductor phase transition in chromium nitride (001) grown by rf-plasma-assisted molecular-beam epitaxy. Appl. Phys. Lett. 2004, 85, 6371–6373. [Google Scholar] [CrossRef]
- Singh, A.; Tešanović, Z. Collective excitations in a doped antiferromagnet. Phys. Rev. B 1990, 41, 614. [Google Scholar] [CrossRef] [PubMed]
- Darradi, R.; Derzhko, O.; Zinke, R.; Schulenburg, J.; Krüger, S.; Richter, J. Ground state phases of the spin-1/2 J 1–J 2 Heisenberg antiferromagnet on the square lattice: A high-order coupled cluster treatment. Phys. Rev. B 2008, 78, 214415. [Google Scholar] [CrossRef]
- Völkel, A.; Mertens, F.; Bishop, A.; Wysin, G. Motion of vortex pairs in the ferromagnetic and antiferromagnetic anisotropic Heisenberg model. Phys. Rev. B 1991, 43, 5992. [Google Scholar] [CrossRef] [PubMed]
- Zieschang, A.M.; Bocarsly, J.D.; Dürrschnabel, M.; Kleebe, H.J.; Seshadri, R.; Albert, B. Low-temperature synthesis and magnetostructural transition in antiferromagnetic, refractory nanoparticles: Chromium nitride, CrN. Chem. Mater. 2018, 30, 1610–1616. [Google Scholar] [CrossRef]
- Nasr-Eddine, M.; Bertaut, E. Etude de la transition de premier ordre dans CrN. Solid State Commun. 1971, 9, 717–723. [Google Scholar] [CrossRef]
- Browne, J.; Liddell, P.; Street, R.; Mills, T. An investigation of the antiferromagnetic transition of CrN. Phys. Status Solidi A 1970, 1, 715–723. [Google Scholar] [CrossRef]
- Quintela, C.; Rivadulla, F.; Rivas, J. Thermoelectric properties of stoichiometric and hole-doped CrN. Appl. Phys. Lett. 2009, 94, 152103. [Google Scholar] [CrossRef]
- Quintela, C.X.; Podkaminer, J.P.; Luckyanova, M.N.; Paudel, T.R.; Thies, E.L.; Hillsberry, D.A.; Tenne, D.A.; Tsymbal, E.Y.; Chen, G.; Eom, C.B.; et al. Epitaxial CrN thin films with high thermoelectric figure of merit. Adv. Mater. 2015, 27, 3032–3037. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Zhang, J.; Wang, L.; Leinenweber, K.; He, D.; Zhao, Y. Synthesis, hardness, and electronic properties of stoichiometric VN and CrN. Cryst. Growth Des. 2016, 16, 351–358. [Google Scholar] [CrossRef]
- Alam, K.; Haider, M.B.; Al-Kuhaili, M.F.; Ziq, K.A.; Haq, B.U. Electronic phase transition in CrN thin films grown by reactive RF magnetron sputtering. Ceram. Int. 2022, 48, 17352–17358. [Google Scholar] [CrossRef]
- Ebad-Allah, J.; Kugelmann, B.; Rivadulla, F.; Kuntscher, C.A. Infrared study of the magnetostructural phase transition in correlated CrN. Phys. Rev. B 2016, 94, 195118. [Google Scholar] [CrossRef]
- Herle, P.S.; Hegde, M.; Vasathacharya, N.; Philip, S.; Rao, M.R.; Sripathi, T. Synthesis of TiN, VN, and CrN from ammonolysis of TiS2, VS2, and Cr2S3. J. Solid State Chem. 1997, 134, 120–127. [Google Scholar] [CrossRef]
- Herwadkar, A.; Lambrecht, W.R. Electronic structure of CrN: A borderline Mott insulator. Phys. Rev. B 2009, 79, 035125. [Google Scholar] [CrossRef]
- Bhobe, P.; Chainani, A.; Taguchi, M.; Takeuchi, T.; Eguchi, R.; Matsunami, M.; Ishizaka, K.; Takata, Y.; Oura, M.; Senba, Y.; et al. Evidence for a correlated insulator to antiferromagnetic metal transition in CrN. Phys. Rev. Lett. 2010, 104, 236404. [Google Scholar] [CrossRef]
- Himmetoglu, B.; Floris, A.; De Gironcoli, S.; Cococcioni, M. Hubbard-corrected DFT energy functionals: The LDA+ U description of correlated systems. Int. J. Quantum Chem. 2014, 114, 14–49. [Google Scholar] [CrossRef]
- Jankovskỳ, O.; Sedmidubskỳ, D.; Huber, Š.; Šimek, P.; Sofer, Z. Synthesis, magnetic and transport properties of oxygen-free CrN ceramics. J. Eur. Ceram. Soc. 2014, 34, 4131–4136. [Google Scholar] [CrossRef]
- Gui, Z.; Gu, C.; Cheng, H.; Zhu, J.; Yu, X.; Guo, E.J.; Wu, L.; Mei, J.; Sheng, J.; Zhang, J.; et al. Improper multiferroiclike transition in a metal. Phys. Rev. B 2022, 105, L180101. [Google Scholar] [CrossRef]
- Singh, D.; Tamrakar, S.; Shrivas, K.; Dewangan, K. Nitridation of Cr–urea complex into nanocrystalline CrN and its antiferromagnetic magnetostructural transition study. New J. Chem. 2022, 46, 20879–20885. [Google Scholar] [CrossRef]
- Lang, X.; Zheng, W.; Jiang, Q. Size and interface effects on ferromagnetic and antiferromagnetic transition temperatures. Phys. Rev. B 2006, 73, 224444. [Google Scholar] [CrossRef]
- Fisher, M.E.; Barber, M.N. Scaling theory for finite-size effects in the critical region. Phys. Rev. Lett. 1972, 28, 1516. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, H.; Wang, Z.; Zhang, Q.; Lin, S.; Roldan, M.A.; Zhao, J.; Wang, J.O.; Chen, S.; He, M.; et al. Strain-Mediated High Conductivity in Ultrathin Antiferromagnetic Metallic Nitrides. Adv. Mater. 2021, 33, 2005920. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, W.; Zhou, X.; Gu, C.; Cheng, H.; Chen, J.; Wu, L.; Zhu, J.; Zhao, Y.; Guo, E.J.; et al. Anomalous finite-size effect on the magnetostructural transition in CrN. Phys. Rev. B 2023, 107, 174112. [Google Scholar] [CrossRef]
- Alam, K.; Ponce-Pérez, R.; Sun, K.; Foley, A.; Takeuchi, N.; Smith, A.R. Investigating the magnetic and atomic interface configuration for a model Fe/CrN bilayer system. J. Vac. Sci. Technol. A 2021, 39, 063209. [Google Scholar] [CrossRef]
- Filippetti, A.; Pickett, W.; Klein, B. Competition between magnetic and structural transitions in CrN. Phys. Rev. B 1999, 59, 7043. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Zhang, J.; Chen, M.; Zhu, J.; Wang, L.; He, D.; Lin, Z.; Zhang, R.; Leinenweber, K.; et al. Experimental invalidation of phase-transition-induced elastic softening in CrN. Phys. Rev. B 2012, 86, 064111. [Google Scholar] [CrossRef]
- Ponce-Pérez, R.; Alam, K.; Cocoletzi, G.H.; Takeuchi, N.; Smith, A.R. Structural, electronic, and magnetic properties of the CrN (001) surface: First-principles studies. Appl. Surf. Sci. 2018, 454, 350–357. [Google Scholar] [CrossRef]
- Hernández, J.C.M.; Ponce-Pérez, R.; Cocoletzi, G.H.; Takeuchi, N.; Hoat, D. Tuning the half-metallicity in reconstructed CrN (111) surfaces. Surf. Interfaces 2022, 35, 102420. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Zhang, J.; He, D.; Zhao, Y. Synthesis of Stoichiometric and Bulk CrN through a Solid-State Ion-Exchange Reaction. Chem. Eur. J. 2012, 18, 15459–15463. [Google Scholar] [CrossRef]
- Rojas, T.; Ulloa, S.E. Strain fields and electronic structure of antiferromagnetic CrN. Phys. Rev. B 2017, 96, 125203. [Google Scholar] [CrossRef]
- Tenelanda-Osorio, L.I.; Vélez, M.E. First principles study of the thermodynamic, mechanical and electronic properties of crystalline phases of Chromium Nitrides. J. Phys. Chem. Solids 2021, 148, 109692. [Google Scholar] [CrossRef]
- Haq, B.U.; Alam, K.; Haider, M.B.; Alsharari, A.M.; Ullah, S.; Kim, S.H. Structural, electronic, magnetic, and optical properties of exfoliated chromium nitride ultrathin films. Phys. E Low Dimens. Syst. Nanostruct. 2023, 150, 115697. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 1980, 45, 566. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+ U study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]
- Alling, B.; Marten, T.; Abrikosov, I. Effect of magnetic disorder and strong electron correlations on the thermodynamics of CrN. Phys. Rev. B 2010, 82, 184430. [Google Scholar] [CrossRef]
- Cheiwchanchamnangij, T.; Lambrecht, W.R. Quasiparticle self-consistent G W band structure of CrN. Phys. Rev. B 2020, 101, 085103. [Google Scholar] [CrossRef]
- Botana, A.S.; Pardo, V.; Baldomir, D.; Blaha, P. Conducting states caused by a surface electric dipole in CrN (001) very thin films. Phys. Rev. B 2013, 87, 075114. [Google Scholar] [CrossRef]
- Kamoru, W.S.; Haider, M.B.; Haq, B.U.; Aleithan, S.H.; Alsharari, A.M.; Ullah, S.; Alam, K. Structural, electronic, and optical properties of chromium oxynitride thin films grown by RF magnetron sputtering. Res. Phys. 2024, 57, 107387. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).