Recent Advances and Future Prospects of Lithium Recovery from Low-Grade Lithium Resources: A Review
Abstract
1. Introduction
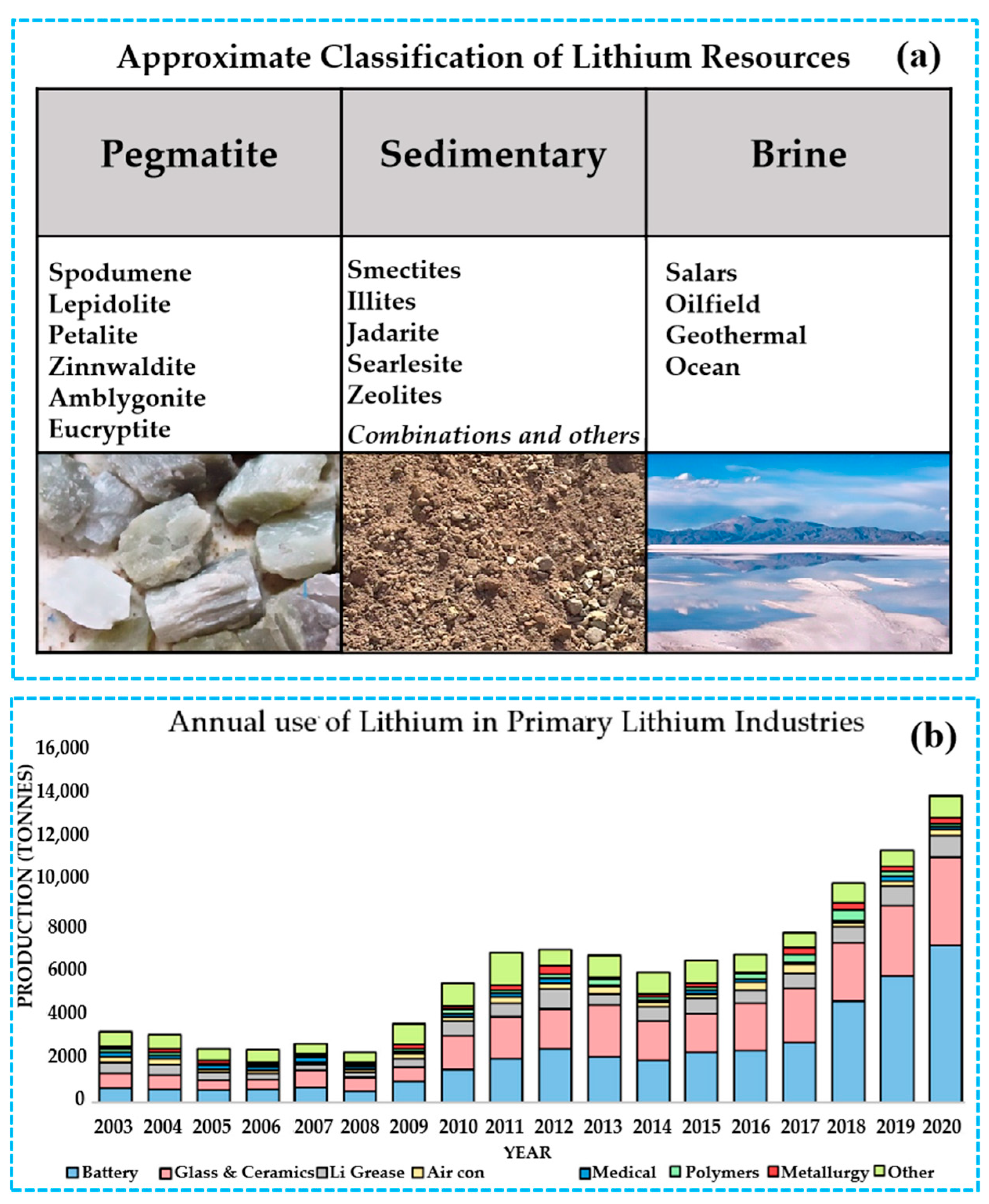
2. Types and Distribution of Low-Grade Lithium Resources
2.1. Lithium-Bearing Clays
2.2. Low-Grade Brines
2.3. Coal and Coal By-Products
2.4. Other Low-Grade Lithium Resources
3. Technologies for Low-Grade Lithium Recovery
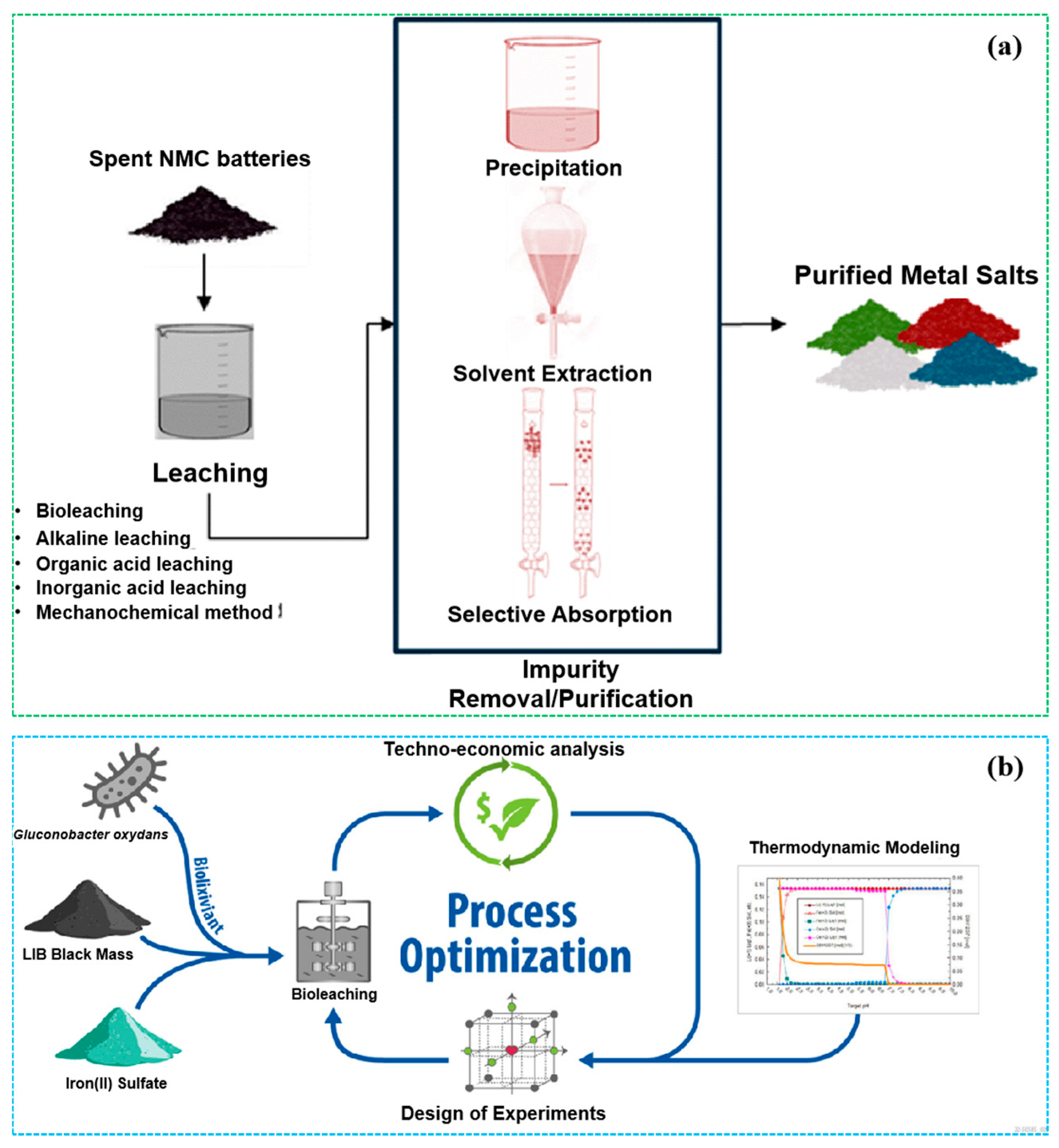
3.1. Leaching Methods
3.1.1. Acid Leaching
3.1.2. Alkaline Leaching
3.1.3. Combined Roasting–Leaching Methods
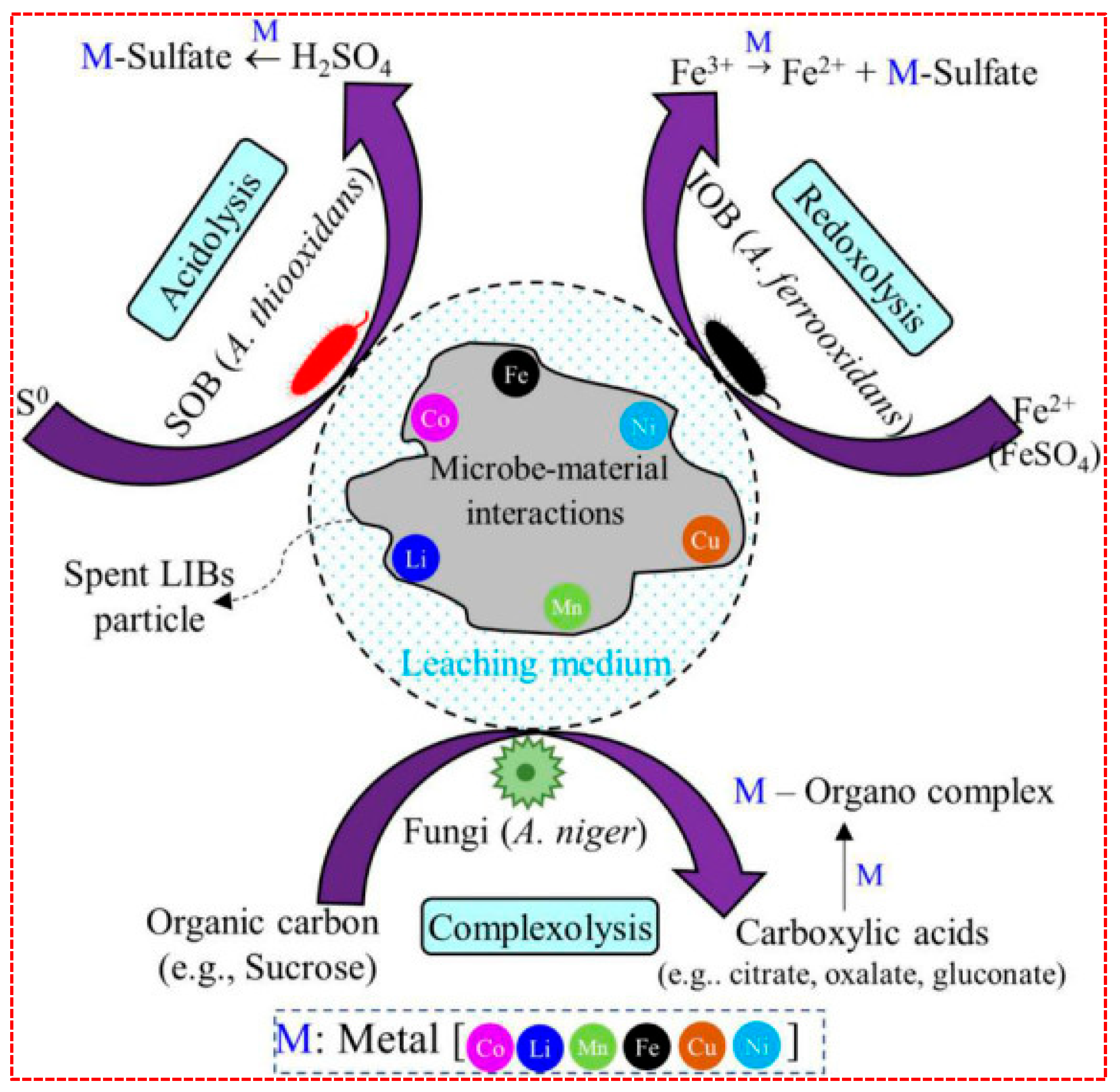
3.1.4. Bioleaching
3.2. Separation and Purification Methods
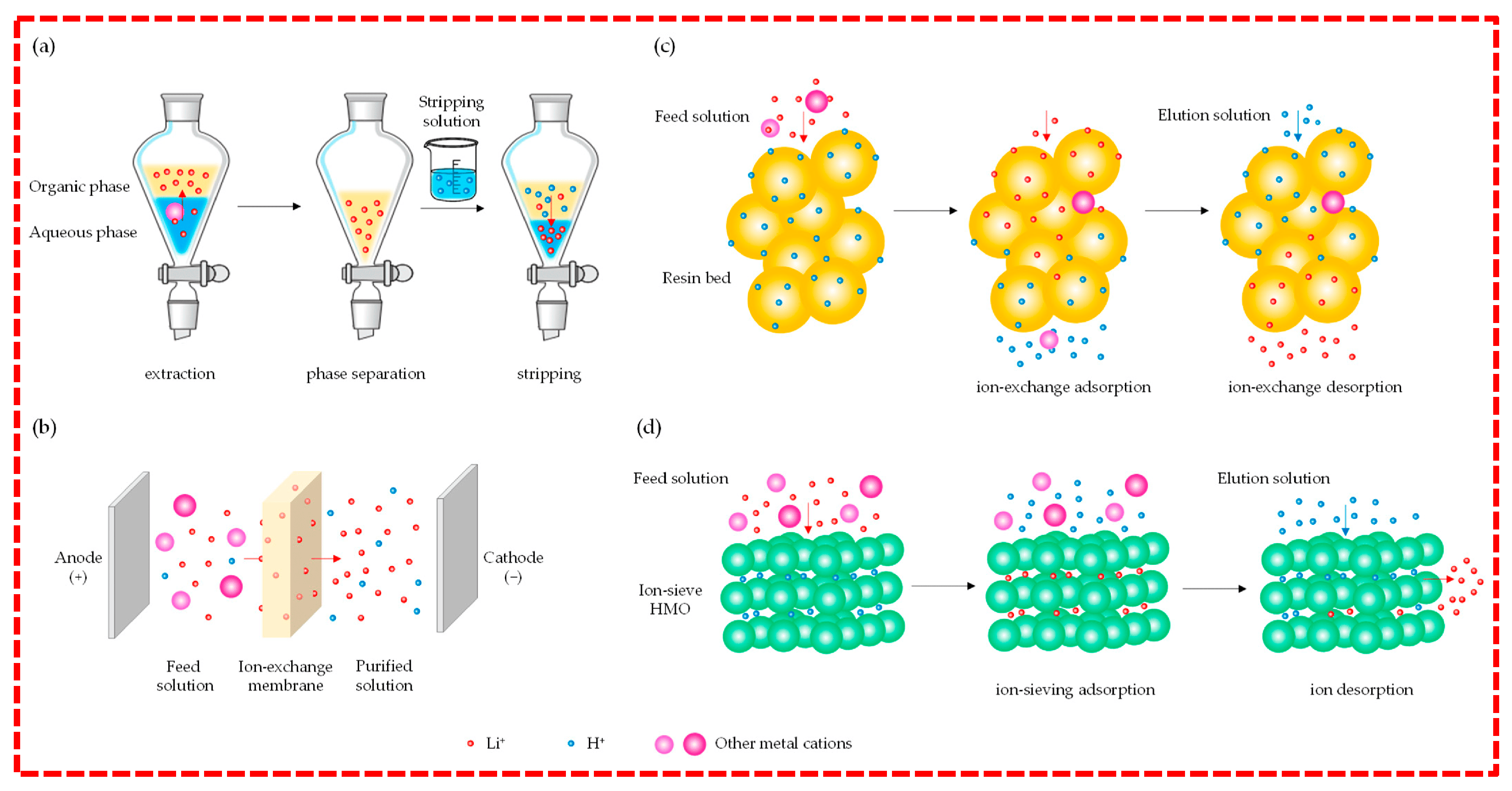
3.2.1. Solvent Extraction
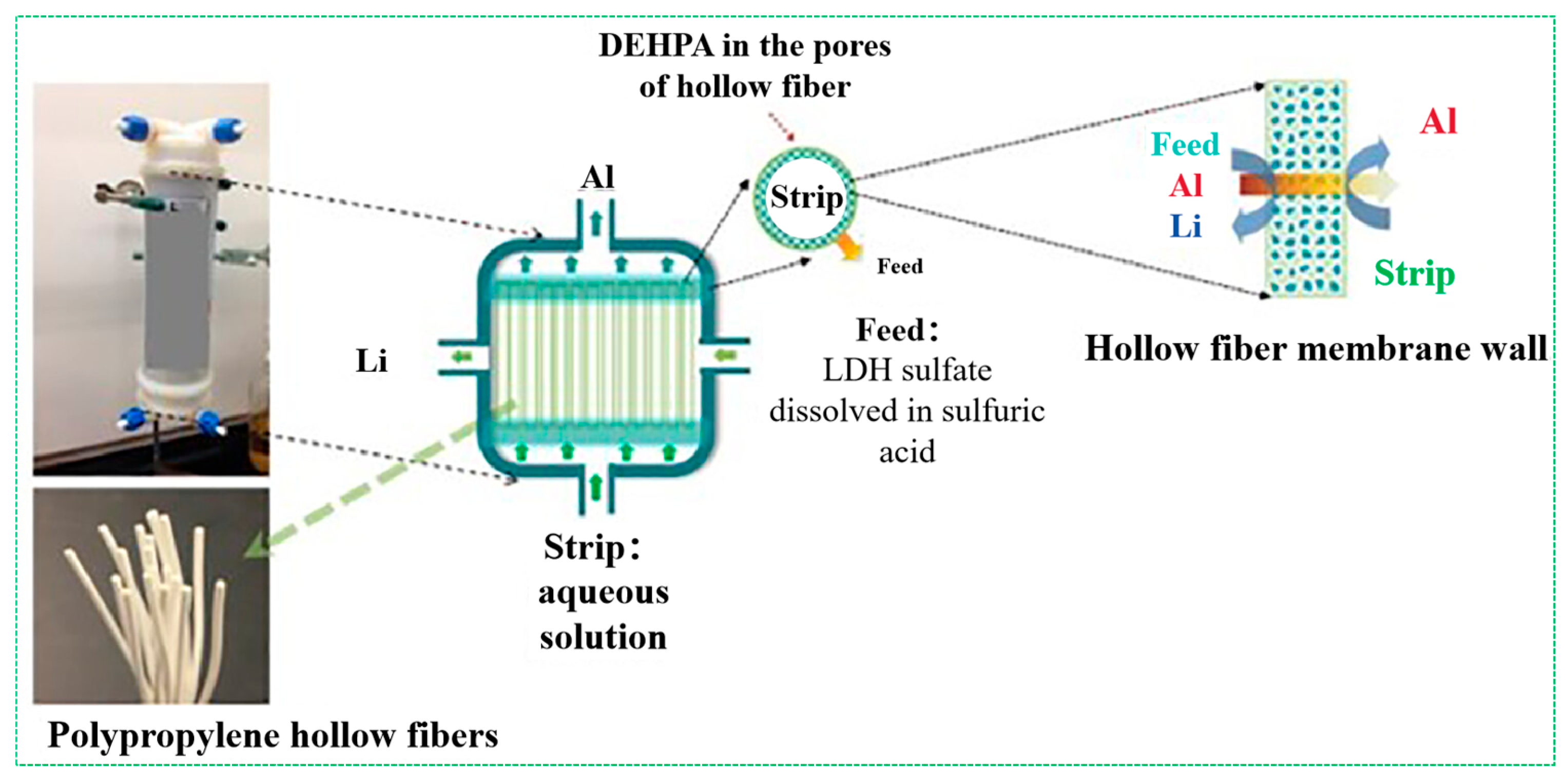
3.2.2. Membrane Separation Technologies
3.2.3. Adsorption-Based Methods
- (a)
- Ion Exchange
- (b)
- Ion-sieving Technology
- (c)
- Electrostatic Adsorption
- (d)
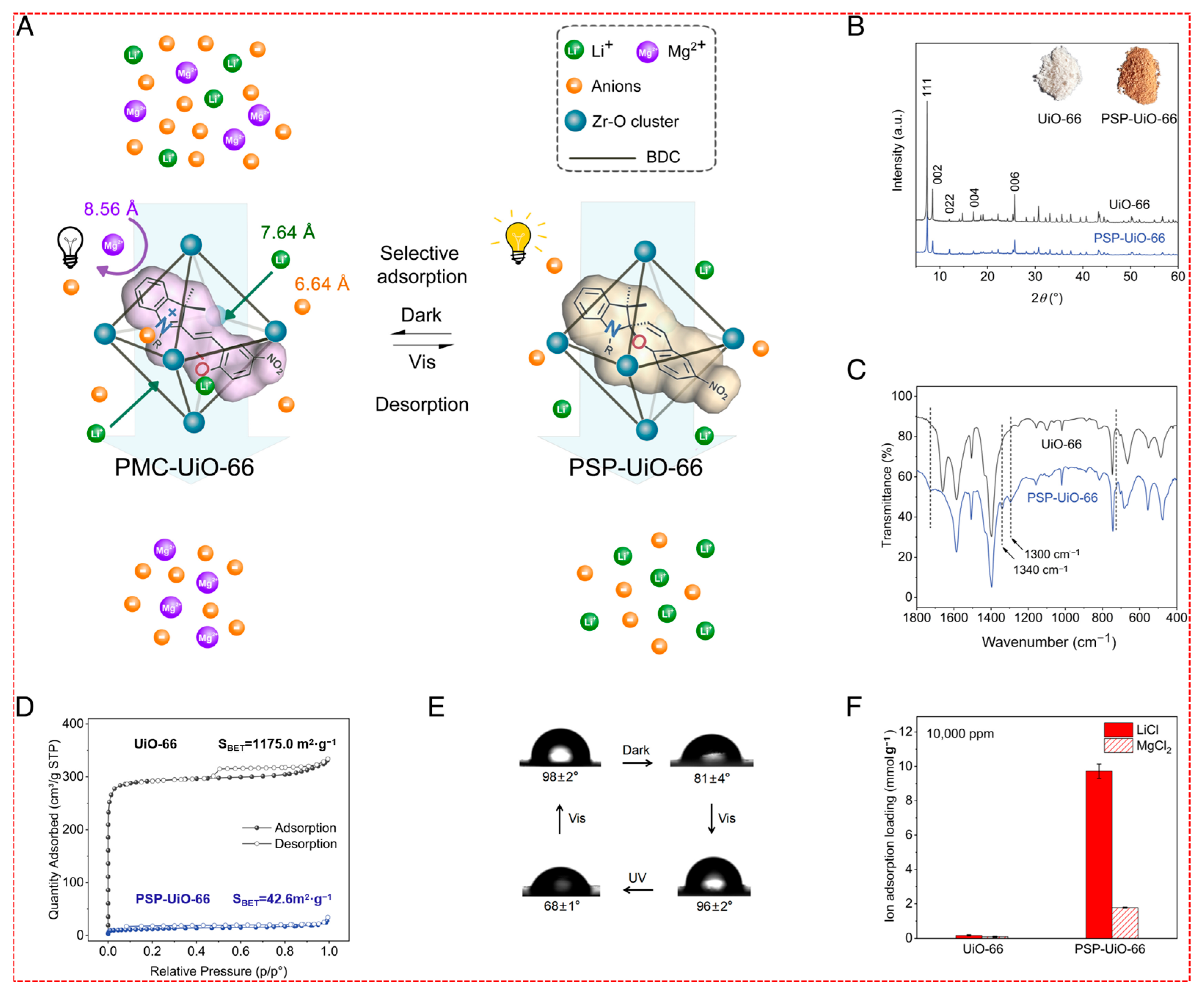
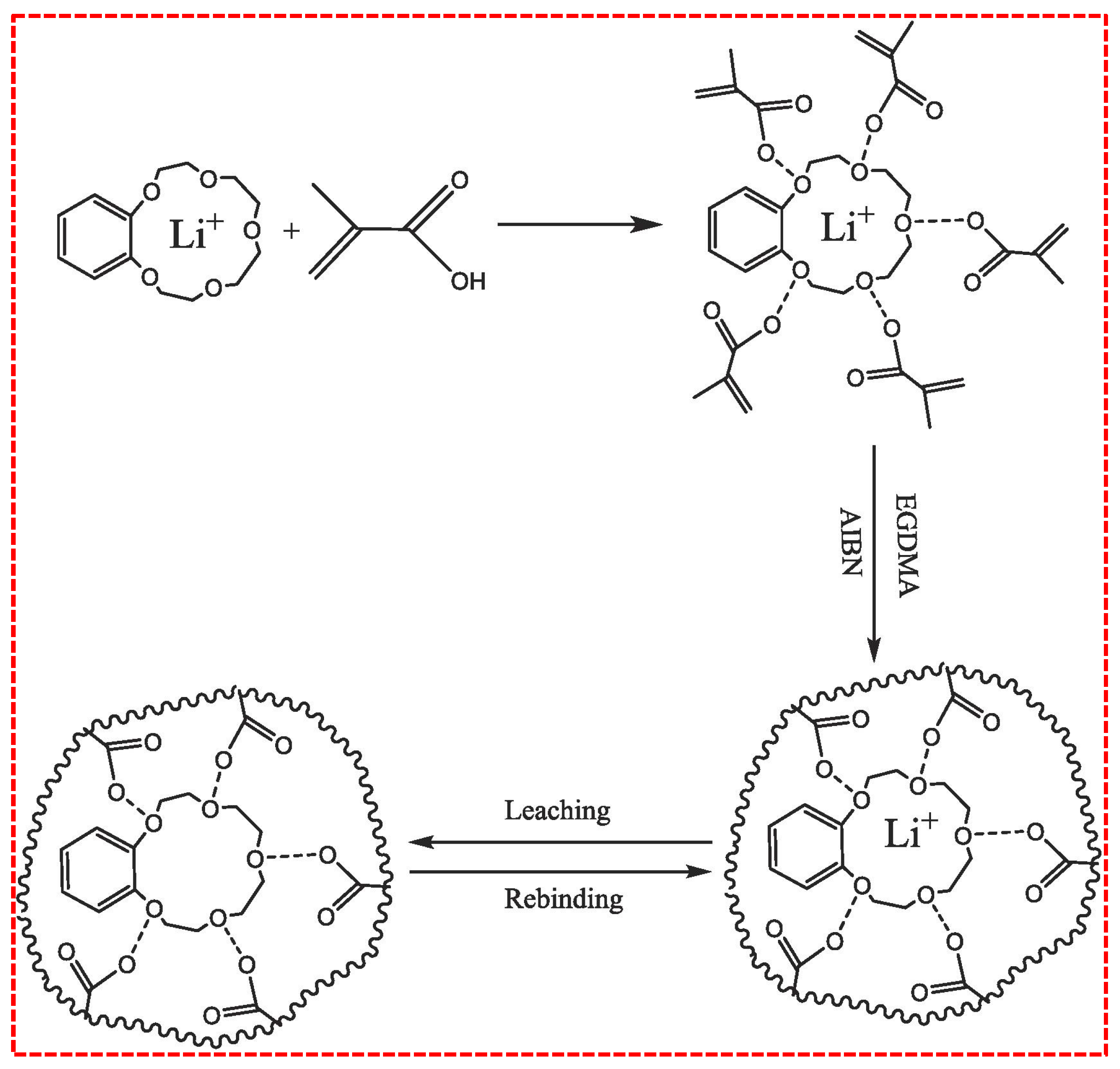
- Ion Imprinting Technology
4. Challenges and Feasibility of Lithium Recovery from Low-Grade Resources
4.1. Technical Challenges
4.1.1. Low Lithium Concentrations
4.1.2. Presence of Impurities
4.1.3. Resource Characteristic Variations
4.1.4. Efficiency of Extraction Technologies
4.2. Economic Feasibility
4.2.1. Cost-Effectiveness of Recovery Technologies
4.2.2. Scaling and Operational Costs
4.2.3. Market Volatility Impact
4.3. Environmental and Sustainability Concerns
4.3.1. Environmental Impact of Recovery Processes
4.3.2. Water Resource Management
4.3.3. Sustainability Considerations
5. Future Outlook and Recommendations
5.1. Research and Development Opportunities
5.1.1. Advancing Extraction and Processing Technologies
5.1.2. Sustainable Practices and Life Cycle Assessment
5.2. Policy Framework for Sustainable Development
5.2.1. Incentives and Regulations
5.2.2. International Cooperation and Knowledge Sharing
5.3. Projected Industry Growth and Market Trends
5.3.1. Demand and Production Outlook
5.3.2. Market Dynamics and Influencing Factors
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prasad, N.E.; Gokhale, A.; Wanhill, R.J.H. Aluminum-Lithium Alloys: Processing, Properties, and Applications; Butterworth-Heinemann: Oxford, UK, 2013; ISBN 978-0-12-401679-8. [Google Scholar]
- Aljarrah, S.; Al-Rawajfeh, A.E.; Shahid, M.K. Qamar Ul Islam Recent Innovations and Patents of Lithium Extraction Techniquesfrom Various Lithium Bearing Solutions. Recent Innov. Chem. Eng. RICE 2023, 16, 241–259. [Google Scholar] [CrossRef]
- Kudryavtsev, P. Lithium in Nature, Application, Methods of Extraction. Sci. Isr. Technol. Advant. 2016, 18, 3–63. [Google Scholar]
- Grant, A. The Sedimentary Lithium Opportunity. Available online: https://www.jadecove.com/research/sedimentarylithium (accessed on 18 December 2024).
- Pavlyshyn, V.I.; Cherniyenko, N.M. LITHIUM in the SUBSOIL of UKRAINE Part 1. Distribution and Forms of Finding Lithium in Mineral Complexes of Ukraine. Mineral. J. 2023, 45, 3–20. [Google Scholar] [CrossRef]
- Ma, Y.; Qin, X.; Pan, T.; Chen, J.; Jiang, Z.; Ding, C.; Zhang, D.; Zhang, F.; Feng, N.; Liu, C.; et al. Sedimentary Environment and Source Analysis of Sedimentary Lithium Deposits in Dezong Mahai Salt Lake, Qaidam Basin. Sustainability 2024, 16, 10561. [Google Scholar] [CrossRef]
- Abdullayev, B.; Askarova, N.; Toshkodirova, R.; Rifky, M.; Ayakulov, N.; Kurbanov, B.; Samadiy, M. Recent Developments in the Extraction of Lithium from Water Resources. AJC 2024, 36, 275–280. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Zhang, X.; Wu, Q.; Liu, X.; Ren, J.; Chen, S. Geothermal-type Lithium Resources in Southern Xizang, China. Acta Geol. Sin. 2021, 95, 860–872. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Graham, J.D.; Rupp, J.A.; Brungard, E. Lithium in the Green Energy Transition: The Quest for Both Sustainability and Security. Sustainability 2021, 13, 11274. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Li, H. Application of Lithium Batteries, Hydrogen Fuel Cells and Solar Energy in Transportation Field. ACE 2023, 26, 245–251. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Zhang, S.E.; Bourdeau, J.E.; Chipangamate, N.S.; Rose, D.H.; Valodia, I.; Nwaila, G.T. The Strategic Role of Lithium in the Green Energy Transition: Towards an OPEC-Style Framework for Green Energy-Mineral Exporting Countries (GEMEC). Resour. Policy 2024, 90, 104737. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bresolin, B.M.; Bontempi, E. Building a Circular Economy for Lithium: Addressing Global Challenges. Glob. Chall. 2024, 8, 2400250. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, Y.; Zhang, Y.; Liu, J.; Hu, Y.; Hu, S.; Wang, Q. Experimental Study of Lithium Extraction in the Solid-Liquid Conversion of Low-Grade Solid Potash Ore. Minerals 2024, 14, 116. [Google Scholar] [CrossRef]
- Rudnik, E. Coal and Coal By-Products as Unconventional Lithium Sources: A Review of Occurrence Modes and Hydrometallurgical Strategies for Metal Recovery. Minerals 2024, 14, 849. [Google Scholar] [CrossRef]
- McShane, E.J.; Gardner, E.J. (Invited) Electroflow: Sustainable Electrochemical Lithium Chemical Production. Electrochem. Soc. Meet. Abstr. 2024, 245, 1483. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, Z.; Tong, X.; Xie, X.; Song, Q.; Fan, P. Review of the Research on the Development and Utilization of Clay-Type Lithium Resources. Particuology 2024, 87, 46–53. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A Review of Lithium Extraction from Natural Resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Reich, R.; Slunitschek, K.; Danisi, R.M.; Eiche, E.; Kolb, J. Lithium Extraction Techniques and the Application Potential of Different Sorbents for Lithium Recovery from Brines. Miner. Process. Extr. Metall. Rev. 2023, 44, 261–280. [Google Scholar] [CrossRef]
- Salaudeen, H.D.; Salaudeen, T.A.; Onaopemipo, Y. Olubunmi Samuel Review of Methods for Lithium Extraction from Geothermal Brines. World J. Adv. Res. Rev. 2024, 24, 948–955. [Google Scholar] [CrossRef]
- Paul, A.; Lizhen, G.; Recheal, T.; Ali, S. Sustainable Lithium and Cobalt Recovery from Spent Lithium-Ion Batteries: Best Practices for the Future. A Review. J. Anal. Tech. Res. 2024, 6, 43–77. [Google Scholar] [CrossRef]
- Jose, S.A.; Stoll, J.L.; Smith, T.; Jackson, C.; Dieleman, T.; Leath, E.; Eastwood, N.; Menezes, P.L. Critical Review of Lithium Recovery Methods: Advancements, Challenges, and Future Directions. Processes 2024, 12, 2203. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, L.; Rao, F.; Tong, L.; Feng, H. Efficient Extraction of Lithium from Calcined Kaolin Lithium Clay with Dilute Sulfuric Acid. Minerals 2024, 14, 359. [Google Scholar] [CrossRef]
- Liu, J.; Xu, R.; Sun, W.; Wang, L.; Zhang, Y. Lithium Extraction from Lithium-Bearing Clay Minerals by Calcination-Leaching Method. Minerals 2024, 14, 248. [Google Scholar] [CrossRef]
- Potts, E. The Clayton Valley Lithium Project: Empowering America’s Lithium Independence. Innovation News Network, 19 January 2024. [Google Scholar]
- Lithium Extraction from Clays Is a Reality: Perez Garibay. Available online: https://mexicobusiness.news/mining/news/lithium-extraction-clays-reality-perez-garibay (accessed on 13 November 2024).
- Energy, U.-B.L.U.D. Chemists Invent a More Efficient Way to Extract Lithium from Mining Sites, Oil Fields, Used Batteries. Available online: https://www.ornl.gov/news/chemists-invent-more-efficient-way-extract-lithium-mining-sites-oil-fields-used-batteries (accessed on 13 November 2024).
- Disu, B.; Rafati, R.; Haddad, A.S.; Fierus, N. Lithium Extraction From North Sea Oilfield Brines Using Ion Exchange Membranes. In Proceedings of the SPE Offshore Europe Conference & Exhibition, Aberdeen, Scotland, UK, 5–6 September 2023; p. D021S004R001. [Google Scholar]
- Shi, Z.; Tan, H.; Xue, F.; Li, Y.; Zhang, X.; Cong, P.; Santosh, M.; Zhang, Y. Hydrochemical Evolution and Source Mechanisms Governing the Unusual Lithium and Boron Enrichment in Salt Lakes of Northern Tibet. Geological Society of America Bulletin 2024, 136, 5174–5190. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Zabłocka-Malicka, M.; Chojnacka, I.; Worsa-Kozak, M. Assessing the Viability of Integrating Evaporation and Solvent Extraction Systems for Lithium Recovery from Low-Grade Brines. Processes 2024, 12, 1453. [Google Scholar] [CrossRef]
- Torres, D.; Pérez, K.; Galleguillos Madrid, F.M.; Leiva, W.H.; Gálvez, E.; Salinas-Rodríguez, E.; Gallegos, S.; Jamett, I.; Castillo, J.; Saldana, M.; et al. Salar de Atacama Lithium and Potassium Productive Process. Metals 2024, 14, 1095. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, S.; Liu, X.; Li, K.; Ma, X.; Zhang, Z.; Li, S.; Xie, J.; Du, Y.; Fu, Z. Separation Study of Magnesium–Lithium from Low-Mg/Li Brine. Energy Technol. 2024, Early View, 2400398. [Google Scholar] [CrossRef]
- Bäuerle, M.; Herrmann, L.; Jeschull, F. Battery Electrode-Based Lithium Recovery from Geothermal Brines. Electrochem. Soc. Meet. Abstr. 2023, 244, 1357. [Google Scholar] [CrossRef]
- Smith, C.L.; Meier, A.L.; Downey, H.D. Lithium in the Brines of Fish Lake Valley and Columbus Salt Marsh, Nevada; U.S. Geological Survey: Reston, VA, USA, 1977. [Google Scholar]
- Murphy, O.; Haji, M.N. A Review of Technologies for Direct Lithium Extraction from Low Li+ Concentration Aqueous Solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.; Srinivasakannan, C.; Li, S.; Yin, S.; Zhang, L.; Jiang, X.; Zhou, G.; Zhang, N. Lithium Extraction from Salt Lake Brines with High Magnesium/Lithium Ratio: A Review. Environ. Chem. Lett. 2023, 21, 1611–1626. [Google Scholar] [CrossRef]
- Ding, T.; Zheng, M.; Nie, Z.; Ma, L.; Ye, C.; Wu, Q.; Zhao, Y.; Yang, D.; Wang, K. Impact of Regional Climate Change on the Development of Lithium Resources in Zabuye Salt Lake, Tibet. Front. Earth Sci. 2022, 10, 865158. [Google Scholar] [CrossRef]
- Danso, D.K.; Bicerano, J.; Heller, D.P.; Barati, R.G. Development of Polyelectrolyte Complex Nanoparticles for Direct Lithium Extraction from Oilfield Brines. In Proceedings of the SPE Energy Transition Symposium, Houston, TX, USA, 5 August 2024; p. D021S009R001. [Google Scholar]
- Bao, L.; Xu, Z.; Guo, W.; Lin, S.; Sun, S. Enhancement of Lithium Extraction from Low Grade Brines by High Hydrophilic Blend Membrane Using MnO2 Ion Sieve as Adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131884. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Su, H.; Yu, J.; Lin, S. Enhancing Lithium Extraction from Low Grade Salt Lake Brines Using High Powder Loading Aluminum-Based Adsorbent Granules. Desalination 2024, 583, 117696. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, J.; Xu, F.; Zheng, L.; De Wolf, S.; Lai, Z.; Lu, X. Solar-Driven Ultrafast Lithium Extraction from Low-Grade Brine Using Microfluidics-Mediated Vortex in Scalable Electrochemical Reactors. Chem. Eng. J. 2023, 454, 140074. [Google Scholar] [CrossRef]
- Wei, Y.; He, W.; Qin, G.; Fan, M.; Cao, D. Lithium Enrichment in the No. 21 Coal of the Hebi No. 6 Mine, Anhe Coalfield, Henan Province, China. Minerals 2020, 10, 521. [Google Scholar] [CrossRef]
- Han, P.; Zhao, F.; Liu, D.; Zhang, Q.; Zhang, Q.; Ullah, S. Occurrence and Favorable Enrichment Environment of Lithium in Gaoping Coal Measures: Evidence from Mineralogy and Geochemistry. Appl. Sci. 2024, 14, 7298. [Google Scholar] [CrossRef]
- Qin, S.; Zhao, C.; Li, Y.; Zhang, Y. Review of Coal as a Promising Source of Lithium. Int. J. Oil Gas Coal Technol. IJOGCT 2015, 9, 215. [Google Scholar] [CrossRef]
- Ma, Z.; Shan, X.; Cheng, F. Distribution Characteristics of Valuable Elements, Al, Li, and Ga, and Rare Earth Elements in Feed Coal, Fly Ash, and Bottom Ash from a 300 MW Circulating Fluidized Bed Boiler. ACS Omega 2019, 4, 6854–6863. [Google Scholar] [CrossRef]
- Li, Y.; Man, J.; Cheng, L.; Panchal, B. Lithium Activation Pretreatment Mechanism and Leaching Process from Coal Fly Ash. Energy Explor. Exploit. 2024, 42, 476–491. [Google Scholar] [CrossRef]
- Robinson, A.; Flynn, S.; Acikalin, S.; Stewart, G. Non-Arid Brine Sources of Li: Using Mine Waters of the North Pennines, England to Test near-Surface Measurements as a Tool for Assessing the Li Resource of Hydrothermal Brines in Granitic Systems. In Proceedings of the Goldschmidt2023 Abstracts, Lyon, France, 9–14 July 2023. [Google Scholar]
- Rinder, T.; Dietzel, M.; Stammeier, J.A.; Leis, A.; Bedoya-González, D.; Hilberg, S. Geochemistry of Coal Mine Drainage, Groundwater, and Brines from the Ibbenbüren Mine, Germany: A Coupled Elemental-Isotopic Approach. Appl. Geochem. 2020, 121, 104693. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Y.; Xu, Z.; Yuan, H.; Yi, H. Long-Term Groundwater Geochemical Evolution Induced by Coal Mining Activities—A Case Study of Floor Confined Limestone Aquifer in Yaoqiao Coal Mine, Jiangsu, China. Environ. Sci. Pollut. Res. 2023, 30, 96252–96271. [Google Scholar] [CrossRef] [PubMed]
- Mousavinezhad, S.; Nili, S.; Fahimi, A.; Vahidi, E. Environmental Impact Assessment of Direct Lithium Extraction from Brine Resources: Global Warming Potential, Land Use, Water Consumption, and Charting Sustainable Scenarios. Resour. Conserv. Recycl. 2024, 205, 107583. [Google Scholar] [CrossRef]
- Mahran, G.M.A.; Gado, M.A.; Fathy, W.M.; ElDeeb, A.B. Eco-Friendly Recycling of Lithium Batteries for Extraction of High-Purity Metals. Materials 2023, 16, 4662. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.; Srinivasan, M. Green Recycling Methods to Treat Lithium-Ion Batteries E-Waste: A Circular Approach to Sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef]
- Javed, A.; Singh, J. Process Intensification for Sustainable Extraction of Metals from E-Waste: Challenges and Opportunities. Environ. Sci. Pollut. Res. 2023, 31, 9886–9919. [Google Scholar] [CrossRef]
- Jiang, M.; He, S.; Zhang, Y. Bioleaching Extraction of Valuable Metal From E-Wastes: A MiniReview. Recent Innov. Chem. Eng. RICE 2023, 16, 306–323. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Jena, S.; Tripathy, S.; Mandre, N.; Venugopal, R.; Farrokhpay, S. Sustainable Use of Copper Resources: Beneficiation of Low-Grade Copper Ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Pakostova, E.; Graves, J.; Latvyte, E.; Maddalena, G.; Horsfall, L. A Novel Closed-Loop Biotechnology for Recovery of Cobalt from a Lithium-Ion Battery Active Cathode Material. Microbiology 2024, 170, 001475. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, L.; Shen, L.; Ran, J.-J.; Zhong, H.; Zhu, Y.-R.; Chen, H. Recent Advancements in Hydrometallurgical Recycling Technologies of Spent Lithium-Ion Battery Cathode Materials. Rare Met. 2024, 43, 879–899. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Qian, X.; Xu, Z.; Xia, H.; Tao, X.; Xu, Z.; Ni, Q.-Q. Materials for Lithium Recovery from Salt Lake Brine. J. Mater. Sci. 2021, 56, 16–63. [Google Scholar] [CrossRef]
- Sun, J.; Liang, D.; Meng, X.; Li, Z. Recent Advances in Lithium Extraction Using Electrode Materials of Li-Ion Battery from Brine/Seawater. Processes 2022, 10, 2654. [Google Scholar] [CrossRef]
- He, J.; Cao, Y.; Wang, X.; Zhao, C.; Huang, J.; Long, W.; Zhou, Z.; Dong, P.; Zhang, Y.; Wang, D.; et al. Short-Process Regeneration of Highly Stable Spherical LiCoO2 Cathode Materials from Spent Lithium-Ion Batteries through Carbonate Precipitation. Chem. A Eur. J. 2024, 30, e202303424. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhao, L.; Wang, W.; Liu, Y.; Ma, Q.; Mu, D.; Li, R.; Dai, C. Optimized Li and Fe Recovery from Spent Lithium-Ion Batteries via a Solution-Precipitation Method. RSC Adv. 2016, 6, 43613–43625. [Google Scholar] [CrossRef]
- Aboelazm, E.A.A.; Mohamed, N.; Ali, G.A.M.; Makhlouf, A.S.H.; Chong, K.F. Recycling of Cobalt Oxides Electrodes from Spent Lithium-Ion Batteries by Electrochemical Method. In Waste Recycling Technologies for Nanomaterials Manufacturing; Makhlouf, A.S.H., Ali, G.A.M., Eds.; Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Switzerland, 2021; pp. 91–123. ISBN 978-3-030-68030-5. [Google Scholar]
- Su, F.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Li, Z.; Wang, H.; Ma, Y. Efficient Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Pyrite Method with Hydrometallurgy Process. Chem. Eng. J. 2023, 455, 140914. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Zeng, L.; Ma, B.; Wang, L.; Chen, Y.; Yang, C. Lithium Extraction and Hydroxysodalite Zeolite Synthesis by Hydrothermal Conversion of α-Spodumene. ACS Sustain. Chem. Eng. 2019, 7, 9498–9505. [Google Scholar] [CrossRef]
- Wu, S.; Tao, W.; Zheng, Y.; Ge, H.; He, J.; Yang, Y.; Wang, Z. A Novel Approach for Lithium Recovery from Waste Lithium-Containing Aluminum Electrolyte by a Roasting-Leaching Process. Waste Manag. 2021, 134, 89–99. [Google Scholar] [CrossRef]
- Zhang, T.; Dao, J.; Wang, J.; Guo, Y.; Wan, R.; Li, C.; Zhou, X.; Zhang, Z. Highly Efficient Recovery of Waste LiNixCoyMnzO2 Cathode Materials Using a Process Involving Pyrometallurgy and Hydrometallurgy. Front. Environ. Sci. Eng. 2024, 18, 25. [Google Scholar] [CrossRef]
- Liu, A.; Hu, G.; Wu, Y.; Guo, F. Life Cycle Environmental Impacts of Pyrometallurgical and Hydrometallurgical Recovery Processes for Spent Lithium-Ion Batteries: Present and Future Perspectives. Clean. Technol. Environ. Policy 2023, 26, 381–400. [Google Scholar] [CrossRef]
- Ashoka Sahadevan, S.; Usen Nazreen, M.S.; Sankarasubramanian, S.; Ramani, V.K. Lithium-Ion Battery Recycling: Ternary Deep Eutectic Solvents Enable Efficient and Selective Electrochemical Recovery of Critical Component Metals. Electrochem. Soc. Meet. Abstr. 2024, 245, 2955. [Google Scholar] [CrossRef]
- Dong, Y.; Ji, H.; Wu, X.; Zheng, N.; Wang, J.; Ji, G.; Chen, Y.; Zhou, G.; Liang, Z. Trends of Sustainable Recycling Technology for Lithium-ion Batteries: Metal Recovery from Conventional Metallurgical Processes to Innovative Direct Recycling. MetalMat 2024, 1, e5. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical Recycling Technologies for NMC Li-Ion Battery Cathodes: Current Industrial Practice and New R&D Trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar] [CrossRef]
- Alipanah, M.; Jin, H.; Zhou, Q.; Barboza, C.; Gazzo, D.; Thompson, V.; Fujita, Y.; Liu, J.; Anderko, A.; Reed, D. Sustainable Bioleaching of Lithium-Ion Batteries for Critical Metal Recovery: Process Optimization through Design of Experiments and Thermodynamic Modeling. Resour. Conserv. Recycl. 2023, 199, 107293. [Google Scholar] [CrossRef]
- Xie, Y.; Ni, C.; Han, Z.; Liao, J.; Xie, W.; Zhong, H.; He, Z. Recovery of Lithium from Coal Gangue Utilizing Acid Baking Followed by Water Leaching. Int. J. Coal Prep. Util. 2024, 44, 82–94. [Google Scholar] [CrossRef]
- Chen, H.; Wen, Z.; Pan, J.; Zhang, L.; He, X.; Valeev, D.; Zhou, C. Study on Leaching Behavior Differences of Rare Earth Elements from Coal Fly Ash during Microwave-Assisted HCl Leaching. Int. J. Coal Prep. Util. 2023, 43, 1993–2015. [Google Scholar] [CrossRef]
- Necke, T.; Stein, J.; Kleebe, H.-J.; Balke-Grünewald, B. Lithium Extraction and Zeolite Synthesis via Mechanochemical Treatment of the Silicate Minerals Lepidolite, Spodumene, and Petalite. Minerals 2023, 13, 1030. [Google Scholar] [CrossRef]
- Braga, P.; França, S.; Neumann, R.; Rodriguez, M.; Rosales, G. Alkaline Process for Extracting Lithium from Spodumene. ChemistrySelect 2024, 9, e202304480. [Google Scholar]
- Biswal, B.K.; Balasubramanian, R. Recovery of Valuable Metals from Spent Lithium-Ion Batteries Using Microbial Agents for Bioleaching: A Review. Front. Microbiol. 2023, 14, 1197081. [Google Scholar] [CrossRef]
- Lalropuia, L.; Kucera, J.; Rassy, W.Y.; Pakostova, E.; Schild, D.; Mandl, M.; Kremser, K.; Guebitz, G.M. Metal Recovery from Spent Lithium-Ion Batteries via Two-Step Bioleaching Using Adapted Chemolithotrophs from an Acidic Mine Pit Lake. Front. Microbiol. 2024, 15, 1347072. [Google Scholar] [CrossRef]
- Kazemian, Z.; Larypoor, M.; Marandi, R. Evaluation of Myco-Leaching Potential of Valuable Metals from Spent Lithium Battery by Penicillium Chrysogenum and Aspergillus Niger. Int. J. Environ. Anal. Chem. 2023, 103, 514–527. [Google Scholar] [CrossRef]
- Rezza, I.; Salinas, E.; Calvente, V.; Benuzzi, D.; Tosetti, M.I.S.D. Extraction of Lithium from Spodumene by Bioleaching. Lett. Appl. Microbiol. 1997, 25, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Dembele, S.; Mishra, S.; Akcil, A.; Agcasulu, İ.; Hazrati, E.; Tuncuk, A.; Malavasi, P.; Gaydardzhiev, S. Small-scale and Scale-up Bioleaching of Li, Co, Ni and Mn from Spent Lithium-ion Batteries. J. Chem. Technol. Biotechnol. 2024, 99, 1069–1082. [Google Scholar] [CrossRef]
- Marcinčáková, R.; Kaduková, J.; Mražíková, A. Bioleaching of Lithium from Lepidolite by the Mixture of Rhodotorula Rubra and Acidithiobacillus Ferrooxidans. J. Pol. Miner. Eng. Soc. 2015, 16, 85–88. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, F.; Zheng, P.; Wang, Y.; Zhao, L.; Xu, C. Selective Recovery of Lithium from High-Aluminum Fly Ash by Using Alkali-Dissolution-Assisted HBTA–TOPO Synergistic Extraction. J. Clean. Prod. 2024, 434, 139998. [Google Scholar] [CrossRef]
- Cui, L.; Feng, L.; Yuan, H.; Cheng, H.; Cheng, F. Efficient Recovery of Aluminum, Lithium, Iron and Gallium from Coal Fly Ash Leachate via Coextraction and Stepwise Stripping. Resour. Conserv. Recycl. 2024, 202, 107380. [Google Scholar] [CrossRef]
- Samadiy, M.; Deng, T. Lithium Recovery from Water Resources by Ion Exchange and Sorption Method. J. Chem. Soc. Pak. 2021, 43, 406. [Google Scholar] [CrossRef]
- Wagh, P.; Islam, S.Z.; Lamichhane, T.N.; Bhave, R.R.; Paranthaman, M.P. Separation of Lithium from Aluminum-Containing Clay Mineral Leachate Solution Using Energy-Efficient Membrane Solvent Extraction. ACS Omega 2023, 8, 46523–46527. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Wang, J.-Z.; Shen, Y.-H. Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals 2023, 13, 285. [Google Scholar] [CrossRef]
- Wesselborg, T.; Virolainen, S.; Sainio, T. Recovery of Lithium from Leach Solutions of Battery Waste Using Direct Solvent Extraction with TBP and FeCl3. Hydrometallurgy 2021, 202, 105593. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Separation of Critical Metals by Membrane Technology under a Circular Economy Framework: A Review of the State-of-the-Art. Processes 2023, 11, 1256. [Google Scholar] [CrossRef]
- Han, J.; Zeng, X.; Wang, K.; Huang, Y.; Meng, Q.; Zhou, L.; Li, Z.; Liu, R.; Zhen, C. Research progress and prospect of membrane method in seawater/brine extraction of lithium. Acta Mater. Compos. Sin. 2022, 39, 2106–2120. [Google Scholar] [CrossRef]
- Wang, R.; Alghanayem, R.; Lin, S. Multipass Nanofiltration for Lithium Separation with High Selectivity and Recovery. Environ. Sci. Technol. 2023, 57, 14464–14471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, M.; Cao, Y.; Yu, K.; Fan, Z.; Cao, Y. Designing Low Toxic Deep Eutectic Solvents for the Green Recycle of Lithium-Ion Batteries Cathodes. ChemSusChem 2024, 17, e202301953. [Google Scholar] [CrossRef] [PubMed]
- Seroka, N.S.; Luo, H.; Khotseng, L. Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review. Batteries 2024, 10, 144. [Google Scholar] [CrossRef]
- Nishihama, S.; Onishi, K.; Yoshizuka, K. Selective Recovery Process of Lithium from Seawater Using Integrated Ion Exchange Methods. Solvent Extr. Ion Exch. 2011, 29, 421–431. [Google Scholar] [CrossRef]
- Gao, J.; Du, Z.; Zhao, Q.; Guo, Y.; Cheng, F. Enhanced Li+ Adsorption by Magnetically Recyclable Iron-Doped Lithium Manganese Oxide Ion-Sieve: Synthesis, Characterization, Adsorption Kinetics and Isotherm. J. Mater. Res. Technol. 2021, 13, 228–240. [Google Scholar] [CrossRef]
- Wang, S.; Chao, X.; Huang, X. A Review of Titanium-Based Lithium-Ion Sieve. In Proceedings of the 2023 8th International Conference on Power and Renewable Energy (ICPRE), Shanghai, China, 22–25 September 2023; pp. 1980–1985. [Google Scholar]
- Roobavannan, S.; Choo, Y.; Truong, D.Q.; Han, D.S.; Shon, H.K.; Naidu, G. Seawater Lithium Mining by Zeolitic Imidazolate Framework Encapsulated Manganese Oxide Ion Sieve Nanomaterial. Chem. Eng. J. 2023, 474, 145957. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Gao, F.; Dong, J.; Zhang, P.; Chen, G. Enhanced Lithium Adsorption on an Anion- and Cation-Codoped Lithium Manganese Oxide Ion Sieve with a Thin Fluoride-Protective Coating Layer. Ind. Eng. Chem. Res. 2024, 63, 9527–9537. [Google Scholar] [CrossRef]
- Shang, X.; Liu, J.; Hu, B.; Nie, P.; Yang, J.; Zhang, B.; Wang, Y.; Zhan, F.; Qiu, J. CNT-Strung LiMn2 O4 for Lithium Extraction with High Selectivity and Stability. Small Methods 2022, 6, 2200508. [Google Scholar] [CrossRef]
- Yang, S.S.; Liu, X.; Shen, J.N.; Gao, C.J. Comparison Study of PVDF-HMn2O4 and PES-HMn2O4 Membrane-Type Adsorbents for Lithium Adsorption/Desorption. AMM 2014, 633–634, 517–520. [Google Scholar] [CrossRef]
- Luo, G.; Wu, Y.; Zeng, X.; Zhou, W.; Wang, P.; Zhang, W. Lithium-Ion-Sieve-Embedded Hybrid Membranes for Anion-Exchange- and Cation-Concentration-Driven Li/Mg Separation. ACS Appl. Mater. Interfaces 2024, 16, 66911–66920. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Shin, G.R.; Kim, S.-C. Electrostatic Adsorption of Uniformly Charged Sphere Ions Confined between Two Charged Plates. J. Korean Phys. Soc. 2019, 74, 30–35. [Google Scholar] [CrossRef]
- Qian, C.; Zheng, M.; Zhang, Y.; Xing, E.; Gui, B. Adsorption Performance and Mechanism of Li+ from Brines Using Lithium/Aluminum Layered Double Hydroxides-SiO2 Bauxite Composite Adsorbents. Front. Chem. 2023, 11, 1265290. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Park, S.-J.; Kang, H.W.; Park, S.B.; Han, J.-I. Recovery of Lithium(I), Strontium(II), and Lanthanum(III) Using Ca–Alginate Beads. J. Chem. Eng. Data 2013, 58, 2455–2464. [Google Scholar] [CrossRef]
- Zhi, K.; Duan, J.; Zhang, J.; Huang, L.; Guo, L.; Wang, L. Progress and Prospect of Ion Imprinting Technology in Targeted Extraction of Lithium. Polymers 2024, 16, 833. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, R. Highly Effective and Low-Cost Ion-Imprinted Polymers Loaded on Pretreated Vermiculite for Lithium Recovery. Ind. Eng. Chem. Res. 2019, 58, 12216–12225. [Google Scholar] [CrossRef]
- Qi, D.; Jin, D.; Tu, Y.; Zhou, Z.; Du, C.; Ren, Z. Synthesis of Lithium Ion-Imprinted Polymers for Selective Recovery of Lithium Ions from Salt Lake Brines. Sep. Purif. Technol. 2024, 340, 126661. [Google Scholar] [CrossRef]
- Li, Y.; Han, N.; He, Q.; Peng, H.; Wu, X.; Meng, Z.; Miao, Z. Nitrogen-Doped Substrate Material Ion Imprinting–Capacitive Deionization Selective Recovery of Lithium Ions from Acidic Solutions. Environ. Sci. Pollut. Res. 2024, 31, 27949–27960. [Google Scholar] [CrossRef]
- Prachi Patel Device Extracts Lithium from Dead Sea Brine. C&EN Glob. Enterp. 2024, 102, 5. [CrossRef]
- Alsabbagh, A.; Aljarrah, S.; Almahasneh, M. Lithium Enrichment Optimization from Dead Sea End Brine by Chemical Precipitation Technique. Miner. Eng. 2021, 170, 107038. [Google Scholar] [CrossRef]
- Aljarrah, S.; Alsabbagh, A.; Almahasneh, M. Selective Recovery of Lithium from Dead Sea End Brines Using UBK10 Ion Exchange Resin. Can. J. Chem. Eng. 2023, 101, 1185–1194. [Google Scholar] [CrossRef]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.-F. Impact of the Impurities on Lithium Extraction from β-Spodumene in the Sulfuric Acid Process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Ji, Z.-Y.; Chen, H.-Y.; Liu, J.; Zhao, Y.-Y.; Li, F.; Yuan, J.-S. Effect of Impurity Ions in the Electrosorption Lithium Extraction Process: Generation and Restriction of “Selective Concentration Polarization”. ACS Sustain. Chem. Eng. 2020, 8, 11834–11844. [Google Scholar] [CrossRef]
- Li, Z.; Mercken, J.; Li, X.; Riaño, S.; Binnemans, K. Efficient and Sustainable Removal of Magnesium from Brines for Lithium/Magnesium Separation Using Binary Extractants. ACS Sustain. Chem. Eng. 2019, 7, 19225–19234. [Google Scholar] [CrossRef]
- Herrmann, L.; Ehrenberg, H.; Graczyk-Zajac, M.; Kaymakci, E.; Kölbel, T.; Kölbel, L.; Tübke, J. Lithium Recovery from Geothermal Brine—An Investigation into the Desorption of Lithium Ions Using Manganese Oxide Adsorbents. Energy Adv. 2022, 1, 877–885. [Google Scholar] [CrossRef]
- Dobson, P.; Araya, N.; Brounce, M.; Busse, M.; Camarillo, M.; English, L.; Humphreys, J.; Kalderon-Asael, B.; McKibben, M.; Millstein, D.; et al. Characterizing the Geothermal Lithium Resource at the Salton Sea; Lawrence Berkeley National Laboratory (LBNL): Berkeley, CA, USA, 2023; LBNL-2001557. [Google Scholar]
- Types of Lithium Brine Deposits|INN. Available online: https://investingnews.com/daily/resource-investing/battery-metals-investing/lithium-investing/lithium-deposit-types-brine-pegmatite-and-sedimentary/ (accessed on 18 November 2024).
- Li, Q.; Fan, Q.; Wang, J.; Qin, Z.; Zhang, X.; Wei, H.; Du, Y.; Shan, F. Hydrochemistry, Distribution and Formation of Lithium-Rich Brines in Salt Lakes on the Qinghai-Tibetan Plateau. Minerals 2019, 9, 528. [Google Scholar] [CrossRef]
- Valdez, S.K.; Flores, H.R.; Orce, A.M.; KwokLeung, H. Influence of The Evaporation Rate Over Lithium Recovery From Brines. World J. Res. Rev. 2016, 3, 66–70. [Google Scholar]
- Xu, R.; Xiao, X.; Zhang, G.; Ye, Y.; Zhang, P.; Yang, Y.; Shuchi, S.B.; Cui, Y. Continuous Lithium Extraction from Brine by Efficient Redox-Couple Electrodialysis. Matter 2024, 7, 3876–3890. [Google Scholar] [CrossRef]
- Forte, F.; Moscardini, E.; Coletta, J.; Toro, L.; Yurramendi, L.; Hidalgo, J.; Ipiñazar, E.; Siriwardana, A.; Riano, S.; Peeters, N.; et al. First Economically and Environmentally Viable Mobile Commercial Metallurgical Plant Based on Advanced Hydrometallurgical and Electrochemical Technologies Able to Produce Cobalt Metal from Spent Lithium-Ion Batteries. Open Res. Eur. 2024, 4, 32. [Google Scholar] [CrossRef]
- Li, Z.; Chen, I.-C.; Cao, L.; Liu, X.; Huang, K.-W.; Lai, Z. Lithium Extraction from Brine through a Decoupled and Membrane-Free Electrochemical Cell Design. Science 2024, 385, 1438–1444. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Exploring the Present and Future of Biomass Recovery Units: Technological Innovation, Policy Incentives and Economic Challenges. Biofuels 2024, 15, 375–387. [Google Scholar] [CrossRef]
- Alipanah, M.; Saha, A.K.; Vahidi, E.; Jin, H. Value Recovery from Spent Lithium-Ion Batteries: A Review on Technologies, Environmental Impacts, Economics, and Supply Chain. CTR Clean Technol. Recycl. 2021, 1, 152–184. [Google Scholar] [CrossRef]
- Varshney, K.P.; Malhotra, S. Recovery of Lithium from Waste Lithium-Ion Batteries-A Review. Res. J. Chem. Environ. 2023, 27, 187–196. [Google Scholar] [CrossRef]
- Yanagida, A.; Webb, E.; Harris, C.E.; Christenson, M.; Comfort, S. Using Electrochemical Oxidation to Remove PFAS in Simulated Investigation-Derived Waste (IDW): Laboratory and Pilot-Scale Experiments. Water 2022, 14, 2708. [Google Scholar] [CrossRef]
- Trigueros, E.; Ramos, C.; Alonso-Riaño, P.; Beltrán, S.; Sanz, M.T. Subcritical Water Treatment for Valorization of the Red Algae Residue after Agar Extraction: Scale-Up from Laboratory to Pilot Plant. Ind. Eng. Chem. Res. 2023, 62, 3503–3514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Challenges and Opportunities of Recycling Cathode Materials from Spent Li-Ion Battery. Int. J. Eng. Technol. IJET 2024, 16, 204–210. [Google Scholar] [CrossRef]
- Flores Fernández, C.; Alba, R. Water or Mineral Resource? Legal Interpretations and Hydrosocial Configurations of Lithium Mining in Chile. Front. Water 2023, 5, 1075139. [Google Scholar] [CrossRef]
- Yan, Z. Research on Lithium Battery Recycling and Utilization. IJMSTS Int. J. Mater. Sci. Technol. Stud. 2024, 1, 1–8. [Google Scholar] [CrossRef]
- Xu, P.; Tan, D.H.S.; Jiao, B.; Gao, H.; Yu, X.; Chen, Z. A Materials Perspective on Direct Recycling of Lithium-Ion Batteries: Principles, Challenges and Opportunities. Adv. Funct. Mater. 2023, 33, 2213168. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Ma, J.; Cheng, H.-M.; Zhou, G. Fundamentals, Status and Challenges of Direct Recycling Technologies for Lithium Ion Batteries. Chem. Soc. Rev. 2023, 52, 8194–8244. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A Perspective on the Sustainability of Cathode Materials Used in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Hu, Y.; Yu, Y.; Huang, K. Environmental Sustainability Assessment of Typical Cathode Materials of Lithium-Ion Battery Based on Three LCA Approaches. Processes 2019, 7, 83. [Google Scholar] [CrossRef]
- Vera, M.L.; Torres, W.R.; Galli, C.I.; Chagnes, A.; Flexer, V. Environmental Impact of Direct Lithium Extraction from Brines. Nat. Rev. Earth Environ. 2023, 4, 149–165. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Dong, G.; Zheng, H. Research Progress of Working Electrode in Electrochemical Extraction of Lithium from Brine. Batteries 2022, 8, 225. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, Z.; Xiao, Y.; Ruan, Q.; Wang, Q.; Zhang, H.; Yao, M.; Zhang, Y. Self-Oxidated Hybrid Conductive Network Enables Efficient Electrochemical Lithium Extraction Under High-Altitude Environment. Small 2024, Early View, 2406607. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Hu, X. Electrochemical Lithium Extraction with Gas Flushing of Porous Electrodes. Nanomaterials 2023, 13, 1471. [Google Scholar] [CrossRef]
- Dong, Q.; Gang, H.; Xu, J.; Li, Z.; Wang, Z. The Technologies of Electrochemical Lithium Extraction Process from Lithium-Containing Solutions. J. Exp. Theor. Anal. JETA 2024, 2, 91–102. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies 2022, 15, 7356. [Google Scholar] [CrossRef]
- Jungi, H.; Podder, S.; Girase, H.; Mitra, J. Sustainable Combination of Waste with Waste: Utilization of Biomass to Recover Critical Metals from Spent Lithium Ion Batteries. Batter. Supercaps 2024, Early View, e202400518. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.; Paz-Garcia, J. Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy. Processes 2024, 12, 1485. [Google Scholar] [CrossRef]
- Sahu, S.; Devi, N. Hydrometallurgical Treatment of Spent Lithium Ion Batteries Using Environmentally Friendly Leachant and Extractant. J. Mater. Cycles Waste Manag. 2023, 25, 3303–3315. [Google Scholar] [CrossRef]
- Korra, C.; Sadhana, V.A.; Khandewal, A. A Framework for Sustainable Lithium-Ion Battery Recycling Facilities, Vol 1. IJERSTE 2023, 13, 164–170. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Thornton, A.W.; Hill, A.J.; Wang, H.; Konstas, K. Lithium Extraction by Emerging Metal–Organic Framework-Based Membranes. Adv. Funct. Mater. 2021, 31, 2105991. [Google Scholar] [CrossRef]
- Lalasari, L.H.; Sulistiyono, E.; Harjanto, S.; Irawan, Y.; Firdiyono, F.; Arini, T.; Andriyah, L.; Suharyanto, A.; Natasha, N.C.; Yunita, F.E. The Effect of pH and Sodium Silicate Dosage on the Separation of Magnesium and Lithium from Artificial Brine Water Using Chemical Precipitation Techniques. Metalurgi 2023, 38, 101. [Google Scholar] [CrossRef]
- Fu, W.; Meng, L.; Qu, J. Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals 2024, 14, 1075. [Google Scholar] [CrossRef]
- Lebit, H.; Brunner, B.; Kharitonova, N.; Deemer, E. Direct Lithium Extraction from Geothermal Brines: The New Oil. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5–8 May 2024; p. D011S011R006. [Google Scholar]
- Vega-Muratalla, V.O.; Ramírez-Márquez, C.; Lira-Barragán, L.F.; Ponce-Ortega, J.M. Review of Lithium as a Strategic Resource for Electric Vehicle Battery Production: Availability, Extraction, and Future Prospects. Resources 2024, 13, 148. [Google Scholar] [CrossRef]
- Du, S.; Gao, F.; Nie, Z.; Liu, Y.; Sun, B.; Gong, X. Comparison of Electric Vehicle Lithium-Ion Battery Recycling Allocation Methods. Environ. Sci. Technol. 2022, 56, 17977–17987. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A Non-Academic Perspective on the Future of Lithium-Based Batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- Popien, J.-L.; Thies, C.; Barke, A.; Spengler, T.S. Comparative Sustainability Assessment of Lithium-Ion, Lithium-Sulfur, and All-Solid-State Traction Batteries. Int. J. Life Cycle Assess. 2023, 28, 462–477. [Google Scholar] [CrossRef]
- Ferreira, D.; Baltazar, M.E.; Santos, L. Developing a Comprehensive Framework for Assessing Airports’ Environmental Sustainability. Sustainability 2024, 16, 6651. [Google Scholar] [CrossRef]
- Abghoui, Y. Hydrogen Fuel Cells vs. Lithium-Ion Batteries in Electric Vehicles. Electrochem. Soc. Meet. Abstr. 2024, 245, 631. [Google Scholar] [CrossRef]
- Buravleva, Y.; Tang, D.; Bethel, B.J. Incentivizing Innovation: The Causal Role of Government Subsidies on Lithium-Ion Battery Research and Development. Sustainability 2021, 13, 8309. [Google Scholar] [CrossRef]
- Esiri, A.E.; Kwakye, J.M.; Ekechukwu, D.E.; Ogundipe, O.B. Augusta Heavens Ikevuje Assessing the Environmental Footprint of the Electric Vehicle Supply Chain. Magna Sci. Adv. Res. Rev. 2023, 8, 219–227. [Google Scholar] [CrossRef]
- Di Leo, A.; Sfodera, F.; Cucari, N.; Mattia, G.; Dezi, L. Sustainability Reporting Practices: An Explorative Analysis of Luxury Fashion Brands. Manag. Decis. 2023, 61, 1274–1297. [Google Scholar] [CrossRef]
- Zhao, X.; Khelifi, F.; Casale, M.; Cavallo, A.; Padoan, E.; Yang, K.; Dino, G.A. Critical Raw Materials Supply: Challenges and Potentialities to Exploit Rare Earth Elements from Siliceous Stones and Extractive Waste. Resources 2024, 13, 97. [Google Scholar] [CrossRef]
- Petavratzi, E.; Sanchez-Lopez, D.; Hughes, A.; Stacey, J.; Ford, J.; Butcher, A. The Impacts of Environmental, Social and Governance (ESG) Issues in Achieving Sustainable Lithium Supply in the Lithium Triangle. Miner. Econ. 2022, 35, 673–699. [Google Scholar] [CrossRef]
- Radchuk, O. Comparative Analysis of Ukrainian and International Standards in the Context of Mining Tailings Dam Design Requirements. IOP Conf. Ser. Earth Environ. Sci. 2024, 1376, 012027. [Google Scholar] [CrossRef]
- Gardiner, N.J.; Jowitt, S.M.; Sykes, J.P. Lithium: Critical, or Not so Critical? Geoenergy 2024, 2, geoenergy2023-045. [Google Scholar] [CrossRef]
- Feng, Q.C.; Ren, Z.J.; Chen, Q.S. The “Rock Candy” Approach for Direct Lithium Extraction. In Proceedings of the 2023 IEEE Integrated STEM Education Conference (ISEC), Laurel, MD, USA, 11 March 2023; pp. 367–374. [Google Scholar]
- Murodjon, S.; Yu, X.; Li, M.; Duo, J.; Deng, T. Lithium Recovery from Brines Including Seawater, Salt Lake Brine, Underground Water and Geothermal Water. In Thermodynamics and Energy Engineering; Vizureanu, P., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-568-5. [Google Scholar]
- Kalungi, P.; Yao, Z.; Huang, H. Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production. Materials 2024, 17, 4389. [Google Scholar] [CrossRef]
- Hasselwander, S.; Meyer, M.; Österle, I. Techno-Economic Analysis of Different Battery Cell Chemistries for the Passenger Vehicle Market. Batteries 2023, 9, 379. [Google Scholar] [CrossRef]
- Harper, G.D.J.; Kendrick, E.; Anderson, P.A.; Mrozik, W.; Christensen, P.; Lambert, S.; Greenwood, D.; Das, P.K.; Ahmeid, M.; Milojevic, Z.; et al. Roadmap for a Sustainable Circular Economy in Lithium-Ion and Future Battery Technologies. J. Phys. Energy 2023, 5, 021501. [Google Scholar] [CrossRef]
- Chala, T.G.; Kornilich, D.O. A Statistical Analysis of the National Market of Lithium-Ion Batteries and the Influence of the Aggression of the Russian Federation on Its Development. Probl. Econ. 2024, 1, 146–152. [Google Scholar] [CrossRef]
- Zou, Q.; Yi, C.; Wang, K.; Yin, X.; Zhang, Y. Global LNG Market: Supply-Demand and Economic Analysis. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 012051. [Google Scholar] [CrossRef]



| Microorganism Type | Representative Species | Leaching Mechanism | Lithium Extraction Efficiency | Applicable Materials | Ref. |
|---|---|---|---|---|---|
| Bacteria | Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans | Oxidation of sulfur and iron compounds, producing sulfuric acid and ferric iron | Up to 100% | Spent lithium-ion batteries | [79] |
| Fungi | Penicillium chrysogenum | Production of organic acids, particularly gluconic acid | Up to 73.31% | Spent lithium-ion batteries | [80] |
| Penicillium purpurogenum | Production of organic acids (gluconic and citric acids) | 10.8 mg % dry weight (accumulated in biomass), 1.26 ppm (in leach liquor) | Spodumene ore | [81] | |
| Aspergillus niger | Production of organic acids (gluconic and citric acids) | 5.1 mg % dry weight (accumulated in biomass), 0.75 ppm (in leach liquor) | Spodumene ore | ||
| Yeast | Rhodotorula rubra | Production of capsular exopolymers | 16.7 mg % dry weight (accumulated in biomass), 1.53 ppm (in leach liquor) | Spodumene ore | |
| Mixed cultures | A. ferrooxidans and A. thiooxidans | Synergistic effect of iron and sulfur oxidation | Up to 80% | Spent lithium-ion batteries | [82] |
| A. ferrooxidans and R. rubra (bacterial-yeast) | Synergistic effect of autotrophic bacteria and heterotrophic yeast | Not specified | Lepidolite | [83] |
| Aspect | Challenges | Opportunities |
|---|---|---|
| Technical | 1. Low lithium concentrations (<100 mg/L) 2. Presence of impurities (e.g., high Mg/Li ratio) 3. Resource characteristic variations 4. Low efficiency of conventional extraction methods | 1. Development of highly selective and efficient extraction techniques 2. Emerging direct lithium extraction (DLE) technologies, particularly electrochemically mediated methods |
| Economic | 1. High-energy consumption and costs of traditional methods 2. Scaling and operational costs 3. Market volatility and price fluctuations | 1. Cost reduction through innovative technologies (e.g., membrane-less electrochemical cells) 2. Pilot-scale trials demonstrating feasibility of large-scale operations 3. Growing global demand for lithium driving price increases |
| Environmental and Sustainability | 1. Environmental impact of traditional extraction methods (e.g., evaporation ponds) 2. Water resource management in water-scarce regions 3. Social impact on local communities | 1. Environmentally friendly alternatives (e.g., electrochemical de-intercalation of lithium) 2. Innovative technologies reducing water consumption and utilizing osmotic energy 3. Exploring green alternatives and energy-efficient extraction methods 4. Development of closed-loop recycling systems for sustainable lithium production 5. Assessing and managing social impacts, ensuring benefits for local communities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Liang, B.; Luo, X.; Zhang, X.; Yuan, W.; Xiao, B.; Tang, X. Recent Advances and Future Prospects of Lithium Recovery from Low-Grade Lithium Resources: A Review. Inorganics 2025, 13, 4. https://doi.org/10.3390/inorganics13010004
Gu J, Liang B, Luo X, Zhang X, Yuan W, Xiao B, Tang X. Recent Advances and Future Prospects of Lithium Recovery from Low-Grade Lithium Resources: A Review. Inorganics. 2025; 13(1):4. https://doi.org/10.3390/inorganics13010004
Chicago/Turabian StyleGu, Jihan, Binjun Liang, Xianping Luo, Xin Zhang, Weiquan Yuan, Bin Xiao, and Xuekun Tang. 2025. "Recent Advances and Future Prospects of Lithium Recovery from Low-Grade Lithium Resources: A Review" Inorganics 13, no. 1: 4. https://doi.org/10.3390/inorganics13010004
APA StyleGu, J., Liang, B., Luo, X., Zhang, X., Yuan, W., Xiao, B., & Tang, X. (2025). Recent Advances and Future Prospects of Lithium Recovery from Low-Grade Lithium Resources: A Review. Inorganics, 13(1), 4. https://doi.org/10.3390/inorganics13010004







