Abstract
Transition metal sulfide (TMS)-based electrocatalysts have received considerable attention in the field of sustainable energy, especially for their high activity in the hydrogen evolution reaction (HER). This review summarizes how researchers have improved the performance of TMSs by adjusting their composition. This review introduces the research background of transition metal sulfides and clarifies the reaction mechanism of the HER and its performance evaluation indicators. Then, it elaborates on the general synthesis techniques for preparing TMS materials, including hydrothermal methods, electrochemical deposition, liquid-phase exfoliation, chemical vapor deposition, and other methods. Moreover, it discusses the realization of excellent electrocatalytic performance in the HER through doping, hole treatment, heterostructures, and multi-sulfides. Finally, this review summarizes the current challenges and future development opportunities of TMS materials in the field of water electrolysis for hydrogen production.
1. Introduction
It is widely acknowledged that as the economy and population expand, problems such as environmental pollution and energy shortages have become increasingly prominent [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. To confront these challenges, there is an urgent necessity to seek new energy substitutes to reduce reliance on fossil energy, conserve resources, and safeguard the environment [5,17,18,19,20,21]. Hydrogen energy, due to its high energy density and zero environmental pollution, has emerged as an ideal alternative to fossil fuels and has garnered extensive attention from researchers over the past several decades [22,23,24]. Among the numerous hydrogen production methods, electrolytic water distinguishes itself by its high conversion efficiency, hydrogen purity, and commercial value. Hydrogen evolution overpotential refers to the phenomenon in which the cathode potential is more negative than the theoretical hydrogen evolution potential in the actual electrolysis process. Consequently, the search for appropriate catalysts to reduce the reaction overpotential at the cathode (HER), thereby effectively accelerating hydrogen generation, is of paramount importance [25,26]. Currently, certain precious metal materials like platinum (Pt), palladium (Pd), iridium (Ir), ruthenium (Ru), along with their alloy compounds, have been comprehensively studied as catalysts for the HER due to their remarkable electrochemical performance and the suitable adsorption energies of the reaction intermediates [26,27,28]. However, the scarcity and high costs of these precious-metal-based materials have hindered their large-scale application. As a result, the development of low-cost and resource-abundant electrocatalysts has become extremely urgent. In recent years, researchers have conducted extensive explorations on low-cost, resource-rich, and high-performance non-precious-metal-based materials, such as transition metal oxides, phosphides [29,30], sulfides, and nitrides [31]. These materials have attracted widespread attention in the context of the HER, owing to their high catalytic performance, tunable electronic structures, and unique physicochemical properties.
Among the various materials under consideration, two-dimensional transition metal dichalcogenides (2D-TMDs) have garnered significant attention on account of their remarkable physical and chemical properties [32,33,34,35]. Structurally, these materials generally adhere to the formula X-M-X. In this formula, “X” denotes an element from the sulfur family, such as sulfur (S), located on the central plane, and “M” represents a transition metal, like molybdenum (Mo) or cobalt (Co), also on the same plane. The basal plane of 2D-TMDs is characterized by strong ionic–covalent bonds, whereas the adjacent layers are connected through relatively weaker van der Waals forces. This structural feature endows them with a huge specific surface area, fine-tuned atomic-level control, outstanding charge transport efficiency, and adjustable physical and chemical properties, making them ideal candidate materials for highly efficient hydrogen evolution reaction (HER) catalysts [36,37,38,39,40,41,42,43].
2. The Introduction of the Hydrogen Evolution Reaction (HER)
Hydrogen production through water electrolysis [44,45] is a fundamental, unique, and promising technology that can utilize renewable energy sources, such as solar and wind energy, and produce hydrogen without carbon dioxide emissions during the hydrogen production process. Hydrogen produced through water electrolysis is carbon-free and is therefore considered a clean energy source. Based on the different electrolysis technologies, it can be divided into alkaline water electrolysis (AWE), solid oxide electrolyzer cell (SOEC), and proton exchange membrane (PEM) electrolysis [46]. In the alkaline water electrolysis (AWE) process, a 20% to 30% aqueous solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH) is typically used as the electrolyte. The electrolysis system consists of an electrolyte, anode, cathode, power supply, and separator. At the cathode, water molecules are reduced to hydrogen gas and released, while hydroxide ions are produced. These ions are then transported to the anode where they are oxidized. AWE technology is already quite mature, with equipment that is durable and suitable for large-scale applications. However, the separator in the electrolyzer cell can isolate gases to prevent mixing, the alkaline electrolyte is corrosive and can react with carbon monoxide to form carbonates, which can affect the efficiency of the reactants and products, leading to a decrease in energy efficiency. Solid oxide electrolyzer cell (SOEC) electrolysis technology operates at high temperatures and pressures, typically using oxygen ions as the conductive medium. This technology has a high energy efficiency, but the operating temperature is higher, and its safety needs to be further improved. Additionally, the materials required for the SOEC electrolyzer cell are strict, leading to a dependence on imported materials for the electrodes and the electrolyzer cell, which results in high costs. Therefore, SOEC technology is currently still in the research stage and has not been widely applied in industrial production.
2.1. The Electrocatalytic Mechanism of HER
Producing hydrogen by electrolyzing water is one of the most promising methods. The operational method is to connect an electrolyzer to an electric power source, and then the water undergoes an electrolytic chemical reaction to generate hydrogen and oxygen. During this process, no greenhouse gases or other harmful gases are produced, making it very environmentally friendly. The electrolysis of water to produce hydrogen involves two reactions, the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). The hydrogen evolution reaction (HER) refers to the reaction in which water is reduced to hydrogen gas during the fracturing process. This reaction exhibits different reaction mechanisms under different pH conditions [47,48], mainly including the Volmer, Tafel, and Heyrovsky reactions. The HER is a two-electron transfer process in acidic electrolytes, where the water–hydrogen ion complex is released to form a hydrogen intermediate (M-H*) and adsorbs on the electrode surface. In alkaline electrolytes, water molecules are the source of protons. Subsequently, hydrogen molecules are produced through two different routes, depending on the coverage of the M-H* intermediate on the electrode surface. If the coverage of H* is high, the Tafel step is the rate-limiting step, because adjacent M-H* molecules can combine to form H2 molecules. If the coverage of H* is low, the Heyrovsky reaction dominates, because a single M-H* atom can simultaneously attract a proton and an electron in an acidic medium, and a water molecule and an electron in a basic medium, as shown in Table 1.

Table 1.
HER equation in acidic and alkaline environments.
2.2. Electrolytic Water Performance Evaluation Parameters
The performance of electrolytic water catalysts can be reflected by looking at the quality of some indicators. For example: the overpotential, Tafel slope, electrochemical impedance, turnover frequency, stability, and so on.
2.2.1. Overpotential
The overpotential (η) is the difference between the actual working potential (Eapplied) of an electrode in an electrochemical reaction and its thermodynamic equilibrium potential (Eeq), that is, η = Eapplied − Eeq. The overpotential is the additional energy required to drive the reaction to overcome the kinetic energy barrier, reflecting the irreversibility of the electrochemical reaction. Its direction is determined by the type of reaction: Anodic overpotential (oxidation reaction): η > 0 (the actual potential is higher than the equilibrium potential). Cathodic overpotential (reduction reaction): η < 0 (the actual potential is lower than the equilibrium potential) [49]. In the HER, overpotential is often used to measure the performance of the catalyst, i.e., the additional voltage required to drive the reaction at a specific current density. The smaller the overpotential, the higher the catalyst’s activity, as it requires less additional energy to achieve the same reaction rate. The overpotential depends on various factors, including the electrode material, the concentration of the electrolyte, the temperature, and other physical and chemical factors. In experiments, by measuring the overpotential under different conditions, one can study the properties of the electrode material and the kinetic parameters of the reaction process, thereby optimizing the reaction conditions.
In an ideal electrochemical environment, the HER (potential relative to the hydrogen electrode) can occur at a potential of 0 V. The additional voltage required beyond this reference potential is called the overpotential (η). In electrochemical research, researchers typically measure the specific overpotential values of η10 and η100. η10 refers to the overpotential required to achieve a current density of 10 mA cm−2, while η100 is the overpotential required to achieve a current density of 100 mA cm−2. These two parameters are important indicators of the catalyst’s performance, with η10 being the entry-level standard for commercial applications, and η100 being used to evaluate the catalyst’s activity in actual applications. It is important to note that to accurately compare the performance of different catalysts, the overpotential curves must be measured under the same test conditions. This includes factors such as the loading of the catalyst, its surface area, the concentration of the electrolyte, and the scan rate during electrochemical testing [50].
2.2.2. Tafel
The Tafel slope in the HER is a key kinetic parameter used in electrochemistry to describe the relationship between the electrochemical reaction rate and electrode potential. The Tafel slope can provide an important reference for exploring the reaction mechanism. By analyzing the Tafel slope, we can infer the speed control step of the electrochemical reaction, that is, the slowest step in the reaction process. The smaller the Tafel slope value, the faster the current density increases, which means that the catalyst’s kinetics are faster, and the catalytic activity is better. Therefore, the Tafel slope can be used as an important index to evaluate catalyst performance.
There exists an inverse relationship between the Tafel slope and the charge transfer coefficient; specifically, a smaller Tafel slope indicates a stronger charge transfer capability of the catalyst. By analyzing the polarization curve, we can determine the Tafel slope and subsequently evaluate the catalytic activity. The Tafel equation is commonly expressed as [49], where η represents the overpotential, b denotes the Tafel slope, j signifies the total current density, and j0 refers to the exchange current density. This equation elucidates the additional overpotential required to increase the current density of the catalyst by one order of magnitude. A lower Tafel slope (b) and a higher current density (j) generally indicate the superior performance of the catalyst in hydrogen evolution reactions (HERs) or oxygen evolution reactions (OERs) [51,52].
The relationship between the Tafel slope and the rate-determining step is as follows: when the rate-determining step involves a single electron transfer (α* = 0.5), the Tafel slope [49]; when the rate-determining step involves no electron transfer (α* = 0), the Tafel slope [49].
2.2.3. Electrochemical Impedance
In the context of the HER, electrochemical impedance spectroscopy (EIS) serves as a critical tool for assessing catalyst performance. EIS elucidates the electrochemical characteristics of the catalyst, including its surface, interface, and coating, thereby reflecting the overall performance of the catalyst. Specifically, EIS can measure charge transfer resistance, a key parameter that directly characterizes the catalyst’s charge transfer capability. In the Nyquist plot of EIS, the diameter of the semicircle corresponds to the inverse of the charge transfer resistance; thus, a smaller semicircle diameter indicates a lower charge transfer resistance, signifying the superior charge transfer performance of the catalyst.
2.2.4. Turnover Frequency
Turnover frequency (TOF) in the HER is an important parameter to measure catalyst performance. It represents the number of reactions occurring per unit of time and per unit of active site, that is, the number of catalytic reactions occurring per unit of active site or the number of target products generated or the number of reactants consumed under a given temperature, pressure, reactant ratio, and a certain degree of reaction. The value of TOF can directly reflect the intrinsic activity of the catalyst and is an important index to evaluate the activity of the catalyst. A high TOF means that the active site of the catalyst is more efficient and can catalyze more reactions per unit of time, which is directly related to the efficiency and economy of the catalyst. In practical applications, a catalyst with a high TOF value can achieve the same reaction rate with a lower dosage, thus reducing costs and improving energy efficiency.
The TOF value is directly proportional to the catalytic activity, enabling the evaluation of the intrinsic activity of the catalyst. During the actual testing process, the hydrogen produced at the interface between the catalyst material and the electrolyte is adsorbed onto the catalyst surface. As a result, the generated gas can have an impact on the catalytic reaction [53].
3. Transition Metal Sulfide Catalysts
Research progress on hydrogen evolution catalysts (HER): The preferred catalysts for hydrogen evolution in the industry are Pt, Pd, and other precious metals. Due to the scarcity of resources and their high cost, developing low-cost, high-catalytic-activity, and stable non-precious metal catalysts has become a research hotspot. It is found that Fe, Co, Ni, Mo, W, and other transition-metal-based compounds have abundant D-orbital holes and multiple valence states, which are expected to replace precious metal electrocatalysts. In particular, transition metal sulfides are widely used in hydrogen evolution electrocatalytic reactions because of their unique two-dimensional layered structure. For example, MoS2 is the best candidate for a hydrogen evolution electrocatalyst to replace Pt-based materials because of its theoretically comparable catalytic performance for the hydrogen evolution reaction, as well as its high natural abundance and low price. In summary, transition metal sulfide catalysts show great potential in the reaction of electrolytic water hydrogen evolution, and researchers are using various strategies to improve their catalytic performance to meet commercial application standards.
3.1. Basic Properties of Transition Metal Sulfides (TMSs)
Transition metal sulfides can be divided into two groups according to their structure, namely layered MS2 (MoS2, WS2) and non-layered MxSy (CoS, FeS, NiS, etc.). A typical layered MS2 is a sandwich structure, where one layer of metal is bonded with two layers of sulfur, and each MS2 cell is stacked vertically together by relatively weak van der Waals forces, which allow MS2 to be stripped layer by layer. Moreover, MS2 has a variety of phase states, such as 2H, 1T, and 3R, and the electronic properties of MS2 in different phase states are significantly different. For example, 2H-MoS2 is a semiconductor, while 1T-MoS2 has metallic properties that are conducive to electrocatalysis. The electronic structure of transition metal sulfides determines their catalytic activity. Due to the involvement of the d orbitals of transition metal atoms in bonding, these materials generally have high catalytic activity. For example, MoS2 and WS2 show good catalytic activity in the hydrogen development reaction (HER). Transition metal sulfides have good electrical conductivity, which allows them to efficiently transport electrons when used as catalysts in electrochemical applications such as water decomposition. The surface activity of transition metal sulfides is closely related to the arrangement and exposure of the surface atoms. Their surface activity can be significantly improved through morphological control (such as the vertical arrangement of nanosheets) and defect engineering (such as the introduction of sulfur vacancies). Transition metal sulfides generally exhibit good chemical stability in acidic or alkaline environments, which is essential for their application in electrochemical water decomposition.
3.2. Synthesis of Transition Metal Sulfides
Due to the great potential of TMSs, the catalytic performance of their derivatives in the field of HER catalysis has been widely discussed. Moreover, TMSs have the characteristics of low cost, good hydrogen evolution performance, good stability, and simple synthesis. The researchers hope to further improve the performance of the HER through doping modification, which also allows transition metal catalysts to replace precious metal catalysts such as Pt and Ru. In this section, we summarized the typical TMS synthesis methods, including the hydrothermal method, electrochemical deposition method, liquid-phase stripping method (LPE), chemical vapor deposition (CVD), two-step synthesis method, etc. Each of these methods has advantages and disadvantages, and the selection of the appropriate synthesis method needs to be determined according to the desired material characteristics and application field. For example, the CVD method is suitable for the preparation of high-quality two-dimensional materials, while the hydrothermal method and electrochemical deposition method are more suitable for the synthesis of nanomaterials with a specific morphology and structure.
3.2.1. Hydrothermal Process
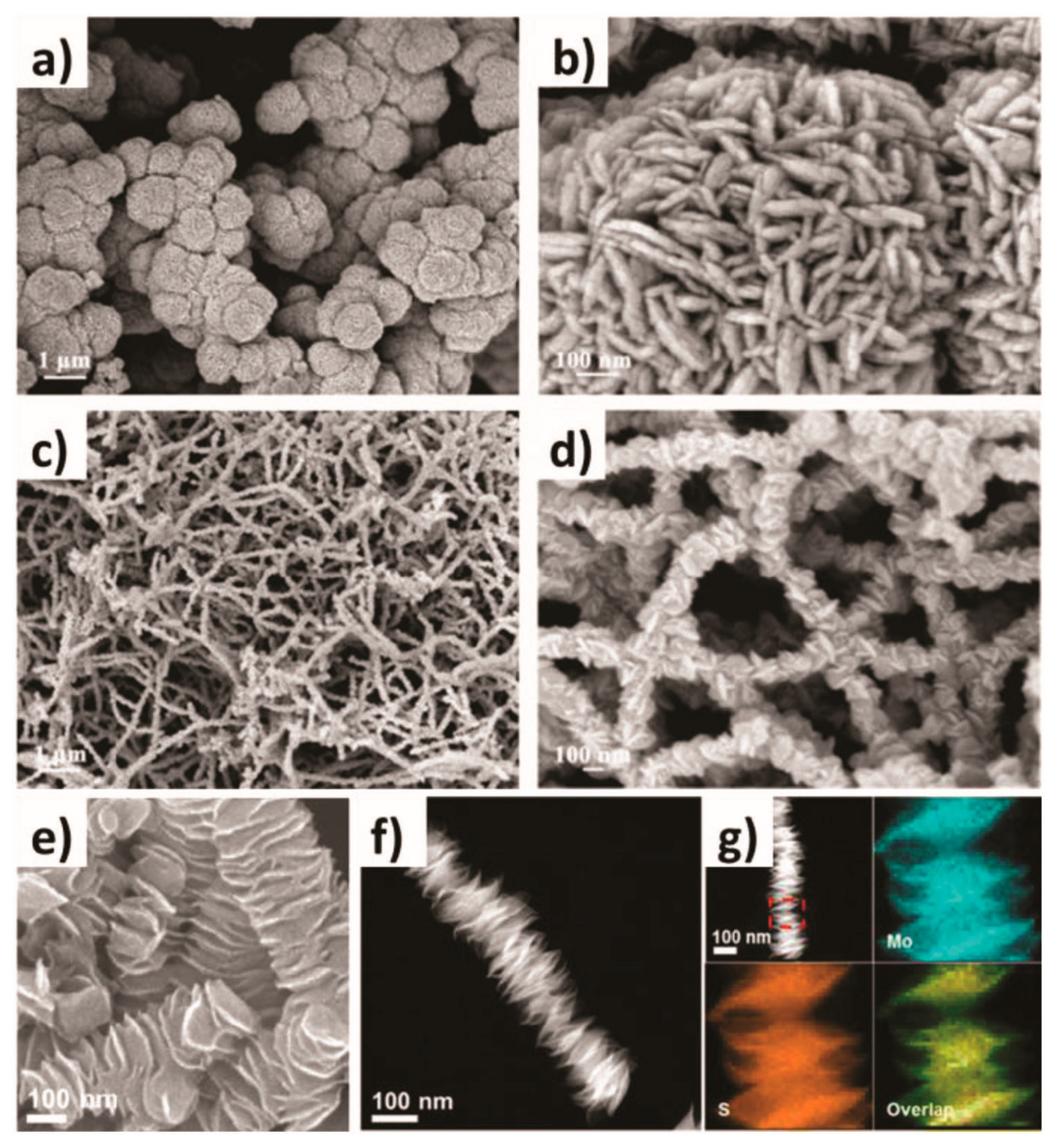
The hydrothermal method, alternatively named the hydrothermal synthesis method, is a chemical reaction process occurring in high-temperature and high-pressure aqueous environments. It uses water as a solvent and reaction medium, uses the special properties of water at high temperatures and pressures to accelerate the reaction rate, and changes the phase and crystal structure of the reaction product. Its underlying principle is to utilize a high-temperature and high-pressure aqueous solution to dissolve substances that are insoluble or sparingly soluble under normal atmospheric conditions, or to cause reactions that generate the dissolved products of these substances. Through controlling the temperature gradient within the autoclave solution to generate convection, a supersaturated state is established, leading to the precipitation and growth of crystals. Hydrothermal crystallization mainly operates based on a solution recrystallization mechanism. The selective synthesis of various transition-metal-phosphide–sulfide (TPS)-based nanostructures can be achieved by choosing the appropriate precursor materials, such as metal salts and sulfur sources, and optimizing the reaction parameters, including reaction time, pH value, temperature, and reactant concentration. For example, with a simple biomolecular-assisted hydrothermal approach, different nanostructured cobalt sulfide (CoS) forms like nanospheres and nanowires can be fabricated merely by modifying the polarity of the ethanol cosolvent in the aqueous solution. There are proposed hypotheses regarding the formation mechanisms of these distinct CoS nanostructures (as shown in Figure 1a–d). It has been ascertained that the shape of the nanostructures has a significant effect on their capacitive properties. Figure 1e–g show the columnar structure of MoS2 consisting of an end-edge MoS2 nanosheet (CLETMoS2), which is also synthesized by a simple hydrothermal reaction. These unique three-dimensional superstructures significantly enhance the accessibility of the active edges of MoS2. Consequently, CLET MoS2 demonstrates an extremely low Tafel slope of 39 mV·dec−1 in the HER, outperforming commercially available MoS2 [54].
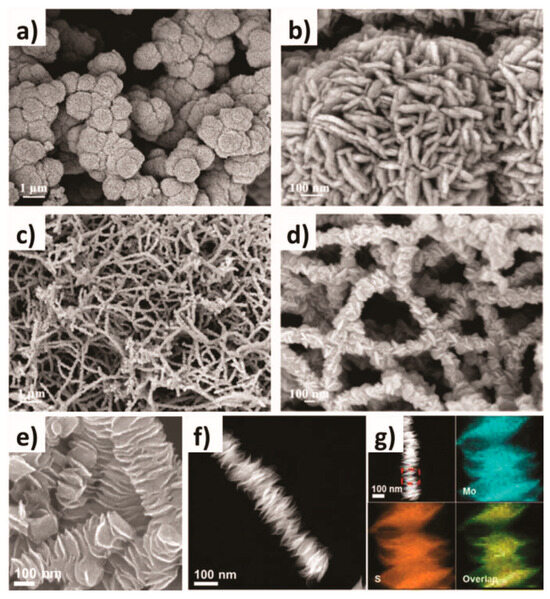
Figure 1.
(a–d) Low-magnification FESEM and high-magnification FESEM of CoS obtained in aqueous solution (a,b), and ethanol aqueous solution (c,d). (e) High-magnification SEM image, (f) STEM image, and (g) HAADF-STEM image and the corresponding EDX elemental mappings of CLET MoS2. (a–d) are reproduced with permission. Ref. [55], Copyright 2008, Elsevier. (e–g) are reproduced with permission. Ref. [54], Copyright 2016, American Chemical Society.
3.2.2. Electrochemical Deposition Method
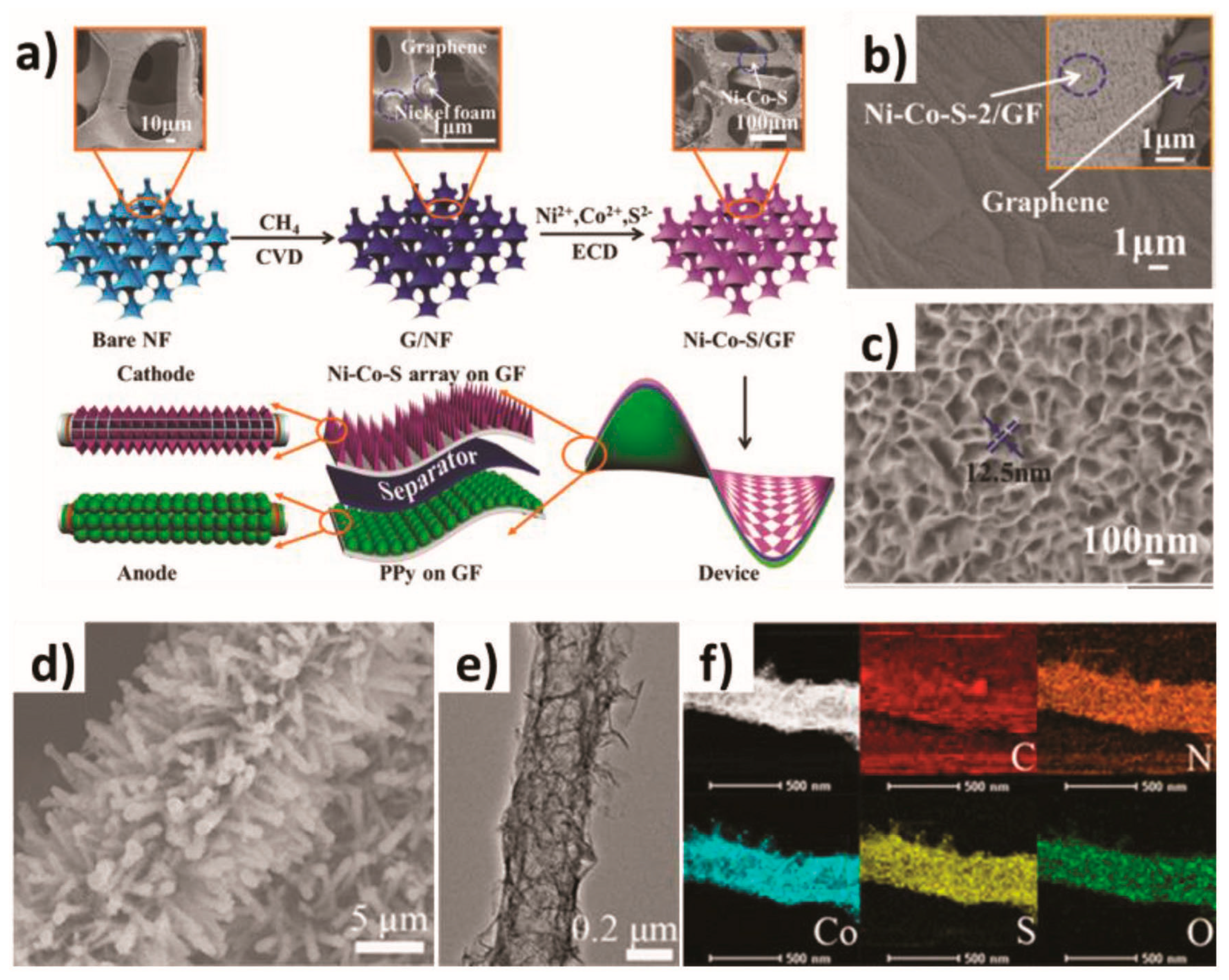
The electrochemical deposition method is a process in which metal ions or other substances in the electrolyte are reduced or oxidized on the electrode by controlling the current or voltage on the surface of the material using the principle of electrochemical reaction and deposited in solid form to form a film or coating. The principle is based on an electrochemical reaction process involving the reduction of metal ions or compounds in electrolyte solutions on the electrode surface through electron transfer and the formation of solid deposits. This involves metal ions in the electrolyte receiving electrons on the cathode to be reduced to metal atoms and deposited on the cathode as a solid substance. Its characteristics are as follows: a low equipment cost, a simple process, easy to operate, environmentally safe, a flexible production mode, suitable for industrial production, and can be precisely controlled. Recently, Zhang et al. used a one-step electrochemical deposition technique to construct an array of nickel–cobalt sulfide nanosheets shaped like layered petals on a three-dimensional porous graphene foam substrate (see Figure 2a) [56]. In this electrochemical deposition process, the concentration of Ni2+ precursors significantly affected the structural characteristics and electrochemical properties of Ni-Co-S/GF. Observations by scanning electron microscopy (SEM) (Figure 2b,c) showed that the ultra-thin and fluffy Ni-Co-S/GF nanosheets were interwoven to form a continuous Ni-Co-S/GF shell arranged vertically with the GF substrate. This unique three-dimensional layered structure provided a wide contact area for REDOX reactions and facilitated ion migration. In addition, Wang et al. used template-assisted electrochemical deposition technology to assemble cobalt sulfide sheets and carbon tubes on carbon paper to form an integrated three-dimensional electrode structure (labeled CP/CTs/Co-S) [57]. It can be seen from the morphology of the CP/CTs/Co-S electrodes of the three-dimensional array shown in Figure 2d–f that this unique three-dimensional structure provided many easily accessible active sites for the catalyst, accelerated the transmission of electrons, and facilitated the release of gas products. These properties make the material excellent in its catalytic activity and stability to water in alkaline media.
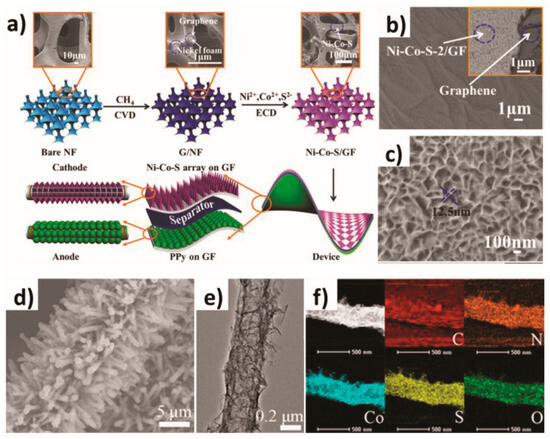
Figure 2.
(a) Schematic illustration of the synthesis of the petal-like Ni–Co–S and the construction of ASC devices, and (b,c) the SEM images of Ni–Co–S/GF. (d) SEM image, (e) TEM image, and (f) element mapping of CP/CTs/Co–S. (a–c) are reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Ref. [56], Copyright 2018, The Authors, Published by Wiley-VCH. (d–f) are reproduced with permission. Ref. [57], Copyright 2016, American Chemical Society.
3.2.3. Liquid-Phase Stripping Method
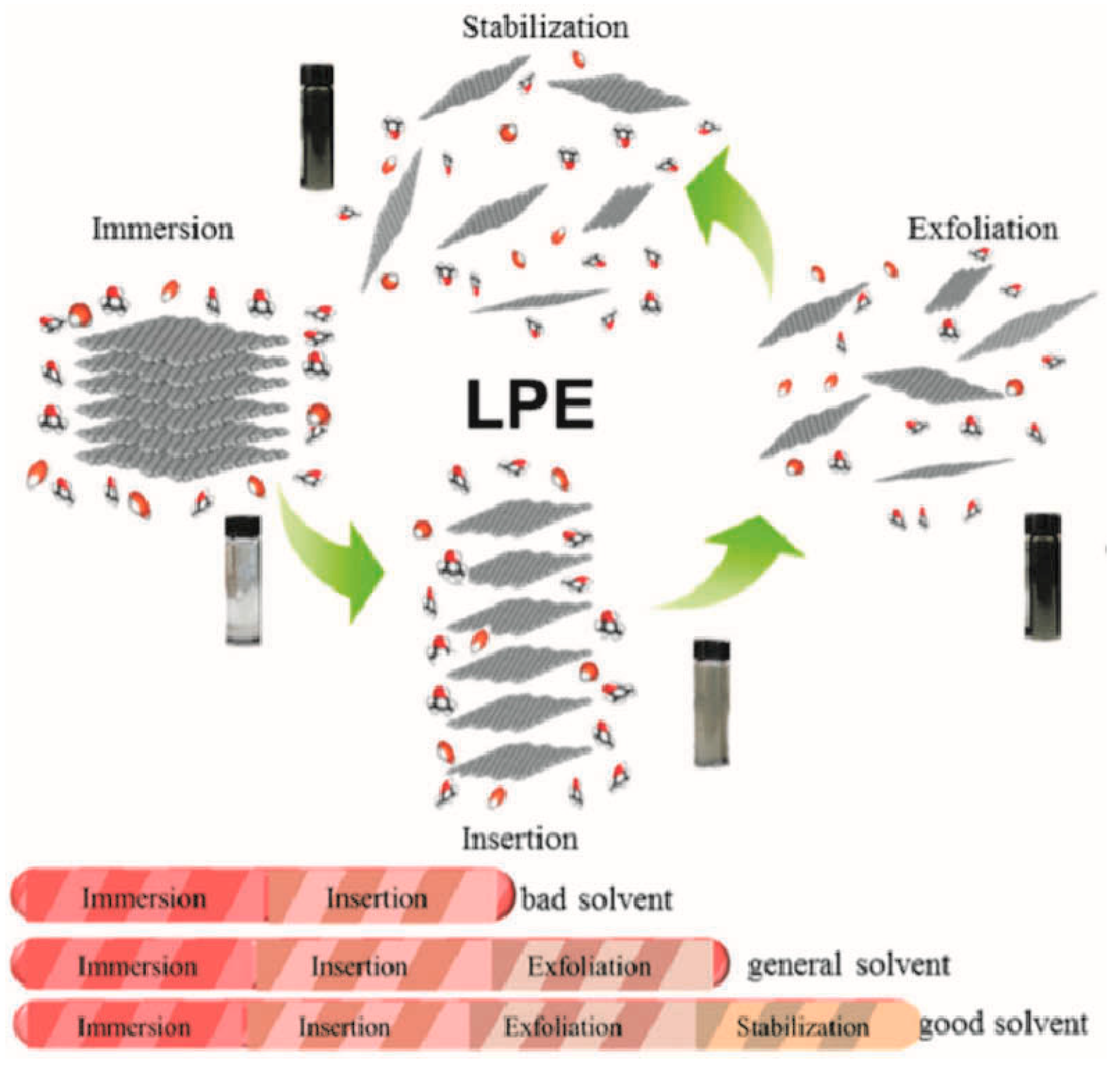
The liquid-phase stripping method is the use of specific solvents or surfactants to disperse graphite, and then through ultrasonic, microwave, shear force, thermal stress, or electrochemical means, assisting in the stripping, to obtain the graphene dispersion method. This method is based on breaking the van der Waals forces between the graphite layers, allowing the graphene layers to peel off from the graphite material. A standard liquid-phase stripping (LPE) process, shown in Figure 3, focuses on the connections of the various steps, including impregnation, intercalation, separation, and stabilization. By blending TMSs with appropriate solvents and polymers, two-dimensional nanostructures can be prepared in large quantities [58]. For instance, Huang et al. [59] successfully synthesized MoS2 nanosheets by employing formamide as a solvent in a solvent heat-treatment-assisted liquid-phase stripping process. The stripping efficiency of N-methyl-2-pyrrolidone (NMP) is enhanced due to the dual effects of the easier embedding of polar solvents and the higher rebound of the treated bulk MoS2 [59]. Zhou et al. [60] effectively stripped MoS2 using a very low-concentration block copolymer alcohol system. Moreover, through conventional ultrasonic methods, the production of nanosheets increased by approximately 5% per hour, and these nanosheets could effectively function as electrocatalysts for hydrogen production. Li et al. [61] prepared and reported that large NiPS3 crystals were exfoliated by electrochemical cathodes to achieve the mass production of large, high-quality, single-layer NiPS3 flakes. The stripped NiPS3 sheets exhibited an unprecedented size (approximately 150 μm2 area) and atomic thickness (approximately 70% monolayer ratio), while maintaining their original structure with very low oxidation and defects.
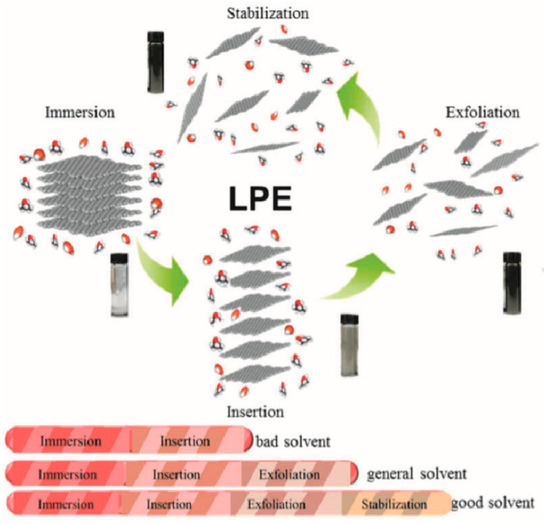
Figure 3.
Scheme of the whole LPE process containing immersion, insertion, exfoliation, and stabilization. Reprinted with permission from Ref. [58], Copyright 2016, Wiley-VCH GmbH.
3.2.4. Chemical Vapor Deposition (CVD)
Chemical vapor deposition (CVD) is an advanced material synthesis technique in which one or more gases (precursors) are heated to high temperatures to break them down, and they are then deposited on the surface of a semiconductor to form the desired film. This method involves a chemical reaction of gaseous reactants on a substrate to produce a solid product. During the CVD process, the diverse precursors required for material synthesis are supplied in the gas phase to the substrate, which is typically positioned within a furnace at a precisely controlled temperature. The precursor decomposes at high temperatures and directly assembles into thermodynamically stable phases on the substrate. CVD serves not only as an outstanding bottom-up synthesis strategy for preparing two-dimensional transition metal sulfide (TMD) films with distinct phases and high quality [62], but can also be used for the wafer-level production of high-quality low-dimensional materials such as nanotubes, nanowires, graphene, and single-layer two-dimensional materials [63]. In the CVD of TMD, sulfur can be introduced either in the form of sulfur-containing gases (e.g., hydrogen sulfide) or as gaseous sulfur generated by evaporating sulfur powder (e.g., sulfur or selenium). The synthesis of atomically thin TMD takes place at high temperatures (up to 700 °C) in a sulfur-containing atmosphere. Furthermore, the CVD of TMD can be accomplished through the direct vulcanization of pre-deposited metal (or metal oxide) films or the gas-phase reaction of evaporated metal (sub-) oxides with gaseous sulfides. This provides an effective approach for the preparation of large-area, high-quality, two-dimensional transition metal sulfide films. TMD synthesized by CVD is characterized by a large specific surface area, uniform thickness, high crystallinity, and excellent electrochemical performance [64]. The synthesis of ternary TMD using CVD enables the modulation and fine-tuning of the hydrogen adsorption free energy (ΔGH), resulting in an abundance of edge sites and reduced resistance, all of which contribute to enhancing the catalytic performance for the HER [65,66].
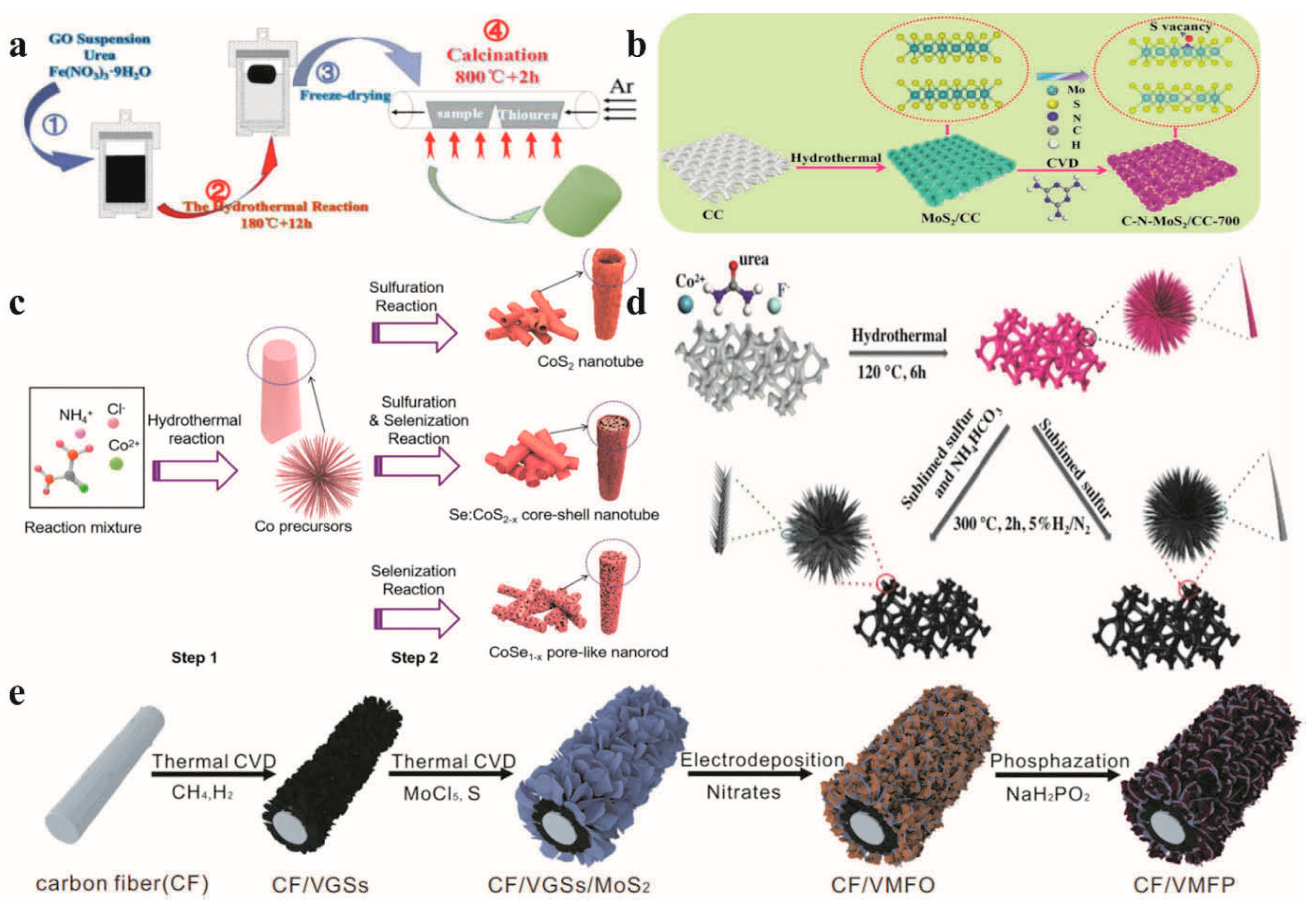
For example, Zhu et al. [67] achieved the successful synthesis of selenium-doped CoS2−x core–shell nanostructures by employing a straightforward two-step method, which combined chemical vapor deposition with hydrothermal treatment (as shown in Figure 4c). Due to the synergistic effects between CoS2 and CoSe1−x and the abundant active sites in the electrode structure, the prepared materials exhibited excellent electrochemical properties. Focusing on the development of catalytic materials with a high current density, Huang et al. fabricated Fe7S8/NGF on N-doped graphene foam by means of hydrothermal technology and chemical vapor deposition (see Figure 4a). In a 1 mol L−1 KOH solution, the Fe7S8/NGF catalyst exhibited a favorable hydrogen evolution reaction (HER) performance. To reach a current density of 100 mA cm−2, only an additional voltage of 2.21 V was required, and the catalyst could maintain its stability for 12 h. Chen et al. [68] developed a high-performance bifunctional electrocatalyst by combining the effects of unsaturated S vacancy and C and N doping in MoS2 nanocatalysts (see Figure 4b). They proposed a new technique for the preparation of C-and N-doped MoS2 at high temperatures. The bifocal catalyst could run stably for 30 h under the condition of 1 mol L−1 KOH and a current density of 10 mA cm−2 without the loss of activity. Ji et al. recently demonstrated the possibility of continuously growing vertical graphene nanosheets (VGSs), layered FeCoNi hydroxide (FeCoNi(OH)x), and MoS2 nanosheets on carbon fiber (see Figure 4e). The preparation process of CF/VGSs/MoS2/FeCoNi(OH)x included the CVD method of growing VGSs on CF and the CVD method of growing MoS2 nanosheets on VGS. The catalyst showed good OER performance at high current densities of 225 and 241 mV and overpotentials of 500 mA cm−2 and 1000 mA Cm−2, respectively.
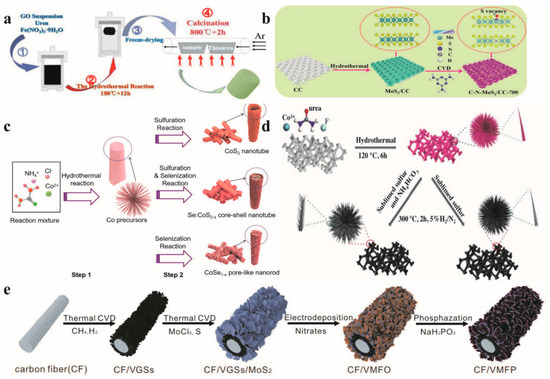
Figure 4.
(a) Synthesis of Fe-S/NGF composite. Reprinted with permission from Ref. [69], Copyright 2021, Science Citation Index Expanded. (b) Synthesis of the C-N-MoS2/CC-700. Reprinted with permission from Ref. [68], Copyright 2021, Royal Society of Chemistry. (c) Schematic illustration of CoS2, Se:CoS2−x, and CoSe1−x electrodes. Reprinted with permission from Ref. [67], Copyright 2021, Royal Society of Chemistry. (d) The synthetic process of N-CoS2 and CoS2. Reprinted with permission from Ref. [70], Copyright 2019, Wiley-VCH GmbH. (e) A schematic diagram showing the preparation process of samples. Reprinted with permission from Ref. [70], Copyright 2021, Springer Nature.
3.2.5. Other Methods
In addition to the above-mentioned techniques, plasma-assisted techniques are also used to synthesize transition metal sulfides. Lu and his team [71] synthesized MoSx containing sulfur ions using plasma-assisted technology. Yang et al. [72] prepared MoS2 nanoarrays with stepped edge and surface-terminating structures by a microwave-assisted hydrothermal method. Ding et al. [73] adopted a simple one-pot method, using thiourea and cobalt chloride as raw materials, to successfully synthesize CoS2 containing sulfur vacancies. Other synthesis methods include solid-state synthesis, electrochemical synthesis [74], liquid-phase growth [75], pyrolysis [76,77], wet chemical solid-state synthesis [78], chemical etching [78], annealing [79,80], calcination, and cation exchange [81,82]. Huang et al. [83] prepared a CoS2 nanowire array supported on carbon cloth (MoS2/CoS2/CC) by a two-step method, which had excellent electrochemical durability.
3.2.6. Comparison of Synthesis Methods
The synthesis strategy of catalysts directly affects their microstructure and catalytic performance. Existing methods have their own characteristics: The hydrothermal method, with its mild reaction conditions, can precisely control the morphology of the nanostructures (such as CoS nanospheres), and it is cost-effective and suitable for large-scale production. However, it has the defect of insufficient crystallinity. The electrochemical deposition method can construct three-dimensional vertical arrays (Ni-Co-S/GF) on conductive substrates (such as graphene foam) through potential regulation, but it is limited by the selectivity of the electrolyte system. The liquid-phase exfoliation method is suitable for preparing single-layer two-dimensional materials (NiPS3), which can effectively retain the intrinsic catalytic sites. However, the exfoliation efficiency and the uniformity of the number of layers still need to be optimized. Although chemical vapor deposition (CVD) can prepare high-quality thin film materials (1T’-RexMo1−xS2), the high equipment cost and strict process conditions limit its industrial application. The plasma-assisted method can quickly introduce sulfur vacancies (MoSx) through the bombardment of high-energy particles, but it may cause damage to the crystal structure. A comparison of the synthesis methods is shown in Table 2.

Table 2.
Comparison of synthesis methods.
3.3. Performance Optimization of Excessive Metal Sulfides
3.3.1. Doping Engineering
Transition metal sulfides exhibit structural diversity primarily governed by distinct metal coordination geometries, manifesting in two predominant crystalline phases: the octahedral 1T-phase and the trigonal prismatic 2H-phase. Current research focuses on two fundamental optimization strategies for enhancing the catalytic performance of these sulfides—either through improving charge transfer kinetics to enhance their electrical conductivity or by maximizing the density of catalytically active sites. For the former approach, synergistic electronic coupling between sulfide catalysts and carbon matrices has been demonstrated to facilitate efficient electron transport pathways, as evidenced by the MoSx nanostructures anchored on carbon nanotube networks that simultaneously enhance hydrogen evolution reaction (HER) activity through improved charge transfer and active site stabilization [84,85]. Regarding active site engineering, optimal catalytic performance requires balanced adsorption/desorption thermodynamics for reaction intermediates, necessitating the precise modulation of binding energies while maintaining structural integrity under operational conditions. Carbon-based substrates offer additional advantages through their tunable physicochemical properties, where the strategic design of porosity and surface architecture can synergistically increase active site exposure and improve mass transport characteristics. This dual-functional optimization—combining electronic structure engineering with nanoscale substrate design—represents a promising paradigm for developing high-performance sulfide-based electrocatalysts with enhanced stability in both acidic and alkaline media.
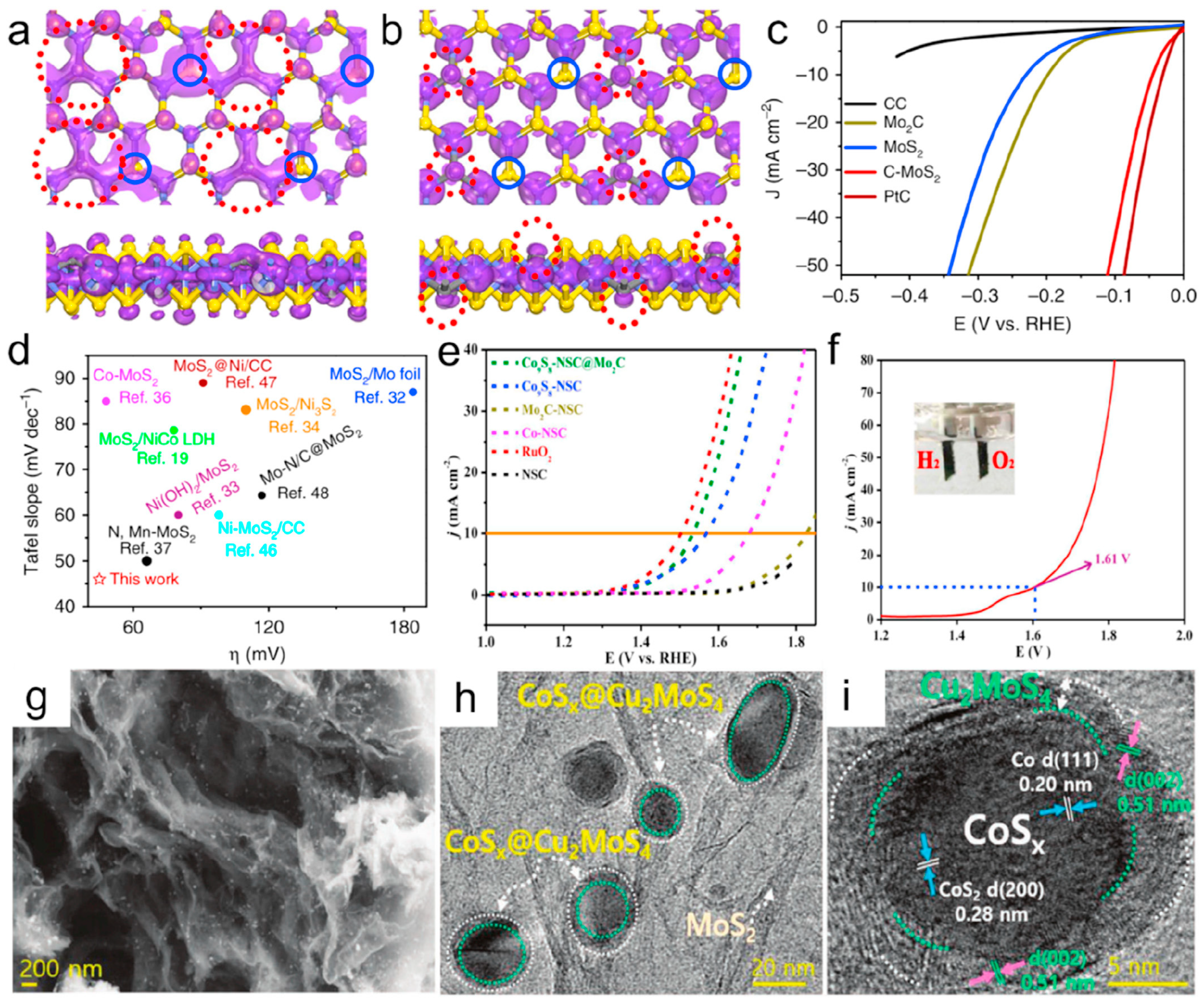
N and S doping: Chemical doping has emerged as an effective strategy to modulate electronic configurations and impart novel functionalities to carbon-based materials. Kim et al. fabricated molybdenum sulfide-doped carbon nanotubes (MoSx-CNTs) through controlled synthesis, demonstrating an overpotential of 110 mV at 10 mA cm−2 for hydrogen evolution reactions [86]. Substantial progress has been achieved in nitrogen-doped graphene systems, with an advanced mechanistic understanding revealing that nitrogen incorporation induces topological defect formation and structural modifications in both edge and basal plane carbon architectures [87]. Beyond monoatomic doping, sulfur has been explored as a complementary dopant, while its synergistic effects in heteroatomic systems have motivated the development of N/S co-doping strategies. For instance, Liu et al. engineered core–shell CoS2@Co nanoparticles encapsulated within N,S-co-doped carbon nanotubes, showcasing their enhanced catalytic activity [88]. The Peng group innovatively synthesized N,Se-co-doped graphene (N,SeG) via in situ calcination and demonstrated its bifunctional capability for overall water splitting [89]. Despite progress in acidic media, the alkaline hydrogen evolution reaction (HER) performance of MoS2 remains suboptimal due to its poorly understood inactive nature. This challenge was addressed by Wang et al., who constructed vertical 2PZ orbitals on MoS2 basal planes through in situ carbon doping (Figure 5a,b). This structural modification facilitated electron transfer and water molecule coupling kinetics, yielding a record-breaking alkaline HER performance (Figure 5c,d) [90]. Cobalt sulfide–carbon composites represent another promising electrocatalytic material class. Recent advancements have been achieved in precursor selection and synthesis optimization for various CoxSy phases (Co9S8, Co4S3, CoS). Notably, Zhang et al. developed sustainable synthesis routes using biowaste-derived carbon/nitrogen sources from shrimp shells, significantly reducing the production complexity [91]. Concurrently, the systematic control of S/N ratios in precursors has enabled precise dopant regulation in catalysts [92]. While bifunctional water electrolysis catalysts attract growing interest, material-specific activity limitations persist. Current research focuses on the strategic integration of complementary materials to overcome the inherent single functionality of most catalysts. Taking Co9S8 as an example, Co9S8 is inactive for HERs in acidic environments, and so a reasonable combination with MoS2 is an effective strategy. Liu et al. designed a hierarchical porous structure of Co9S8eNSC@Mo2C [76] (see Figure 5e,f), and Lee synthesized a core–shell structure of CoSx@Cu2MoS4 [93] (see Figure 5g–i), both of which demonstrated outstanding overall water splitting performance.
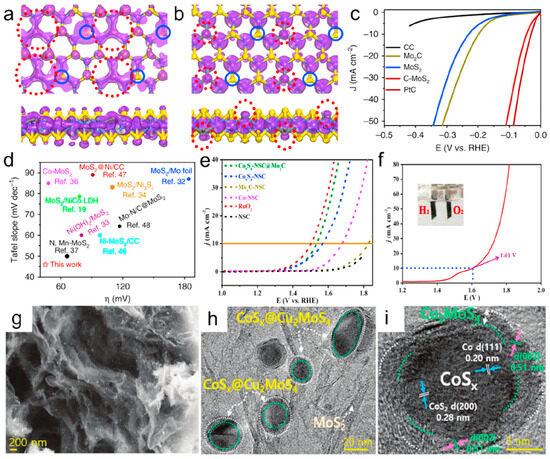
Figure 5.
The top view and side view of SP2 hybrid orbitals (highlighted by red dash circle) at the top of the valence band (a), and the empty 2p orbitals (highlighted by red dash circle) perpendicular to the basal plane at the bottom of the conduction band (b) of CeMoS2. (c) The LSV curves of CC, Mo2C, CeMoS2, MoS2, and Pt/C with IR correction. (d) Performance comparison of CeMoS2 with the ever-reported MoS2-based catalysts in alkaline conditions [90], Copyright 2019, Nat. Commun. (e) OER polarization curves of the Co9S8eNSC@Mo2C, Co9S8eNSC, CoeNSC, Mo2CeNSC, RuO2, and NSC. (f) Polarization curves of water electrolysis for the Co9S8eNSC@Mo2C/NF||Co9S8eNSC@Mo2C/NF in 1.0 M KOH [76], Copyright 2018, ACS Appl. Mater. Interfaces. (g) FEeSEM image and (h,i) HReTEM image of CoSx@Cu2MoS4eMoS2/NSG hybrid [93], Copyright 2020, Adv. Energy Mater. For the interpretation of the references to color in this Figure legend, the reader is referred to the web version of this article.
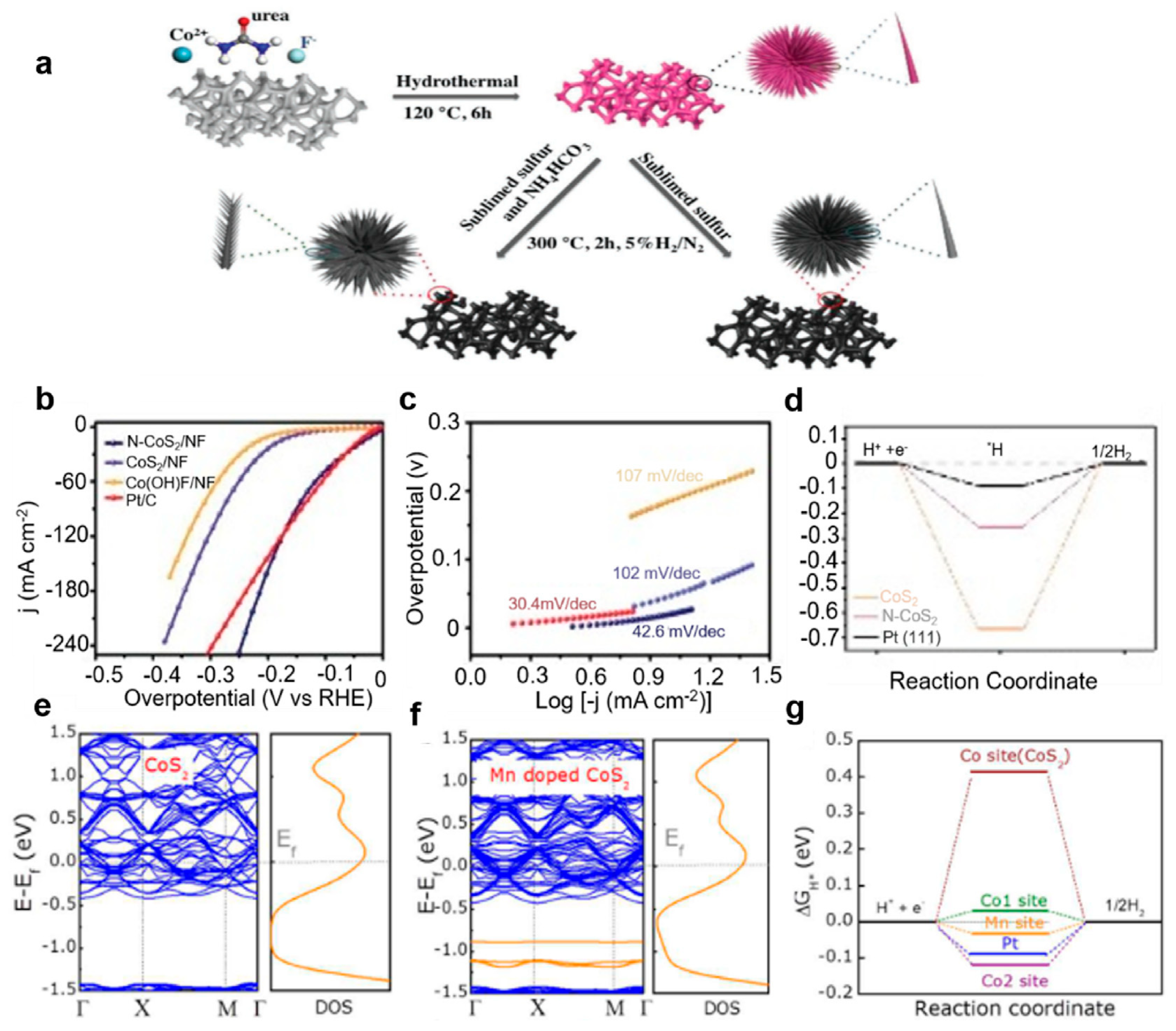
N doping: The Luo group [70] achieved a remarkable enhancement in the HER performance of CoS2 by engineering a three-dimensional, dandelion-flower-like, nitrogen-doped CoS2 self-supported electrode (Figure 6a). This innovative architecture demonstrated exceptional catalytic efficiency, requiring only 28 mV overpotential to deliver a current density of 10 mA cm−2 in alkaline media (Figure 6b–e), surpassing most reported transition metal sulfide (TMS)-based electrocatalysts. Mechanistic insights from density functional theory (DFT) calculations reveal that nitrogen doping strategically modulates the d-band center of CoS2, simultaneously optimizing hydrogen intermediate adsorption free energy (ΔG*H) and water molecule activation energy (ΔG*H2O), thereby synergistically accelerating alkaline HER kinetics (Figure 6d). Parallel efforts by Zhang et al. [94] employed phosphorus doping via hydrothermal synthesis to fabricate CoS2-based catalysts, achieving a competitive overpotential of 53 mV at 10 mA cm−2. Their study highlighted the dual advantages of P doping: enhancing active site density while preserving the intrinsic metallic conductivity of CoS2. Furthermore, phosphorus incorporation effectively weakened hydrogen intermediate binding at Co sites (ΔG*H), contributing to the improved HER activity. Catalyst interface engineering emerged as another critical design strategy. The Tan group [95] developed selenium-doped CoS2 nanowire arrays with electronically reconfigured surfaces that optimized intermediate adsorption. Notably, the engineered superhydrophobic interface facilitated rapid hydrogen bubble detachment, significantly reducing mass transfer limitations and boosting operational efficiency. Beyond anion doping, cation substitution strategies have demonstrated profound impacts on catalytic stability. Transition metal doping strengthens metal–support interactions by eliminating antibonding orbital occupancy while maintaining favorable electronic structures [96]. Systematic investigations by Gao et al. [97] established a volcano-type correlation between computed hydrogen adsorption free energies (ΔGH*) on transition-metal-doped CoS2 (001) surfaces and experimental exchange current densities (Figure 6e–g). Theoretical predictions identified Mn-doped CoS2 as an optimal system, where Mn incorporation simultaneously reduced ΔGH at both the Co and Mn active centers while retaining bulk metallic conductivity. Experimental validation confirmed exceptional performance for the 5% Mn-doped catalyst, exhibiting a 43 mV overpotential at 10 mA cm−2 with outstanding operational stability. This work further elucidated the thermodynamic feasibility of doping processes through formation energy calculations, establishing a predictive framework for catalyst design.
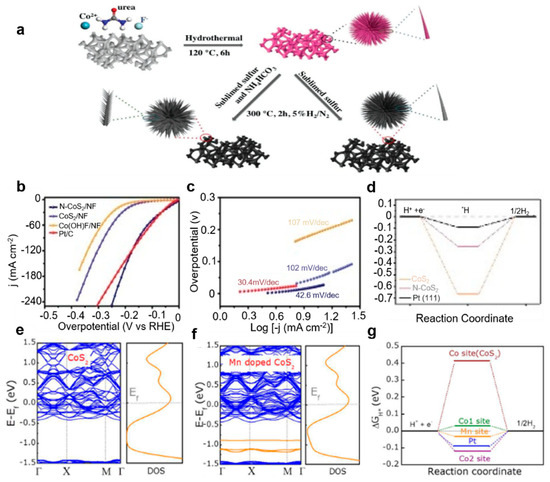
Figure 6.
(a) Schematic illustration of the synthetic process of N-CoS2 and CoS2. (b) LSV curves, (c) Tafel slope, and (d) free energy of Co(OH)F, N-CoS2, CoS2, and Pt/C [70]. With permission from John Wiley & Sons, Inc. (e,f) Electronic band and density of state (DOS) results and (g) free energy of H adsorption of CoS2 and Mn-CoS2 [97]. By the license of the American Chemical Society.
3.3.2. Engineering Vacancy Defects
In the field of two-dimensional transition metal dichalcogenide (2D-TMD) research, the intentional engineering of vacancy defects has emerged as an effective strategy for performance optimization in catalytic applications. Substantial studies have demonstrated that the controlled introduction of vacancy defects enables the precise modulation of key material characteristics, including localized electronic states, morphological configurations, elemental distributions, and crystallographic arrangements, thereby significantly enhancing the intrinsic catalytic properties of TMD-based materials [98,99,100,101,102,103,104]. From a structural classification perspective, these engineered vacancy defects are broadly categorized into two principal types: anion-deficient vacancies and cation-deficient vacancies, each exhibiting distinct physicochemical properties that influence catalytic behavior.
Cation vacancies, such as Mo vacancies [105], Ni vacancies [106], and Re vacancies [107,108], are typically generated through the selective removal of metal cations from transition metal dichalcogenide lattices. These structural imperfections have demonstrated tunable electrocatalytic hydrogen evolution reaction (HER) activity through precise electronic structure modulation [109,110]. Specifically, He et al. fabricated Ni-deficient Ni3S2 nanosheet arrays on nickel foam substrates via controlled vacancy engineering, achieving an exceptional alkaline HER overpotential of 35 mV at 10 mA cm−2 [111]. Density functional theory (DFT) calculations revealed that the extraction of interior Ni atoms destabilized the atomic framework of Ni3S2, triggering electronic reconfiguration and reducing charge transfer barriers, thereby enhancing hydrogen evolution reaction (HER) kinetics. Zeng et al. demonstrated that cobalt vacancy engineering in nitrogen-doped CoS, achieved via the in situ sulfidation of Co3O4 precursors, yielded an efficient HER catalyst [112]. Computational analyses indicated that sulfur atoms adjacent to vacancy sites functioned as supplementary active centers for the HER intermediates. Furthermore, the coordinated effects of cobalt vacancy formation and nitrogen doping induced bandgap narrowing in cobalt sulfides, optimizing the charge/mass transport dynamics during catalytic processes.
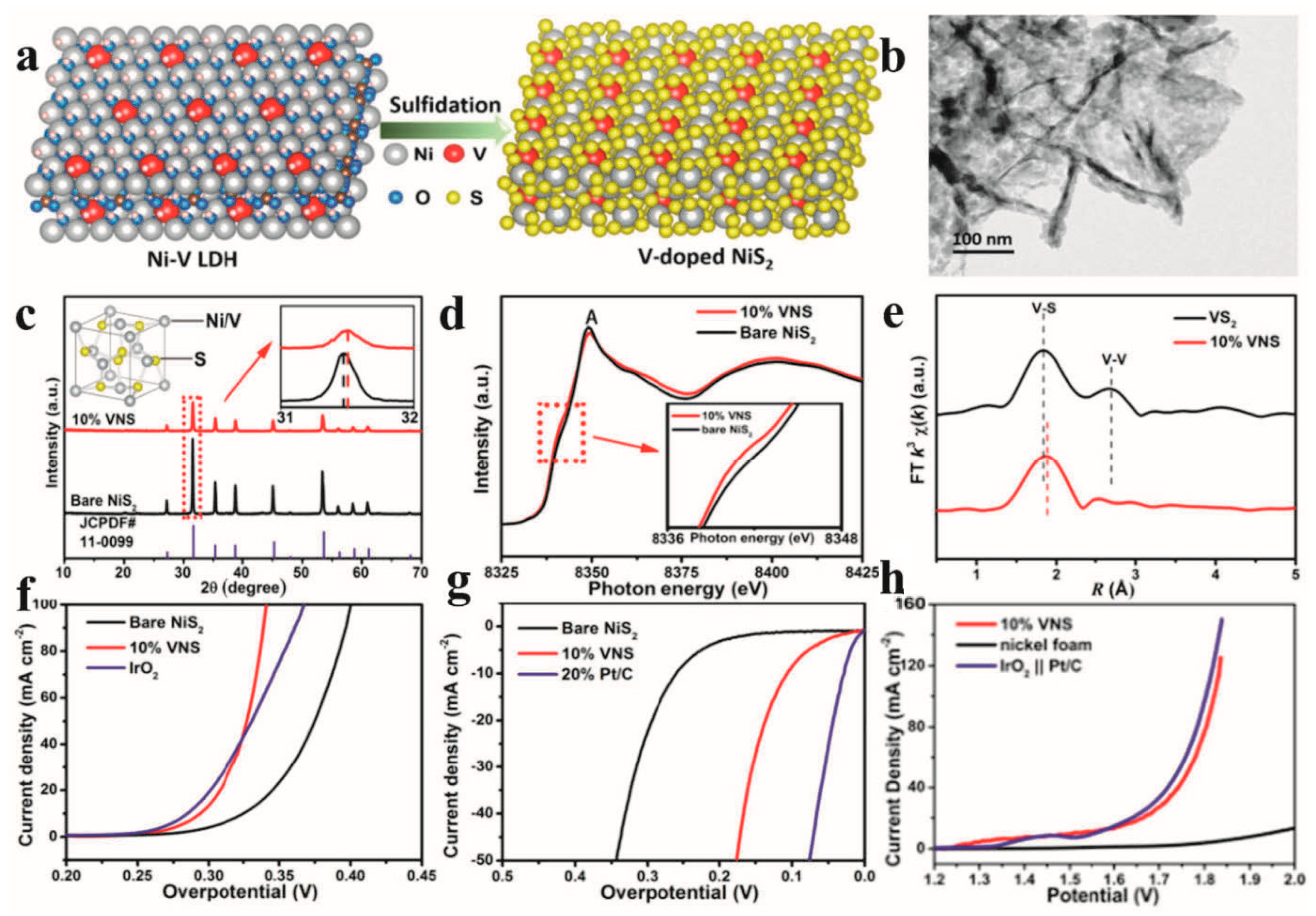
Liu et al. [113] synthesized vanadium-doped pyrite NiS2 nanosheets via the sulfidation of nickel–vanadium layered double hydroxide (Ni-V LDH) precursors, demonstrating their bifunctional electrocatalytic activity for HERs (Figure 7a). Structural characterization revealed that the 10% V-doped NiS2 retained the characteristic pyrite morphology (Figure 7b), while X-ray diffraction (XRD) analysis exhibited a slight shift in diffraction peaks toward higher angles compared to pristine NiS2 (Figure 7c), suggesting that lattice contraction was induced by the substitution of smaller V atoms for Ni vacancies. Crucially, combined experimental and DFT investigations disclosed that vanadium substitutional defects induced a semiconductor-to-metallic transition in the electronic structure of NiS2. X-ray absorption spectroscopy (XAS) further confirmed the electron redistribution from V dopants to adjacent Ni sites (Figure 7d,e), effectively enriching the electron density at Ni active centers. The optimized V-doped NiS2 nanosheets achieved exceptional catalytic performance, requiring overpotentials of merely 110 mV for an HER at 10 mA cm−2 (Figure 7f,g), coupled with robust operational stability. Remarkably, the material demonstrated superior efficiency in overall water splitting when configured as a dual-functional electrode (Figure 7h). In a complementary study, Li et al. [114] developed a scalable two-step synthesis protocol to fabricate Ni-Fe-P-Ni3S2 heterostructures on nickel foam (Ni-Fe-P-Ni3S2/NF). The engineered catalyst exhibited abundant lattice defects, hierarchical porosity, and synergistic electronic interactions between the Ni-Fe-P alloy and Ni3S2 phases. These structural advantages endowed the material with exceptional catalytic activity and durability for HER, OER, and overall water splitting in alkaline electrolytes, attributed to its high surface area, exposed Ni edge sites, and optimized charge transfer kinetics.
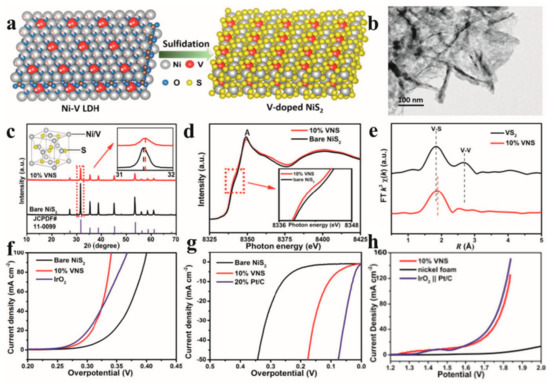
Figure 7.
(a) Schematic illustration of the synthesis process for V-doped NiS2. (b) TEM image of 10% VNS. (c) PXRD patterns of bare NiS2 and 10% VNS (inset: zoomed-in view of XRD patterns). (d) Ni K-edge XANES of bare NiS2 and 10% VNS (inset: zoomed-in view of the Ni K-edge XANES). (e) FT V K-edge FT(k3(χ(k))) of VS2 and 10% VNS. (f,g) LSV polarization curves of bare NiS2, IrO2, Pt/C, and 10% VNS for OER and HER. (h) LSV curves of the typical two-electrode system using nickel foam supported 10% VNS as both the cathode and anode in 1 mol L−1 KOH. Reprinted with permission from Ref. [113], Copyright 2017, American Chemical Society.
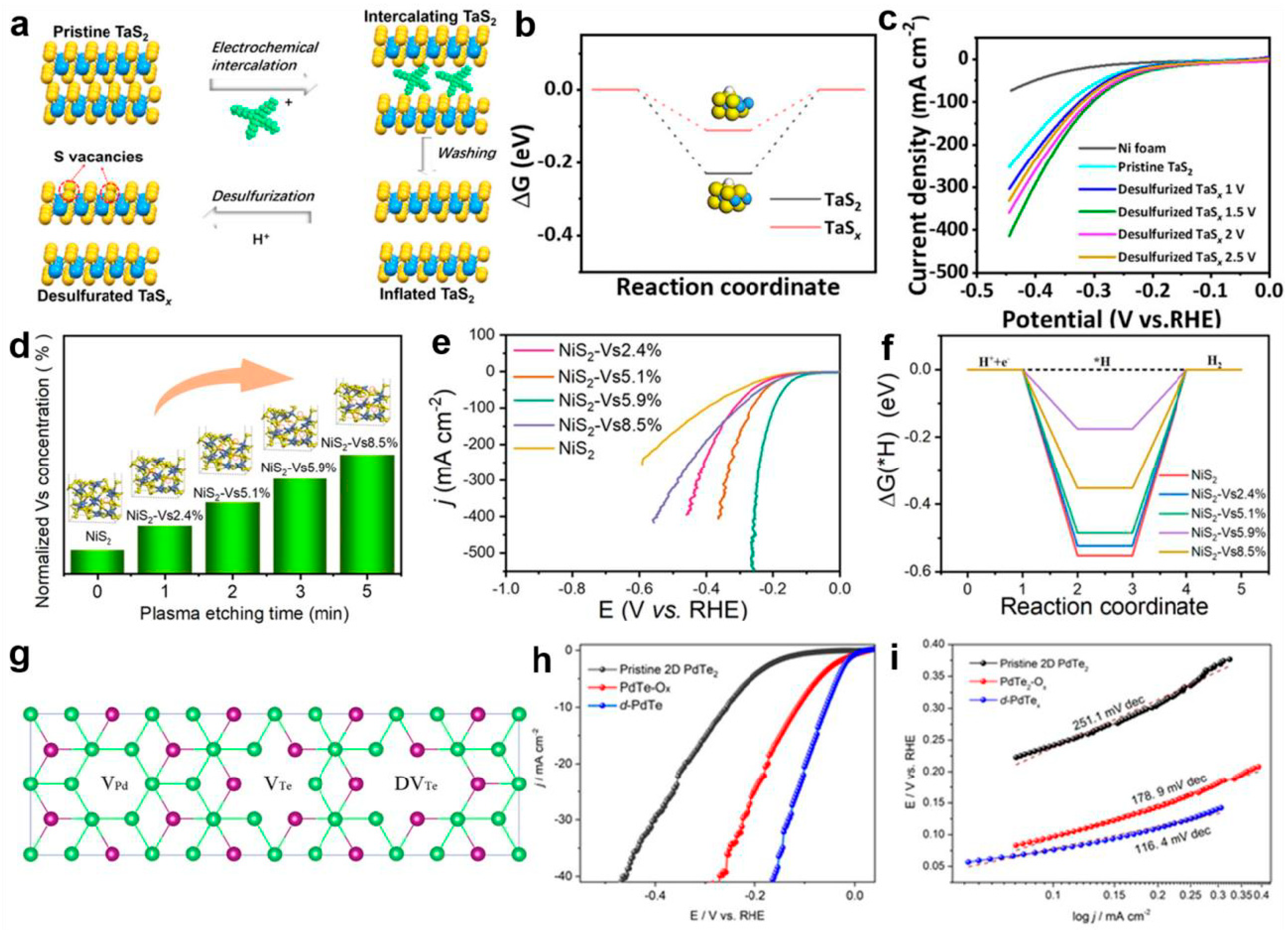
Anion vacancy: Because the carbon atom has a lighter atomic mass, it has fewer coordination structures. Specifically, in TMD systems, this characteristic leads to the prevalence of S vacancies. These vacancies on the TMD basal plane induce local structural changes, providing more active sites for catalytic reactions, which is an important aspect of TMD vacancy defect engineering. They can be introduced through various methods, such as electrochemical reduction [115], PH3 thermal chemical annealing, H2O2 chemical etching, Ar plasma treatment [116], and liquid-phase ligand exchange [117]. Cao et al. prepared desulfurized tantalum sulfide nanosheets (denoted as TaSx NSs) at room temperature using an electrochemical reduction method, as shown in Figure 8a [115]. First-principles calculations revealed that introducing sulfur vacancies substantially modulated the hydrogen adsorption free energy (ΔGH*) in TaSx systems, elevating its value from −0.231 eV in pristine TaS2 to −0.109 eV (Figure 8b). This electronic structure modification enhanced the kinetics of electron transfer between adsorbed hydrogen species and the TaSx matrix while reducing the activation barrier for the hydrogen evolution reaction (HER). The optimized TaSx catalyst demonstrated superior electrocatalytic performance under acidic conditions, achieving a low overpotential of 99 mV at 10 mA cm−2, accompanied by a favorable Tafel slope of 39 mV dec−1 (Figure 8c). Recent advancements by Jin et al. [116] employed Ar plasma etching to engineer uniform sulfur vacancies on NiS2 nanosheet surfaces. Through the systematic regulation of the etching duration, they achieved precise control over the vacancy density and corresponding HER activity. The optimal sample with 5.9% sulfur vacancies (3 min etching, Figure 8d,e) exhibited an onset potential of 68 mV and sustained operational stability over 100 h in alkaline media. Computational analyses confirmed that controlled sulfur vacancy concentrations optimized H* adsorption energetics at active sulfur sites, with a calculated ΔGH* of −0.17 eV (Figure 8f), leading to an enhanced HER performance. Beyond monovacancy engineering, recent studies demonstrated that the strategic introduction of mixed anion vacancies (Se [118], Te [119], or hybrid vacancy configurations [120]) can synergistically optimize HER activity through tailored spatial distribution in transition metal dichalcogenides (TMDs). Lai et al. [121] systematically investigated 1T’ phase ReS2xSe2(1-x) (x = 0–1) nanodots synthesized via chemical vapor transport coupled with lithium intercalation. These asymmetric nanostructures maintained metastable 1T’ phase characteristics while hosting multiple anion vacancy types. Combined experimental and theoretical evidence revealed that low-coordination Re sites adjacent to sulfur vacancies exhibited enhanced charge density, effectively activating the basal plane of 1T’-ReSSe for catalytic processes. The rational design of multivalent vacancy systems presents new opportunities for defect engineering optimization [122]. Zuo et al. [100] conducted comparative studies on three distinct vacancy types in 2D-PdTe2 systems: palladium monovacancies (VPd), tellurium monovacancies (VTe), and ditellurium divacancies (DVTe) (Figure 8g). A characteristic volcano relationship emerged between the exchange current density (i0) and ΔGH*, confirming the vacancy-induced enhancement of charge transfer kinetics [123]. Electrochemical characterization revealed that optimized d-PdTex catalysts achieved an overpotential of 76 mV with a Tafel slope of 116.4 mV dec−1 in acidic media (Figure 8h,i), consistent with theoretical predictions. Similar defect engineering strategies have been successfully applied to VSe2 [102] and MoSe2 systems [124], where the controlled introduction of vanadium/selenium and molybdenum/selenium vacancies, respectively, generated intermediate energy states near the Fermi level, facilitating efficient electron transfer pathways. Notably, advanced synthesis techniques such as ion beam irradiation enabled the precise creation of multiple vacancy types on MoSe2 basal planes, simultaneously increasing active site density and improving electrical conductivity [125]. Collectively, these investigations demonstrate that vacancy defects serve as critical modulators of TMD electronic structures, significantly influencing the HER’s thermodynamics and kinetics. However, achieving optimal catalytic performance requires meticulous control over defect concentrations—insufficient vacancies provide limited electronic modulation, while excessive defect densities may induce structural degradation and charge transport barriers. Future research should focus on developing advanced characterization techniques coupled with machine-learning-assisted synthesis optimization to establish quantitative structure–activity relationships. Such efforts will enable the rational design of TMD-based catalysts that balance vacancy-induced activity enhancement with long-term structural integrity and charge transfer efficiency.
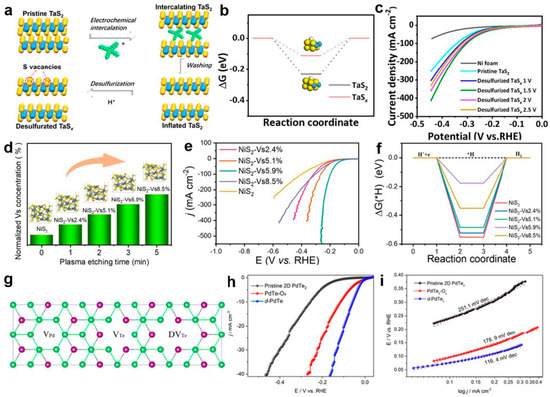
Figure 8.
(a) Schematic synthetic procedure of TaS2 nanosheets by the electrochemical exfoliation of the TaS2 crystal followed by a desulfurization reaction. (b) ΔGH* diagrams of TaS2 NSs and TaSx NSs at zero potential and pH = 0. (c) Polarization curves without the iR correction of TaS2 NSs and TaSx NSs at different desulfurization voltages in 0.5 M H2SO4 at a scan rate of 5 mV s−1. Adapted with permission [115], Copyright 2023, American Chemical Society. (d) Histogram of the calculated S vacancy concentrations. (e) Polarization curves with iR correction, and (f) ΔGH* diagrams of the NiS2 nanosheets with a series of S vacancy concentrations. Adapted with permission [116], Copyright 2024, Springer Nature. (g) Illustration diagram of three different possible vacancy defects in a PdTe2 crystal, including single-vacancy of the Pd atom (VPd), single-vacancy of the Te atom (VTe), and double Te vacancy (DVTe). (h) The polarization curves and (i) the corresponding Tafel plots of the three catalysts in 0.5 M H2SO4 solution at a scan rate of 5 mV s−1. Adapted with permission [100], Copyright 2023, American Chemical Society.
3.3.3. Constructing Heterostructures
Heterostructures are typically constructed to leverage the synergistic effects among different materials for the development of highly efficient hydrogen evolution reaction (HER) catalysts. The intense interaction between distinct materials can modify the charge distribution and electron transfer velocity at the heterointerface, thereby influencing the adsorption capacity for reaction intermediates [126,127,128].
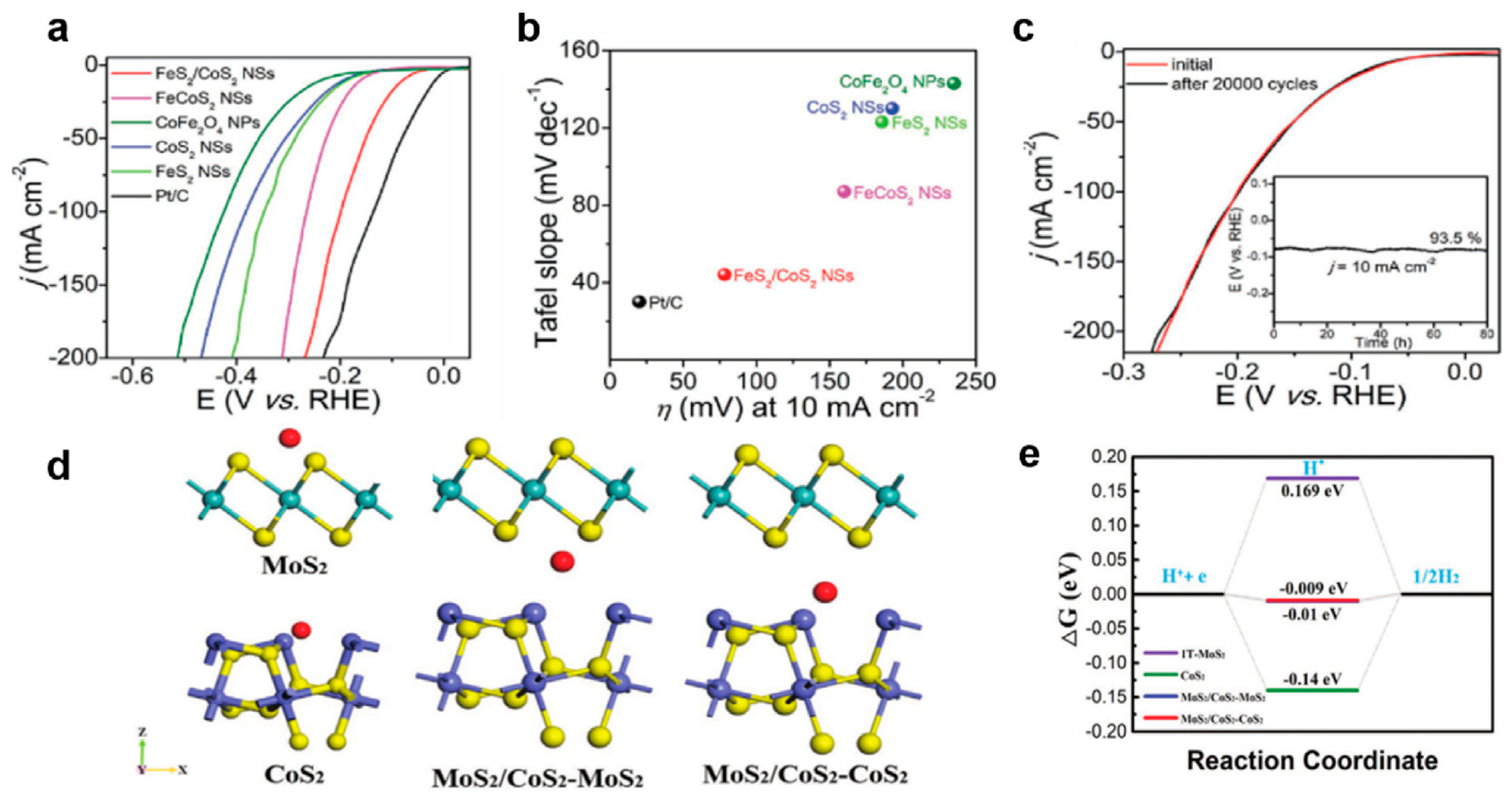
Multi-dimensional heterogeneous structure: The researchers Li et al. [129] successfully fabricated a heterostructure of defect-rich FeS2 and CoS2. Studies have indicated that the two-dimensional structure can expose more active edge sites, and the defect sites at the interface are conducive to the adsorption of active substances. The FeS2/CoS2NSs manifested an overpotential of 78.2 mV at 10 mA cm−2 for the HER (see Figure 9a–e) and could stably operate for 80 h in 1 M KOH solution. When the current density reached 100 mA cm−2, the overpotential for OER was 302 mV, and the voltage only decreased by 1% after 70 h of cycling. The catalyst demonstrated structural integrity following prolonged water electrolysis operation (Figure 9c). Interface-specific characterization revealed the selective oxidation of cobalt and sulfur elements during the oxygen evolution reaction (OER), while these components remained chemically stable under the hydrogen evolution reaction (HER) conditions. Song et al. [130] developed an advanced three-dimensional heterostructure through the in situ growth of MoS2/CoS2 heterointerface nanosheets on one-dimensional nanorod arrays. This configuration induced a phase transformation in MoS2 (2H → 1T phase) through strong interfacial electronic coupling, simultaneously increasing active site density and improving charge transfer efficiency. The optimized 1T-MoS2/CoS2 catalyst achieved exceptional overpotentials of 71 mV (alkaline) and 26 mV (acidic) at 10 mA cm−2 current density. Density functional theory (DFT) calculations revealed near-optimal hydrogen adsorption energy (ΔGH* = 0.009 eV) at the heterojunction interface, confirming its superior HER catalytic performance (Figure 9d–e). Current heterostructure design strategies typically employ high-surface-area conductive substrates (e.g., nickel foam, carbon paper, carbon cloth) to maximize interface exposure. However, these conventional substrates exhibit limited intrinsic catalytic activity. Recent studies have suggested that substrates combining both high conductivity and catalytic functionality could enable more efficient HER systems. Following this principle, Guo et al. [131] engineered a CoS2/CoSe2 composite by immobilizing size-controlled CoS2 nanoparticles on catalytically active CoSe2/diethylenetriamine (DETA) nanobelts. The amino-functionalized surface mediated nanoparticle nucleation while preventing aggregation. Benefiting from strong CoS2-CoSe2 synergism, this architecture demonstrated a remarkable acidic HER performance with an 80 mV overpotential at 10 mA cm−2 and a Tafel slope of 33.6 mV dec−1, alongside enhanced durability from the optimized nanoparticle dimensions. Alternative interface engineering approaches include electronic structure modulation through carbon hybridization. Xie et al. [132] developed a GDY/CoS2/CC heterostructure by coating graphdiyne (GDY) on CoS nanowire arrays supported by carbon cloth. Computational analysis revealed that sp-hybridized carbon atoms cooperatively reduced the electron transfer barrier at unsaturated sulfur sites on the (211) plane, facilitating hydrogen adsorption/desorption kinetics. In a separate strategy, Tang et al. [133] synthesized hollow CoS2@MoS2 nanowire arrays using Co-MOF precursors. This hierarchical architecture promoted rapid ion/charge transport through shortened diffusion pathways while achieving pH-universal HER activity via interfacial electronic optimization between the CoS2 and MoS2 components.
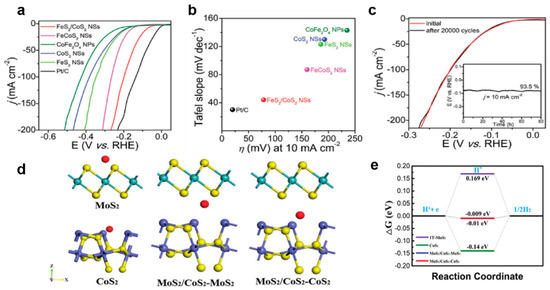
Figure 9.
(a) LSV curves and (b) Tafel slopes of various catalysts. (c) LSV curves of FeS2/CoS2 NSs before and after 20,000 cycles. With permission from John Wiley & Sons, Inc. [129]. (d) Theoretical models for different adsorption sites and (e) Gibbs free energy of different active sites [130]. By the license of John Wiley & Sons, Inc.
LDH heterogeneous structure: Recent advances in heterostructural engineering have demonstrated the efficacy of integrating transition metal dichalcogenides (MS2) with robust metal hydroxides to optimize alkaline hydrogen evolution catalysis. As a representative example, Hu et al. [134] developed a MoS2/NiCo-LDH [134,135,136] heterojunction system through the hybridization of molybdenum disulfide nanosheets with nickel–cobalt layered double hydroxide. This architecture exhibited exceptional alkaline HER performance, requiring only 78 mV overpotential to reach 10 mA cm−2 current density. Mechanistic analysis through density functional theory (DFT) simulations revealed that the interfacial coupling between MoS2 and NiCo-LDH facilitated the synergistic enhancement of hydrogen adsorption energetics on both components while simultaneously accelerating the rate-limiting water dissociation step, thereby improving overall HER kinetics in alkaline media. Furthermore, heterostructure fabrication through conductive template-supported growth has emerged as an effective strategy for kinetic enhancement. Chen et al. [137] achieved significantly improved HER activity (η10 = 139 mV) by embedding FeS2 nanoparticles within a graphene oxide matrix. The subsequent optimization of the catalyst composition and electrode architecture enabled the development of Fe0.54Co0.46S0.92-decorated carbon nanotubes on carbon cloth (CC), which demonstrated superior performance with an ultralow overpotential of 70 mV at 10 mA cm−2. The implementation of surface modification strategies, particularly phosphidation treatments (P/Co-FeS2), further enhanced catalytic functionality, yielding a system with η20 = 60 mV and a Tafel slope of 41 mV dec−1, indicative of optimized charge transfer kinetics [138].
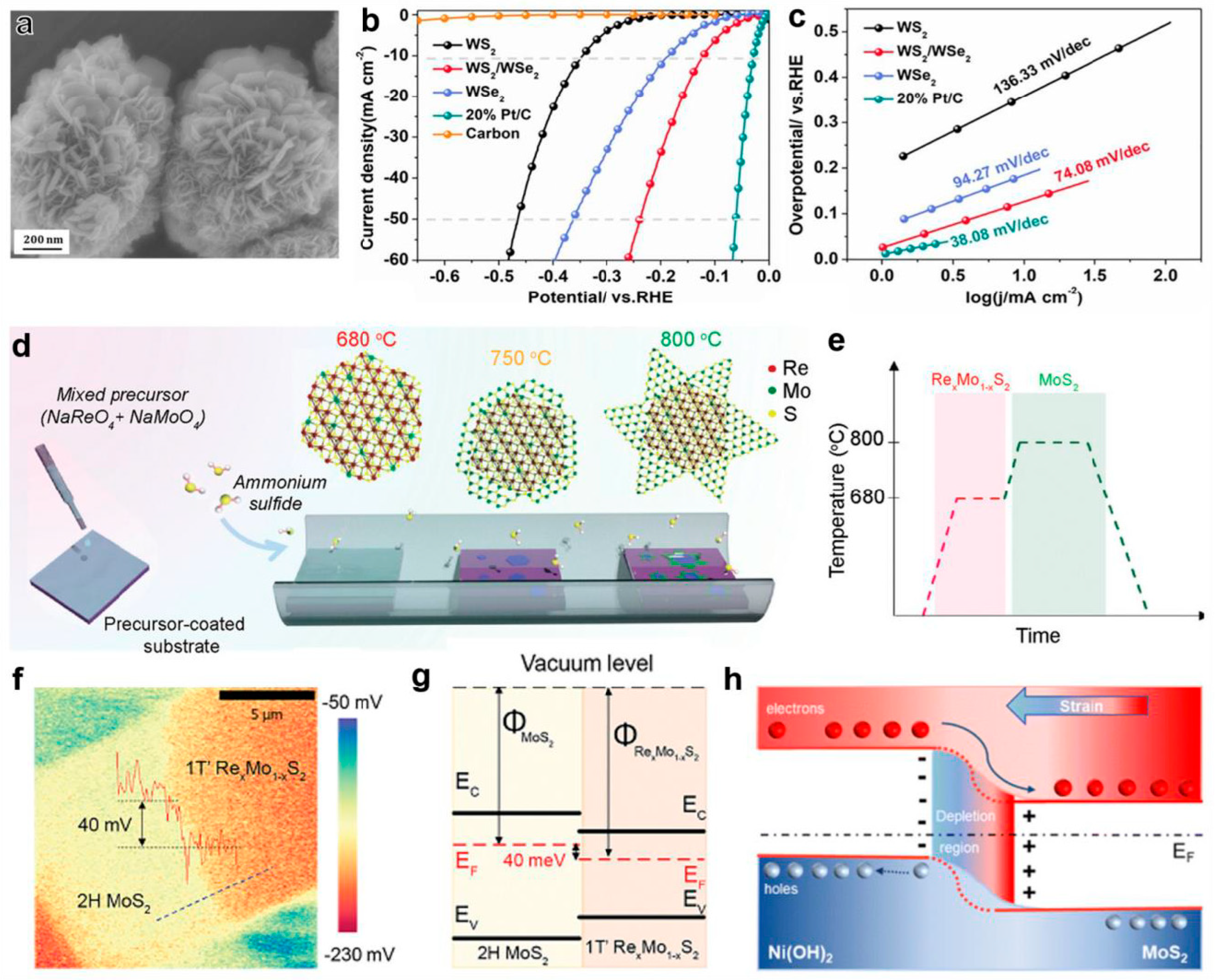
Floral heterostructure: Sun et al. prepared flower-like WS2 heterostructures via a simple one-step solvothermal method (Figure 10a) [139]. Electrochemical tests demonstrated that this catalyst exhibited excellent hydrogen evolution performance in acidic media: a low overpotential of only 121 mV at a current density of 10 mA cm−2, a Tafel slope of 74.08 mV dec−1, and a good stability (Figure 10b,c). The study pointed out that the similarity in crystal structure of WS2 facilitated the construction of the heterointerface, promoting the redistribution of charge density at the interface and effectively enhancing the electrocatalytic performance. Another study successfully fabricated a 1T’RexMo1-xS2/2H MoS2 lateral heterojunction via a two-step chemical vapor deposition method [140]. The specific process involved synthesizing the 1T’ phase matrix at 680 °C, followed by growing the 2H phase structure at its edge region at 800 °C (Figure 10d,e). This heterostructure achieved a low overpotential of 84 mV at 10 mA cm−2 and a Tafel slope of 58 mV dec−1 under acidic conditions. Surface potential tests (Figure 10f) revealed a 40 mV potential difference at the heterointerface, confirming the effective transfer of electrons from the 2H phase with a lower work function to the 1T’ phase (Figure 10g), which is the key mechanism for enhancing the HER efficiency. In addition, the FeV3O8/2H-MoS2 heterostructure (FVO/MS), prepared by the two-step hydrothermal method developed by Huang’s team, exhibited an overpotential of 160 mV at 10 mA cm−2 and an excellent kinetic parameter of 53 mV dec−1 under acidic conditions. The performance improvement was attributed to the synergistic effect between the materials: increased active site density, improved electrical conductivity, and an expanded BET-specific surface area. The Ni(OH)2/MoS2-NF heterostructure designed by Le et al. (for alkaline HERs) successfully induced an expansion of the MoS2 interlayer spacing and generated interfacial tensile strain by regulating the size of the Ni(OH)2 nanoparticles [141]. Theoretical calculations indicated that this strain-induced piezoelectric potential changed the electron transfer direction (Figure 10h), reduced the kinetic barrier, and simultaneously optimized the water dissociation process and hydrogen adsorption kinetics. It is worth noting that the in-plane MoS2/α-MoC heterostructure developed by the Cheng team, prepared by the thermal treatment of α-MoO3 nanoribbons with thiourea, has achieved highly efficient HERs under all pH conditions [142]. The performance improvement mechanism involves dual factors: the cooperative adsorption of hydrogen/hydroxyl species at the heterointerface between MoS2 and α-MoC (α-MoC promotes the cleavage of the H-OH bond), and the strain effect induced by lattice mismatch. This strain not only regulates the electronic structure but also generates a large number of molybdenum active sites in the interface region, significantly promoting the proton adsorption/desorption process.
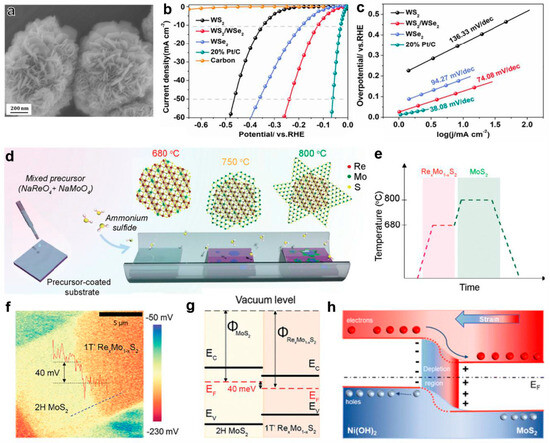
Figure 10.
(a) Scanning electron microscope image of the flower-like WS2/WSe2 heterostructure. (b) The polarization curves and (c) the corresponding Tafel plots of different catalysts for the HER in 0.5 M H2SO4. Adapted with permission [139], Copyright 2022, Elsevier. (d,e) Schematic illustration of the synthetic procedure and temperature profile of the 1T RexMo1−xS2-2H MoS2 lateral heterojunctions through an in situ two-step CVD growth. (f) Surface potential mapping image of the 1T’ RexMo1−xS2-2H MoS2 lateral heterostructure. The line profile across the interface in the inset shows a surface potential difference of 40 mV. (g) The band alignment of 2H MoS2 and 1T’ RexMo1−xS2. Adapted with permission [140], Copyright 2022, Wiley-VCH. (h) The electron transfer process of Ni(OH)2/MoS2 NFs with strain. Adapted with permission [141], Copyright 2023, Royal Society of Chemistry.
3.3.4. Ternary Sulfides
Among the single-metal sulfides, MoS2, due to its unique two-dimensional layered structure and abundant active edge sites, exhibits excellent hydrogen evolution reaction (HER) performance (η10 ≈ 39 mV) in an acidic environment. Catalytic activity can be further enhanced through element doping. For example, the overpotential of nitrogen-doped CoS2 (N-CoS2) under alkaline conditions is reduced to 28 mV, which is significantly better than that of conventional single-metal sulfides. Nickel-based sulfides (such as V-Ni3S2) regulate the electronic structure through vacancy engineering, and the overpotential for the alkaline HER reaches 35 mV. For binary/ternary sulfides, NiCo2S4 achieves η10 ≈ 89.7 mV (alkaline) through the bimetallic synergistic effect, while FeNiS nanosheets obtain an overpotential of 105 mV and a low Tafel slope of 40 mV dec−1 under acidic conditions by means of a topological transformation strategy. It is worth noting that the design of heterostructures, such as Co9S8@Mo2C, not only enhances stability (η10 ≈ 89 mV) but also optimizes the reaction kinetics through interfacial electron coupling. In terms of defect engineering, sulfur vacancy regulation (such as TaSx) and heteroatom doping (N/P-CoS2) significantly improve the intrinsic activity by optimizing the position of the d-band center. Through a comprehensive comparison, it is found that MoS2-based composite catalysts (such as 1T-MoS2/CoS2) have the best performance (η10 ≈ 26 mV) in an acidic system, while doped materials (N-CoS2) and heterostructure materials (MoS2/NiCo-LDH) have more advantages (η10 ≈ 28–78 mV) in an alkaline environment. Ternary sulfides, with their multi-metal synergistic effect and abundant active sites, exhibit a more excellent comprehensive performance than single-metal sulfides.
Ternary iron–nickel sulfides have also been confirmed to have better HER activity than single-metal sulfides. Long et al. designed a sulfide of iron and nickel (INS) with an excellent HER performance by using the topological transformation reaction of Ni-LDH [143]. Particularly, the metal α-INS nanosheets exhibited a low overpotential of 105 mA to reach a current density of 10 mA cm−2, with a very small Tafel slope of 40 mV dec−1. Piontek et al. further explored the influence of the Fe: Ni ratio and the HER operational temperature on the activity in (FexNi1−x)9S8 [144], and found that (FexNi1−x)9S8 with an Fe: Ni ratio of 1:1 had the most active sites, which endowed it with an excellent HER performance. Jiang et al. synthesized NiCo2S4 double-shell hollow spheres (NiCo2S4 BHSs) [145], and due to their superior chemical composition and unique structural features, NiCo2S4 BHSs had an overpotential of 89.7 mA to reach a current density of 10 mA cm−2 in an alkaline solution. Wu et al. proposed a general strategy of doping N into NiCo2S4 to weaken the strong H-S interaction and obtain a high HER activity [146]; the obtained N-NiCo2S4 had a Tafel slope of 37 mV dec−1 and an overpotential of 41 mA for the HER process to reach a current density of 10 mA cm−2. Sheng et al. assembled NiCo2S4/Pd heterostructures [147] and compared them with NiCo2S4; the prepared NiCo2S4/Pd exhibited stronger HER catalytic activity in both acidic and alkaline media, with the same overpotential of 83 mA at a current density of 10 mA cm−2. The contents of different sulfide catalysts are shown in Table 3.

Table 3.
Contents of different sulfide catalysts.
The long-term stability of catalysts is a key indicator determining their practical application value. Among MoS2-based materials, the 1T-MoS2/CoS2 heterostructure maintains stable operation for 30 h in acidic and alkaline media, respectively, and the MoS2/NiCo-LDH composite system shows no performance degradation after 50 h under alkaline conditions. For CoS2-based catalysts, nitrogen doping (N-CoS2) controls the increase in overpotential within 2% in 1 M KOH for 12 h, and the core–shell-structured CoS2@CoSe2 maintains good structural integrity for 20 h under acidic conditions. Ternary sulfides exhibit more excellent cycle stability. The NiCo2S4 double-shell hollow spheres maintain 90% of their activity after 5000 cycles, and the FeS2/CoS2 heterostructure achieves continuous operation for 80 h in 1 M KOH (η100 ≈ 302 mV). The main strategies for improving stability include the following: optimizing the electronic structure through sulfur/metal vacancy regulation to reduce the inactivation of active sites, constructing heterointerfaces (such as MoS2/α-MoC) to enhance corrosion resistance, and using conductive substrates such as carbon cloth/nickel foam to improve mechanical stability. Currently, alkaline-adapted materials such as N-CoS2 and NiCo2S4 have demonstrated the potential for application in industrial electrolyzers, while 1T-MoS2/CoS2 and P/Co-FeS2 perform prominently in proton exchange membrane electrolysis systems. In the field of flexible devices, the VGS/MoS2/FeCoNi(OH)x composite material supported on carbon fiber shows good prospects for wearable applications. However, it should be noted that most of the existing studies have been verified at the laboratory scale (<100 h), and there is an urgent need to establish a stability evaluation system at the thousand-hour level. At the same time, the high cost of the CVD and plasma methods restricts large-scale production, and it is necessary to develop process scaling-up schemes for the hydrothermal/electrodeposition methods to balance their performance and cost. Future research should focus on developing self-healing catalyst systems and establishing standardized analysis methods for degradation mechanisms. To compare the catalytic activity of these TMSs for HERs, the main parameters related to electrochemical performance, including overpotential at a given current density and Tafel slope, were obtained and are provided in Table 4.

Table 4.
Performance research of HER electrocatalysts based on TMSs.
4. Conclusions
Electrocatalytic overall water splitting for hydrogen evolution has emerged as a prominent research focus in sustainable energy technologies, owing to its environmentally benign nature, high-purity hydrogen yield, and simplicity in system design. Within this domain, transition metal sulfides (TMSs) have demonstrated catalytic performance comparable to benchmark noble metal catalysts (e.g., Pt for HERs), attributed to their cost-effectiveness, favorable overpotential characteristics, and exceptional operational stability. Furthermore, recent advances in mechanistic investigations have elucidated the fundamental catalytic pathways of TMS-based systems, thereby establishing a robust theoretical framework for the rational design of commercially viable electrocatalysts.
In terms of preparation methods, the synthesis technology for transition metal sulfides includes the hydrothermal method, the electrochemical deposition method, the liquid-phase stripping method, the chemical vapor deposition method, and so on. Through these methods, combined with modification methods such as recombination, introduction of defects, vacancy, and multiform regulation, the electronic structure can be adjusted, the number of active sites can be increased, the adsorption or desorption energy of reaction intermediates can be optimized, and the reaction energy barrier of the OER process can be reduced to improve the electrochemical performance of monometallic sulfide and polymetallic sulfide.
The results show that the relationship between the structure, composition, and properties of transition metal sulfides in the HER process is complex and profound. When more metals are introduced, the electronic structure and coordination environment near the active center change, especially in polymetallic sulfides, where the active site may be a metal cation. To improve the effectiveness of the catalyst, it may be necessary to use a mixture of multiple metals.
Despite the excellent performance of transition metal sulfides in HER processes, the understanding of the HER mechanism of bimetallic sulfides is still in the preliminary stage. Challenges including the economy, high performance, stability, and environmental friendliness need to be overcome before large-scale commercial applications can be realized. This involves the in-depth study of the design, preparation, and performance regulation of electrocatalysts, as well as the exploration of the mechanism of electrocatalysis, to promote the continuous development of the field of electrocatalysis.
Funding
No external funding was provided for this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
H. X. acknowledges support from the Hunan Provincial Department of Education project (No. 24C0264).
Conflicts of Interest
There is no conflict of interest in this article.
References
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Li, N.; Gao, Y.; Ge, L. NiCu alloys anchored defect-rich NiFe layered double-hydroxides as efficient electrocatalysts for overall water splitting. Chem. Eng. J. 2022, 446, 137226. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition metal-based bimetallic MOFs and MOF-derived catalysts for electrochemical oxygen evolution reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Zhang, Y.; Tian, N.; Ma, T.; Huang, H. Inside-and-Out Semiconductor Engineering for CO2 Photoreduction: From Recent Advances to New Trends. Small Struct. 2021, 2, 2000061. [Google Scholar] [CrossRef]
- Li, X.-L.; He, R.-B.; Dai, Y.-J.; Li, S.-S.; Xiao, N.; Wang, A.-X.; Gao, Y.-Q.; Li, N.; Gao, J.-F.; Zhang, L.-H.; et al. Design and fabrication of Co9S8/Zn0.5Cd0.5S hollow nanocages with significantly enhanced photocatalytic hydrogen production activity. Chem. Eng. J. 2020, 400, 125474. [Google Scholar] [CrossRef]
- Li, X.-L.; Yang, G.-Q.; Li, S.-S.; Xiao, N.; Li, N.; Gao, Y.-Q.; Lv, D.; Ge, L. Novel dual co-catalysts decorated Au@HCS@PdS hybrids with spatially separated charge carriers and enhanced photocatalytic hydrogen evolution activity. Chem. Eng. J. 2020, 379, 122350. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Liu, S.; Xu, B.; Xiao, N.; Gao, Y.; Song, W.; Ge, L.; Liu, J. In Situ Synthesis of Strongly Coupled Co2P-CdS Nanohybrids: An Effective Strategy To Regulate Photocatalytic Hydrogen Evolution Activity. ACS Sustain. Chem. Eng. 2018, 6, 9940–9950. [Google Scholar] [CrossRef]
- Jiang, J.; Li, F.; Su, H.; Gao, Y.; Li, N.; Ge, L. Flower-like NiCo2S4/NiFeP/NF composite material as an effective electrocatalyst with high overall water splitting performance. Chin. Chem. Lett. 2022, 33, 4367–4374. [Google Scholar] [CrossRef]
- Feng, B.; Guo, R.; Cai, Q.; Song, Y.; Li, N.; Fu, Y.; Chen, D.-L.; Zhang, J.; Zhu, W.; Zhang, F. Construction of isolated Ni sites on nitrogen-doped hollow carbon spheres with Ni–N3 configuration for enhanced reduction of nitroarenes. Nano Res. 2022, 15, 6001–6009. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Ma, W.; Wan, H.; Zhang, X.; Zhang, X.; Jiang, S.; Zheng, J.Y.; Zhou, Z. In Situ Anchoring Massive Isolated Pt Atoms at Cationic Vacancies of α-NixFe1−x(OH)2 to Regulate the Electronic Structure for Overall Water Splitting. Adv. Funct. Mater. 2022, 32, 2203342. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Jiang, S.; Zhang, L.; Ma, W.; Ma, R.; Zhou, Z. Controllable fabrication and structure evolution of hierarchical 1T-MoS2 nanospheres for efficient hydrogen evolution. Green Energy Environ. 2022, 7, 314–323. [Google Scholar] [CrossRef]
- Li, X.; Song, S.; Gao, Y.; Ge, L.; Song, W.; Ma, T.; Liu, J. Identification of the Charge Transfer Channel in Cobalt Encapsulated Hollow Nitrogen-Doped Carbon Matrix@CdS Heterostructure for Photocatalytic Hydrogen Evolution. Small 2021, 17, 2101315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, G.; Liu, X.; Ning, B.; Shi, C.; Pan, L.; Zhang, X.; Huang, Z.-F.; Zou, J.-J. Self-supporting NiFe LDH-MoSx integrated electrode for highly efficient water splitting at the industrial electrolysis conditions. Chin. J. Catal. 2021, 42, 1732–1741. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Atomically dispersed catalysts for hydrogen/oxygen evolution reactions and overall water splitting. J. Power Sources 2020, 471, 228446. [Google Scholar] [CrossRef]
- Peng, J.; Dong, W.; Wang, Z.; Meng, Y.; Liu, W.; Song, P.; Liu, Z. Recent advances in 2D transition metal compounds for electrocatalytic full water splitting in neutral media. Mater. Today Adv. 2020, 8, 100081. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Gao, Y.; Ge, L. Design and applications of hollow-structured nanomaterials for photocatalytic H2 evolution and CO2 reduction. Chin. J. Catal. 2022, 43, 679–707. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Deng, F.; Huang, J.; Han, L.; He, C.; Chen, Z.; Luo, Y.; Zhu, Y. Double-defect-induced polarization enhanced OV-BiOBr/Cu2−xS high-low junction for boosted photoelectrochemical hydrogen evolution. Appl. Catal. B Environ. 2022, 314, 121502. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.; Yang, H.; Ge, L.; Song, W.; Liu, J.; Ma, T.; et al. Electron Redistributed S-Doped Nickel Iron Phosphides Derived from One-Step Phosphatization of MOFs for Significantly Boosting Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2200733. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, L.; Li, X.; Gao, Y.; Li, N.; Lu, G.; Ge, L. Synthesis of ternary Ni2P@UiO-66-NH2/Zn0.5Cd0.5S composite materials with significantly improved photocatalytic H2 production performance. Chin. J. Catal. 2022, 43, 1295–1305. [Google Scholar] [CrossRef]
- Guan, J.; Bai, X.; Tang, T. Recent progress and prospect of carbon-free single-site catalysts for the hydrogen and oxygen evolution reactions. Nano Res. 2022, 15, 818–837. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Ultrafine Pt-Based Nanowires for Advanced Catalysis. Adv. Funct. Mater. 2020, 30, 2000793. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Low-Dimensional Metallic Nanomaterials for Advanced Electrocatalysis. Adv. Funct. Mater. 2020, 30, 2006317. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Zhao, Z.-J.; Li, A.; Chang, X.; Gong, J. Synergism of Geometric Construction and Electronic Regulation: 3D Se-(NiCo)S/(OH) Nanosheets for Highly Efficient Overall Water Splitting. Adv. Mater. 2018, 30, 1705538. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Sun, L.; Guo, H.; Tai, X.; Li, D.; Liu, L.; Ling, C.; Sun, X. Facilitating active species by decorating CeO2 on Ni3S2 nanosheets for efficient water oxidation electrocatalysis. Chin. J. Catal. 2021, 42, 482–489. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, X.; Sun, X.; Zhang, Y.; Wei, Q.; Liu, X.; Wu, D. In situ evolution of surface Co2CrO4 to CoOOH/CrOOH by electrochemical method: Toward boosting electrocatalytic water oxidation. Chin. J. Catal. 2021, 42, 1096–1101. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.; Xu, R. In situ formation of amorphous Fe-based bimetallic hydroxides from metal-organic frameworks as efficient oxygen evolution catalysts. Chin. J. Catal. 2021, 42, 1370–1378. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Xue, H.; Gao, J.; Peng, Y.; Song, W.; Ge, L. Mechanism investigation of PtPd decorated Zn0.5Cd0.5S nanorods with efficient photocatalytic hydrogen production combining with kinetics and thermodynamics. Chin. J. Catal. 2021, 42, 1677–1688. [Google Scholar] [CrossRef]
- Peng, X.; Qasim, A.M.; Jin, W.; Wang, L.; Hu, L.; Miao, Y.; Li, W.; Li, Y.; Liu, Z.; Huo, K.; et al. Ni-doped amorphous iron phosphide nanoparticles on TiN nanowire arrays: An advanced alkaline hydrogen evolution electrocatalyst. Nano Energy 2018, 53, 66–73. [Google Scholar] [CrossRef]
- Huang, C.; Pi, C.; Zhang, X.; Ding, K.; Qin, P.; Fu, J.; Peng, X.; Gao, B.; Chu, P.K.; Huo, K. In Situ Synthesis of MoP Nanoflakes Intercalated N-Doped Graphene Nanobelts from MoO3–Amine Hybrid for High-Efficient Hydrogen Evolution Reaction. Small 2018, 14, 1800667. [Google Scholar] [CrossRef]
- Li, Y.; Hu, L.; Zheng, W.; Peng, X.; Liu, M.; Chu, P.K.; Lee, L.Y.S. Ni/Co-based nanosheet arrays for efficient oxygen evolution reaction. Nano Energy 2018, 52, 360–368. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiong, C.; Chen, M.-M.; Wang, S.; Fu, L.; Zhang, X. Structure modulation of two-dimensional transition metal chalcogenides: Recent advances in methodology, mechanism and applications. Chem. Soc. Rev. 2023, 52, 1215–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Xue, Y.; Ji, Y.; Wang, X.; Wang, H.; Chen, X.; Zhang, X.; Tian, J. Heterostructuring noble-metal-free 1T’ phase MoS2 with g-C3N4 hollow nanocages to improve the photocatalytic H2 evolution activity. Green Energy Environ. 2023, 8, 864–873. [Google Scholar] [CrossRef]
- Wang, S.; Geng, Z.; Bi, S.; Wang, Y.; Gao, Z.; Jin, L.; Zhang, C. Recent advances and future prospects on Ni3S2-Based electrocatalysts for efficient alkaline water electrolysis. Green Energy Environ. 2024, 9, 659–683. [Google Scholar] [CrossRef]
- Yu, J.; Le, T.A.; Tran, N.Q.; Lee, H. Earth-Abundant Transition-Metal-Based Bifunctional Electrocatalysts for Overall Water Splitting in Alkaline Media. Chem.—A Eur. J. 2020, 26, 6423–6436. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Rao, T.; Wang, H.; Zeng, Y.-J.; Guo, Z.; Zhang, H.; Liao, W. Phase Transitions and Water Splitting Applications of 2D Transition Metal Dichalcogenides and Metal Phosphorous Trichalcogenides. Adv. Sci. 2021, 8, 2002284. [Google Scholar] [CrossRef]
- Hong, J.; Chen, X.; Li, P.; Koshino, M.; Li, S.; Xu, H.; Hu, Z.; Ding, F.; Suenaga, K. Multiple 2D Phase Transformations in Monolayer Transition Metal Chalcogenides. Adv. Mater. 2022, 34, 2200643. [Google Scholar] [CrossRef]
- Yu, J.; Seo, S.; Luo, Y.; Sun, Y.; Oh, S.; Nguyen, C.T.K.; Seo, C.; Kim, J.-H.; Kim, J.; Lee, H. Efficient and Stable Solar Hydrogen Generation of Hydrophilic Rhenium-Disulfide-Based Photocatalysts via Chemically Controlled Charge Transfer Paths. ACS Nano 2020, 14, 1715–1726. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Liu, X.; Zhao, W.; Liu, S.; Huang, X.; Zhao, Q. Tetra-Coordinated W2S3 for Efficient Dual-pH Hydrogen Production. Angew. Chem. Int. Ed. 2024, 63, e202316306. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, J.; Chen, K.; Chao, W.; Zhuang, Z.; Feng, S.; Chen, J.; Xie, L.; Wang, L.; Zhao, Q. MoSx nanowire networks derived from [Mo3S13]2−clusters for efficient electrocatalytic hydrogen evolution. Nano Res. 2024, 17, 6910–6915. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Sun, N.; Xie, L.; Zhi, T.; Zhang, Q.; Luo, Z.; Liu, X.; Liu, S.; Zhao, Q. Boosting hydrogen evolution on MoS2 via synergistic regulation of interlayer dislocations and interlayer spacing. Chem. Eng. J. 2023, 474, 145792. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Sun, M.-L.; Ren, J.-T.; Yuan, Z.-Y. Circumventing Challenges: Design of Anodic Electrocatalysts for Hybrid Water Electrolysis Systems. Adv. Energy Mater. 2023, 13, 2203568. [Google Scholar] [CrossRef]
- Ren, J.-T.; Chen, L.; Wang, L.; Song, X.-L.; Kong, Q.-H.; Yuan, Z.-Y. Multifunctional metal-phosphide-based electrocatalysts for highly efficient solar hydrogen production integrated devices. J. Mater. Chem. A 2023, 11, 2899–2909. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Lv, Z.; Liu, J.; Zhou, H.; Xu, C. Green hydrogen: A promising way to the carbon-free society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chinese J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Wirth, S.; Harnisch, F.; Weinmann, M.; Schröder, U. Comparative study of IVB–VIB transition metal compound electrocatalysts for the hydrogen evolution reaction. Appl. Catal. B Environ. 2012, 126, 225–230. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F. Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 14433–14437. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhou, Y.; Li, Y.; Guo, S.; Huang, X. MoS2 Nanosheet Assembling Superstructure with a Three-Dimensional Ion Accessible Site: A New Class of Bifunctional Materials for Batteries and Electrocatalysis. Chem. Mater. 2016, 28, 2074–2080. [Google Scholar] [CrossRef]
- Bao, S.-J.; Li, C.M.; Zang, J.-F.; Cui, X.-Q.; Qiao, Y.; Guo, J. New Nanostructured TiO2 for Direct Electrochemistry and Glucose Sensor Applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, X.; Qian, Y.; Jiang, H.; Zhou, L.; Li, B.; Lai, L.; Shen, Z.; Huang, W. Electrochemically Synthesis of Nickel Cobalt Sulfide for High-Performance Flexible Asymmetric Supercapacitors. Adv. Sci. 2018, 5, 1700375. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.-X.; Wang, Z.-L.; Meng, F.-L.; Zhang, X.-B. Integrated Three-Dimensional Carbon Paper/Carbon Tubes/Cobalt-Sulfide Sheets as an Efficient Electrode for Overall Water Splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef]
- Shen, J.; Wu, J.; Wang, M.; Dong, P.; Xu, J.; Li, X.; Zhang, X.; Yuan, J.; Wang, X.; Ye, M.; et al. Surface Tension Components Based Selection of Cosolvents for Efficient Liquid Phase Exfoliation of 2D Materials. Small 2016, 12, 2741–2749. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wang, Y.X.; Zeng, L.Y.; Yang, J.Z.; Song, X.L.; Rao, W.W.; Li, H.H.; Ning, Y.P.; He, H.B.; Li, T.; et al. Prevalence and Correlation of Anxiety, Insomnia and Somatic Symptoms in a Chinese Population During the COVID-19 Epidemic. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Zhou, D.; Yin, J.Z. Steer the Rheology of Solvent with Little Surfactan to Exfoliate MoS2 Nanosheet by Liquid Phase Exfoliation Method. Nano 2020, 15, 2050118. [Google Scholar] [CrossRef]
- Li, X.Z.; Fang, Y.Y.; Wang, J.; Wei, B.; Qi, K.; Hoh, H.Y.; Hao, Q.Y.; Sun, T.; Wang, Z.C.; Yin, Z.Y.; et al. High-Yield Electrochemical Production of Large-Sized and Thinly Layered NiPS3 Flakes for Overall Water Splitting. Small 2019, 15, e1902427. [Google Scholar] [CrossRef]
- Yun, Q.B.; Ge, Y.Y.; Shi, Z.Y.; Liu, J.W.; Wang, X.X.; Zhang, A.; Huang, B.; Yao, Y.; Luo, Q.X.; Zhai, L.; et al. Recent Progress on Phase Engineering of Nanomaterials. Chem. Rev. 2023, 123, 13489–13692. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Tanwar, S.; Arya, A.; Gaur, A.; Sharma, A.L. Transition metal dichalcogenide (TMDs) electrodes for supercapacitors: A comprehensive review. J. Phys.-Condens. Matter 2021, 33, 303002. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.W.; Hong, W.; Yang, S.Y.; Choi, S.Y. Tuning the catalytic functionality of transition metal dichalcogenides grown by chemical vapour deposition. J. Mater. Chem. A 2017, 5, 14950–14968. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Wang, F.; Shifa, T.A.; Wen, Y.; Wang, F.; Xu, K.; Wang, Z.; Jiang, C.; He, J. Dendritic growth of monolayer ternary WS2(1−x)Se2x flakes for enhanced hydrogen evolution reaction. Nanoscale 2017, 9, 5641–5647. [Google Scholar] [CrossRef]
- Shi, Z.; Qi, X.; Zhang, Z.; Song, Y.; Zhang, J.; Guo, C.; Xu, W.; Liu, K.; Zhu, Z. Interface engineering of cobalt–sulfide–selenium core–shell nanostructures as bifunctional electrocatalysts toward overall water splitting. Nanoscale 2021, 13, 6890–6901. [Google Scholar] [CrossRef]
- Chen, W.; Wei, W.; Wang, K.; Cui, J.; Zhu, X.; Ostrikov, K. Partial sulfur vacancies created by carbon–nitrogen deposition of MoS2 for high-performance overall electrocatalytic water splitting. Nanoscale 2021, 13, 14506–14517. [Google Scholar] [CrossRef]
- Balamurugan, K.; Rajakumaran, R.; Chen, S.M.; Chen, T.W.; Huang, P.J. Co-precipitation synthesis and characterization of rare-earth pyrochlore Gadolinium stannate; A novel electrocatalyst for the determination of furazolidone in water samples. Int. J. Electrochem. Sci. 2021, 16, 210368. [Google Scholar] [CrossRef]
- Yao, N.; Li, P.; Zhou, Z.; Meng, R.; Cheng, G.; Luo, W. Nitrogen Engineering on 3D Dandelion-Flower-Like CoS2 for High-Performance Overall Water Splitting. Small 2019, 15, 1901993. [Google Scholar] [CrossRef]
- Lu, A.Y.; Yang, X.L.; Tseng, C.C.; Min, S.X.; Lin, S.H.; Hsu, C.L.; Li, H.N.; Idriss, H.C.; Kuo, J.L.; Huang, K.W.; et al. High-Sulfur-Vacancy Amorphous Molybdenum Sulfide as a High Current Electrocatalyst in Hydrogen Evolution. Small 2016, 12, 5530–5537. [Google Scholar] [CrossRef]
- Hu, J.; Huang, B.; Zhang, C.; Wang, Z.; An, Y.; Zhou, D.; Lin, H.; Leung, M.K.H.; Yang, S. Engineering stepped edge surface structures of MoS2 sheet stacks to accelerate the hydrogen evolution reaction. Energy Environ. Sci. 2017, 10, 593–603. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, W.; Xi, P.X.; Xi, S.B.; Du, Y.H.; Gao, D.Q.; Ding, J. Activating and Optimizing Activity of CoS2 for Hydrogen Evolution Reaction through the Synergic Effect of N Dopants and S Vacancies. ACS Energy Lett. 2017, 2, 1022–1028. [Google Scholar] [CrossRef]
- Prasannachandran, R.; Vineesh, T.V.; Lithin, M.B.; Nandakishore, R.; Shaijumon, M.M. Phosphorene-quantum-dot-interspersed few-layered MoS2hybrids as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution. Chem. Commun. 2020, 56, 8623–8626. [Google Scholar] [CrossRef] [PubMed]
- Souleymen, R.; Wang, Z.T.; Qiao, C.; Naveed, M.; Cao, C.B. Microwave-assisted synthesis of graphene-like cobalt sulfide freestanding sheets as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 7592–7607. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhou, Q.L.; Du, S.; Li, J.; Zhong, J.W.; Deng, X.L.; Liu, Y.L. Porous Co9S8/Nitrogen, Sulfur-Doped Carbon@Mo2C Dual Catalyst for Efficient Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 22291–22302. [Google Scholar] [CrossRef]
- Shang, D.; Li, D.; Chen, B.; Luo, B.; Huang, Y.; Shi, W. 2D–2D SnS2/Covalent Organic Framework Heterojunction Photocatalysts for Highly Enhanced Solar-Driven Hydrogen Evolution without Cocatalysts. ACS Sustain. Chem. Eng. 2021, 9, 14238–14248. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, M.; Hu, N.; Zhang, W.; Luo, Z.; Wang, R.; Wang, J.; Huang, L.; Suo, Y.; Wang, J. Traditional NiCo2S4 Phase with Porous Nanosheets Array Topology on Carbon Cloth: A Flexible, Versatile and Fabulous Electrocatalyst for Overall Water and Urea Electrolysis. ACS Sustain. Chem. Eng. 2018, 6, 5011–5020. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Y.; Yu, B.; Zhang, X.J.; Yang, D.X.; Chen, Y.F. Heterogeneous CoFe-Co8FeS8 nanoparticles embedded in CNT networks as highly efficient and stable electrocatalysts for oxygen evolution reaction. J. Power Sources 2019, 433, 126688. [Google Scholar] [CrossRef]
- Ahn, I.-K.; Joo, W.; Lee, J.-H.; Kim, H.G.; Lee, S.-Y.; Jung, Y.; Kim, J.-Y.; Lee, G.-B.; Kim, M.; Joo, Y.-C. Metal-organic Framework-driven Porous Cobalt Disulfide Nanoparticles Fabricated by Gaseous Sulfurization as Bifunctional Electrocatalysts for Overall Water Splitting. Sci. Rep. 2019, 9, 19539. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Okyay, M.S.; Kim, M.; Lee, M.H.; Park, N.; Lee, J.S. Bifunctional sulfur-doped cobalt phosphide electrocatalyst outperforms all-noble-metal electrocatalysts in alkaline electrolyzer for overall water splitting. Nano Energy 2018, 53, 286–295. [Google Scholar] [CrossRef]
- Feng, X.T.; Jiao, Q.Z.; Cui, H.R.; Yin, M.M.; Li, Q.; Zhao, Y.; Li, H.S.; Zhou, W.; Feng, C.H. One-Pot Synthesis of NiCo2S4 Hollow Spheres via Sequential Ion Exchange as an Enhanced Oxygen Bifunctional Electrocatalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2018, 10, 29521–29531. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hou, D.; Zhou, Y.; Zhou, W.; Li, G.; Tang, Z.; Li, L.; Chen, S. MoS2 nanosheet-coated CoS2 nanowire arrays on carbon cloth as three-dimensional electrodes for efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 22886–22891. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X.; Owusu, K.A.; Monestel, H.G.R.; Boakye, F.O.; Zhang, H.; Mu, S. Multifunctional Mo–N/C@MoS2 Electrocatalysts for HER, OER, ORR, and Zn–Air Batteries. Adv. Funct. Mater. 2017, 27, 1702300. [Google Scholar] [CrossRef]
- Ye, Z.F.; Yang, J.; Li, B.; Shi, L.; Ji, H.X.; Song, L.; Xu, H.X. Amorphous Molybdenum Sulfide/Carbon Nanotubes Hybrid Nanospheres Prepared by Ultrasonic Spray Pyrolysis for Electrocatalytic Hydrogen Evolution. Small 2017, 13, 1700111. [Google Scholar] [CrossRef]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum Sulfide/N-Doped CNT Forest Hybrid Catalysts for High-Performance Hydrogen Evolution Reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef]
- Lee, W.J.; Lim, J.; Kim, S.O. Nitrogen Dopants in Carbon Nanomaterials: Defects or a New Opportunity? Small Methods 2017, 1, 1600014. [Google Scholar] [CrossRef]
- Wang, J.Y.; Ouyang, T.; Li, N.; Ma, T.Y.; Liu, Z.Q. N co-doped carbon nanotube-encapsulated core-shelled CoS2@Co nanoparticles: Efficient and stable bifunctional catalysts for overall water splitting. Sci. Bull. 2018, 63, 1130–1140. [Google Scholar] [CrossRef]
- Li, X.T.; Duan, X.G.; Han, C.; Fan, X.B.; Li, Y.; Zhang, F.B.; Zhang, G.L.; Peng, W.C.; Wang, S.B. Chemical activation of nitrogen and sulfur co-doped graphene as defect-rich carbocatalyst for electrochemical water splitting. Carbon 2019, 148, 540–549. [Google Scholar] [CrossRef]
- Zang, Y.P.; Niu, S.W.; Wu, Y.S.; Zheng, X.S.; Cai, J.Y.; Yee, J.; Xie, Y.F.; Liu, Y.; Zhou, J.B.; Zhu, J.F.; et al. Tuning orbital orientation endows molybdenum disulfide with exceptional alkaline hydrogen evolution capability. Nat. Commun. 2019, 10, 1217. [Google Scholar] [CrossRef]
- Zheng, F.Y.; Li, R.S.; Ge, S.Y.; Xu, W.R.; Zhang, Y.C. Nitrogen and phosphorus co-doped carbon networks derived from shrimp shells as an efficient oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2020, 446, 227356. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.W.; Zang, Y.P.; Liu, R.R.; Liu, G.Q.; Wang, G.Z.; Zhang, Y.X.; Zhang, H.M.; Zhao, H.J. Co/Co9S8@S,N-doped porous graphene sheets derived from S, N dual organic ligands assembled Co-MOFs as superior electrocatalysts for full water splitting in alkaline media. Nano Energy 2016, 30, 93–102. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Tran, D.T.; Doan, T.L.L.; Kim, D.H.; Kim, N.H.; Lee, J.H. Rational Design of Core@shell Structured CoSx@Cu2MoS4 Hybridized MoS2/N,S-Codoped Graphene as Advanced Electrocatalyst for Water Splitting and Zn-Air Battery. Adv. Energy Mater. 2020, 10, 1903289. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, Y.C.; Xia, B.R.; Sun, C.Q.; Liu, Y.G.; Liu, P.T.; Gao, D.Q. Facile one-step synthesis of phosphorus-doped CoS2as efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 259, 955–961. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, Y.J.; Yin, X.H.; Lan, C.H.; Wang, Y.; Hu, F.L.; Huang, Q.; Mi, Y. A “Superaerophobic” Se-Doped CoS2 Porous Nanowires Array for Cost-Saving Hydrogen Evolution. Catalysts 2021, 11, 169. [Google Scholar] [CrossRef]
- Cabán-Acevedo, M.; Stone, M.L.; Schmidt, J.R.; Thomas, J.G.; Ding, Q.; Chang, H.C.; Tsai, M.L.; He, J.H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015, 14, 1245–1251. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, Y.C.; Sun, C.Q.; Xi, P.X.; Peng, S.L.; Gao, D.Q.; Xue, D.S. Accelerated Hydrogen Evolution Reaction in CoS2 by Transition-Metal Doping. ACS Energy Lett. 2018, 3, 779–786. [Google Scholar] [CrossRef]
- Xu, J.; Shao, G.L.; Tang, X.; Lv, F.; Xiang, H.Y.; Jing, C.F.; Liu, S.; Dai, S.; Li, Y.G.; Luo, J.; et al. Frenkel-defected monolayer MoS2 catalysts for efficient hydrogen evolution. Nat. Commun. 2022, 13, 2193. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Zhang, Y.W.; Sun, Y.; Ma, K.K.; Xie, Y.; Zheng, W.H.; Tian, Z.; Kang, Z.; Zhang, Y. Vacancy Defects in 2D Transition Metal Dichalcogenide Electrocatalysts: From Aggregated to Atomic Configuration. Adv. Mater. 2023, 35, e2206576. [Google Scholar] [CrossRef]
- Sofer, Z.; Zuo, Y.; Antonatos, N.; Dekanovsky, L.; Luxa, J.; Elliott, J.D.; Gianolio, D.; Saturala, J.; Guzzetta, F.; Mourdikoudis, S.; et al. Defect Engineering in Two-Dimensional Layered PdTe2 for Enhanced Hydrogen Evolution Reaction. ACS Catal. 2023, 13, 2601–2609. [Google Scholar] [CrossRef]
- Liu, Y.; Bui, H.T.D.; Jadhav, A.R.; Yang, T.; Saqlain, S.; Luo, Y.; Yu, J.; Kumar, A.; Wang, H.; Wang, L.; et al. Revealing the Synergy of Cation and Anion Vacancies on Improving Overall Water Splitting Kinetics. Adv. Funct. Mater. 2021, 31, 2010718. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.D.; Huang, H.J.; Chen, W.; Cui, Y.; Li, Y.H.; Mao, W.W.; Zhu, X.B.; Li, X.A. Spatial Relation Controllable Di-Defects Synergy Boosts Electrocatalytic Hydrogen Evolution Reaction over VSe2 Nanoflakes in All pH Electrolytes. Small 2022, 18, e2204557. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.W.; Si, H.N.; Zhang, Q.H.; Wu, J.; Gao, L.; Wei, X.F.; Sun, Y.; Liao, Q.L.; Zhang, Z.; et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. J. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yao, J.H.; Xie, F.; Wang, X.Q.; Wan, H.; Shen, X.J.; Zhang, L.L.; Jiao, M.G.; Zhou, Z. Optimizing electronic structure through point defect engineering for enhanced electrocatalytic energy conversion. Green Energy Environ. 2025, 10, 109–131. [Google Scholar] [CrossRef]
- Shao, G.L.; Xu, J.; Gao, S.S.; Zhang, Z.; Liu, S.; Zhang, X.; Zhou, Z. Unsaturated bi-heterometal clusters in metal-vacancy sites of 2D MoS2 for efficient hydrogen evolution. Carbon Energy 2024, 6, e417. [Google Scholar] [CrossRef]
- Wang, G.J.; Guo, X.M.; Chen, H.Y.; Zhu, Y.Z.; Min, Y. Ce3+-Induced metal vacancies engineering of NiSe2 with needle-like structure for alkaline hydrogen evolution. Appl. Surf. Sci. 2023, 640, 158364. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, E.H.; Zhou, J.D.; Lin, J.H.; Ma, R.G.; Wang, Y.W.; Qiu, W.J.; Shen, R.X.; Suenaga, K.; Liu, Q.; et al. Auto-optimizing Hydrogen Evolution Catalytic Activity of ReS2 through Intrinsic Charge Engineering. ACS Nano 2018, 12, 4486–4493. [Google Scholar] [CrossRef]
- Zhou, G.; Guo, Z.J.; Shan, Y.; Wu, S.Y.; Zhang, J.L.; Yan, K.; Liu, L.Z.; Chu, P.K.; Wu, X.L. High-efficiency hydrogen evolution from seawater using hetero-structured T/Td phase ReS2 nanosheets with cationic vacancies. Nano Energy 2019, 55, 42–48. [Google Scholar] [CrossRef]
- Han, X.; Niu, M.; Luo, Y.; Li, R.; Dan, J.; Hong, Y.; Wu, X.; Trukhanov, A.V.; Ji, W.; Wang, Y.; et al. Atomically engineering metal vacancies in monolayer transition metal dichalcogenides. Nat. Synth. 2024, 3, 586–594. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, S.Z.; Gao, S.R.; Zhu, R.H.; Tan, Y.R.; Tang, X.L.; Yi, H.H. Recent advances of metal vacancies in energy and environmental catalysis: Synthesis, characterization, and roles. Green Energy Environ. 2025, 10, 84–108. [Google Scholar] [CrossRef]
- He, W.J.; Zhang, R.; Zhang, J.Y.; Wang, F.Q.; Li, Y.; Zhao, J.L.; Chen, C.; Liu, H.; Xin, H.L. Promoting the water dissociation of nickel sulfide electrocatalyst through introducing cationic vacancies for accelerated hydrogen evolution kinetics in alkaline media. J. Catal. 2022, 410, 112–120. [Google Scholar] [CrossRef]
- Zeng, P.; Meng, Y.; Liu, Z.; Sun, G.Q.; Li, X.Y.; Yang, X.Y.; Ye, C.F.; Li, Y.; Liu, J.P.; Chen, L.H.; et al. N-Doping Coupled with Co-Vacancies Activating Sulfur Atoms and Narrowing Bandgap for CoS Toward Synergistically Accelerating Hydrogen Evolution. Small 2023, 19, e2301279. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; He, Q.; Jiang, H.L.; Lin, Y.X.; Zhang, Y.K.; Habib, M.; Chen, S.M.; Song, L. Electronic Structure Reconfiguration toward Pyrite NiS2 via Engineered Heteroatom Defect Boosting Overall Water Splitting. ACS Nano 2017, 11, 11574–11583. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Wang, K.H.; Tan, X.; Liu, X.; Wang, G.X.; Xie, G.W.; Jiang, L.H. Defect-enriched multistage skeleton morphology Ni-Fe-P-Ni3S2 heterogeneous catalyst on Ni foam for efficient overall water splitting. Chem. Eng. J. 2021, 424, 130390. [Google Scholar] [CrossRef]
- Cao, Y.W.; Li, L.; Yu, X.X.; Tahir, M.; Xiang, Z.Y.; Kong, W.; Lu, Z.; Xing, X.; Song, Y. Engineering Vacancies at the 2D Nanocrystals for Robust Bifunctional Electrocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 56725–56734. [Google Scholar] [CrossRef]
- Jin, J.; Wang, X.Y.; Hu, Y.; Zhang, Z.; Liu, H.B.; Yin, J.; Xi, P.X. Precisely Control Relationship between Sulfur Vacancy and H Absorption for Boosting Hydrogen Evolution Reaction. Nano-Micro Lett. 2024, 16. [Google Scholar] [CrossRef]
- Lee, J.; Heo, J.; Lim, H.Y.; Seo, J.; Kim, Y.; Kim, J.; Kim, U.; Choi, Y.; Kim, S.H.; Yoon, Y.J.; et al. Defect-Induced in Situ Atomic Doping in Transition Metal Dichalcogenides via Liquid-Phase Synthesis toward Efficient Electrochemical Activity. ACS Nano 2020, 14, 17114–17124. [Google Scholar] [CrossRef]
- Tan, C.L.; Luo, Z.M.; Chaturvedi, A.; Cai, Y.Q.; Du, Y.H.; Gong, Y.; Huang, Y.; Lai, Z.C.; Zhang, X.; Zheng, L.R.; et al. Preparation of High-Percentage 1T-Phase Transition Metal Dichalcogenide Nanodots for Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef]
- Li, X.Z.; Fang, Y.Y.; Wang, J.; Fang, H.Y.; Xi, S.B.; Zhao, X.X.; Xu, D.Y.; Xu, H.M.; Yu, W.; Hai, X.; et al. Ordered clustering of single atomic Te vacancies in atomically thin PtTe2 promotes hydrogen evolution catalysis. Nat. Commun. 2021, 12, 2351. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Qiu, Y.; Zhang, J.; Liu, G.; Zheng, W.; Feng, W.; Cao, W.; Hu, P.; Hu, W. Controlled growth of vertical 3D MoS2(1−x)Se2x nanosheets for an efficient and stable hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 18060–18066. [Google Scholar] [CrossRef]
- Lai, Z.; Chaturvedi, A.; Wang, Y.; Tran, T.H.; Liu, X.; Tan, C.; Luo, Z.; Chen, B.; Huang, Y.; Nam, G.-H.; et al. Preparation of 1T′-Phase ReS2xSe2(1-x) (x = 0–1) Nanodots for Highly Efficient Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 8563–8568. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Debela, T.T.; Kim, J.Y.; Yoo, S.J.; Kim, J.G.; Park, J.; Kang, H.S. Phase-Transition Mo1−xVxSe2 Alloy Nanosheets with Rich V-Se Vacancies and Their Enhanced Catalytic Performance of Hydrogen Evolution Reaction. ACS Nano 2021, 15, 14672–14682. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Fan, X.F.; Singh, D.J.; Zheng, W.T. Modulation of Hydrogen Evolution Catalytic Activity of Basal Plane in Monolayer Platinum and Palladium Dichalcogenides. ACS Omega 2018, 3, 10058–10065. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Q.; Xia, B.R.; Wang, Y.Y.; Xiao, W.; Xi, P.X.; Xue, D.S.; Ding, J. Dual-Native Vacancy Activated Basal Plane and Conductivity of MoSe2 with High-Efficiency Hydrogen Evolution Reaction. Small 2018, 14, e1704150. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.R.; Wang, T.T.; Jiang, X.D.; Zhang, T.M.; Li, J.; Xiao, W.; Xi, P.X.; Gao, D.Q.; Xue, D.S.; Ding, J. Ar2+ Beam Irradiation-Induced Multivancancies in MoSe2 Nanosheet for Enhanced Electrochemical Hydrogen Evolution. ACS Energy Lett. 2018, 3, 2167–2172. [Google Scholar] [CrossRef]
- Shao, Z.Y.; Zhu, Q.; Sun, Y.; Zhang, Y.; Jiang, Y.L.; Deng, S.Q.; Zhang, W.; Huang, K.K.; Feng, S.H. Phase-Reconfiguration-Induced NiS/NiFe2O4 Composite for Performance-Enhanced Zinc-Air Batteries. Adv. Mater. 2022, 34, 2110172. [Google Scholar] [CrossRef]
- Li, X.; Kou, Z.K.; Xi, S.B.; Zang, W.J.; Yang, T.; Zhang, L.; Wang, J. Porous NiCo2S4/FeOOH nanowire arrays with rich sulfide/hydroxide interfaces enable high OER activity. Nano Energy 2020, 78, 105230. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Srivastava, V.K.; Kumar, P.; Wang, J.; Yu, A.M. Graphene-supported 2D transition metal oxide heterostructures. J. Mater. Chem. A 2018, 6, 13509–13537. [Google Scholar] [CrossRef]
- Li, Y.X.; Yin, J.; An, L.; Lu, M.; Sun, K.; Zhao, Y.Q.; Gao, D.Q.; Cheng, F.Y.; Xi, P.X. FeS2/CoS2 Interface Nanosheets as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Small 2018, 14, e1801070. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Zhang, T.; Zhang, J.H.; Fan, H.; He, C.; Song, J.X. 3D 1T-MoS2/CoS2 Heterostructure via Interface Engineering for Ultrafast Hydrogen Evolution Reaction. Small 2020, 16, e2002850. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, C.; Wang, E. An efficient CoS2/CoSe2 hybrid catalyst for electrocatalytic hydrogen evolution. J. Mater. Chem. A 2017, 5, 2504–2507. [Google Scholar] [CrossRef]
- Xie, W.J.; Liu, K.; Shi, G.D.; Fu, X.L.; Chen, X.J.; Fan, Z.X.; Liu, M.; Yuan, M.J.; Wang, M. CoS2 nanowires supported graphdiyne for highly efficient hydrogen evolution reaction. J. Energy Chem. 2021, 60, 272–278. [Google Scholar] [CrossRef]
- Tang, B.; Yu, Z.G.; Zhang, Y.; Tang, C.; Seng, H.L.; Seh, Z.W.; Zhang, Y.-W.; Pennycook, S.J.; Gong, H.; Yang, W. Metal–organic framework-derived hierarchical MoS2/CoS2 nanotube arrays as pH-universal electrocatalysts for efficient hydrogen evolution. J. Mater. Chem. A 2019, 7, 13339–13346. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.X.; Jiang, L.; Lin, H.; An, Y.M.; Zhou, D.; Leung, M.K.H.; Yang, S.H. Nanohybridization of MoS2 with Layered Double Hydroxides Efficiently Synergizes the Hydrogen Evolution in Alkaline Media. Joule 2017, 1, 383–393. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Wang, J.S.; Ruan, Y.J.; Ji, X.; Xu, K.; Chen, C.; Wan, H.Z.; Miao, L.; Jiang, J.J. Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution. Nano Energy 2017, 37, 74–80. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.Y. Nickel Hydr(oxy)oxide Nanoparticles on Metallic MoS2 Nanosheets: A Synergistic Electrocatalyst for Hydrogen Evolution Reaction. Adv. Sci. 2018, 5, 1700644. [Google Scholar] [CrossRef]
- Chen, Y.N.; Xu, S.M.; Li, Y.C.; Jacob, R.J.; Kuang, Y.D.; Liu, B.Y.; Wang, Y.L.; Pastel, G.; Salamanca-Riba, L.G.; Zachariah, M.R.; et al. FeS2 Nanoparticles Embedded in Reduced Graphene Oxide toward Robust, High-Performance Electrocatalysts. Adv. Energy Mater. 2017, 7, 1700482. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, W.T.; Liao, H.J.; Yang, Y.H.; Yen, H.C.; Liao, T.W.; Wen, C.Y.; Lee, Y.C.; Chen, C.C.; Wang, D.Y. Improving Hydrogen Evolution Activity of Earth-Abundant Cobalt-Doped Iron Pyrite Catalysts by Surface Modification with Phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.Z.; Cheng, Z.Y.; Zheng, D.H.; Zhou, Q.N.; Yang, S.K.; Lin, J.J. A heterostructured WS2/WSe2 catalyst by heterojunction engineering towards boosting hydrogen evolution reaction. Chem. Eng. J. 2022, 443, 136348. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Adofo, L.A.; Yang, S.H.; Kim, H.J.; Choi, S.H.; Kirubasankar, B.; Cho, B.W.; Ben-Smith, A.; Kang, J.H.; Kim, Y.M.; et al. 1T’ RexMo1−xS2-2H MoS2 Lateral Heterojunction for Enhanced Hydrogen Evolution Reaction Performance. Adv. Funct. Mater. 2023, 33, 2209572. [Google Scholar] [CrossRef]
- Le, K.T.; Pham, N.N.T.; Liao, Y.-S.; Ranjan, A.; Lin, H.-Y.; Chen, P.-H.; Nguyen, H.; Lu, M.Y.; Lee, S.G.; Wu, J.M. Piezoelectricity of strain-induced overall water splitting of Ni(OH)2/MoS2 heterostructure. J. Mater. Chem. A 2023, 11, 3481–3492. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Xiao, Y.K.; Wu, W.P.; Zhang, X.Q.; Fu, Q.; Zhao, Y.; Qu, L.T. All-pH-Tolerant In-Plane Heterostructures for Efficient Hydrogen Evolution Reaction. ACS Nano 2021, 15, 11417–11427. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Li, G.X.; Wang, Z.L.; Zhu, H.Y.; Zhang, T.; Xiao, S.; Guo, W.Y.; Yang, S.H. Metallic Iron-Nickel Sulfide Ultrathin Nanosheets As a Highly Active Electrocatalyst for Hydrogen Evolution Reaction in Acidic Media. J. Am. Chem. Soc. 2015, 137, 11900–11903. [Google Scholar] [CrossRef]
- Piontek, S.; Andronescu, C.; Zaichenko, A.; Konkena, B.; Puring, K.J.; Marler, B.; Antoni, H.; Sinev, I.; Muhler, M.; Mollenhauer, D.; et al. Influence of the Fe:Ni Ratio and Reaction Temperature on the Efficiency of (FexNi1−x)9S8 Electrocatalysts Applied in the Hydrogen Evolution Reaction. ACS Catal. 2018, 8, 987–996. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Qian, X.; Zhu, C.L.; Liu, H.Y.; Hou, L.X. Nickel Cobalt Sulfide Double-Shelled Hollow Nanospheres as Superior Bifunctional Electrocatalysts for Photovoltaics and Alkaline Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 9379–9389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Han, D.; Song, X.; Shi, L.; Song, Y.; Niu, S.; Xie, Y.; Cai, J.; Wu, S.; et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Chen, J.; Li, Y.; Ye, H.; Hu, Z.; Fu, X.-Z.; Sun, R.; Huang, W.; Wong, C.-P. Flowerlike NiCo2S4 Hollow Sub-Microspheres with Mesoporous Nanoshells Support Pd Nanoparticles for Enhanced Hydrogen Evolution Reaction Electrocatalysis in Both Acidic and Alkaline Conditions. ACS Appl. Mater. Interfaces 2018, 10, 22248–22256. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, P.; Xu, X.; Guo, S.; Pang, K.; Guo, H.; Zhang, Z.; Wu, X.; Zheng, L.; Song, R. S-Edge-rich MoxSy arrays vertically grown on carbon aerogels as superior bifunctional HER/OER electrocatalysts. Nanoscale 2019, 11, 20284–20294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).