Straightforward Synthesis and Characterization of Analcime@Nickel Orthosilicate Novel Nanocomposite for Efficient Removal of Rhodamine B Dye from Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Adsorption of Rhodamine B Dye from Aqueous Media
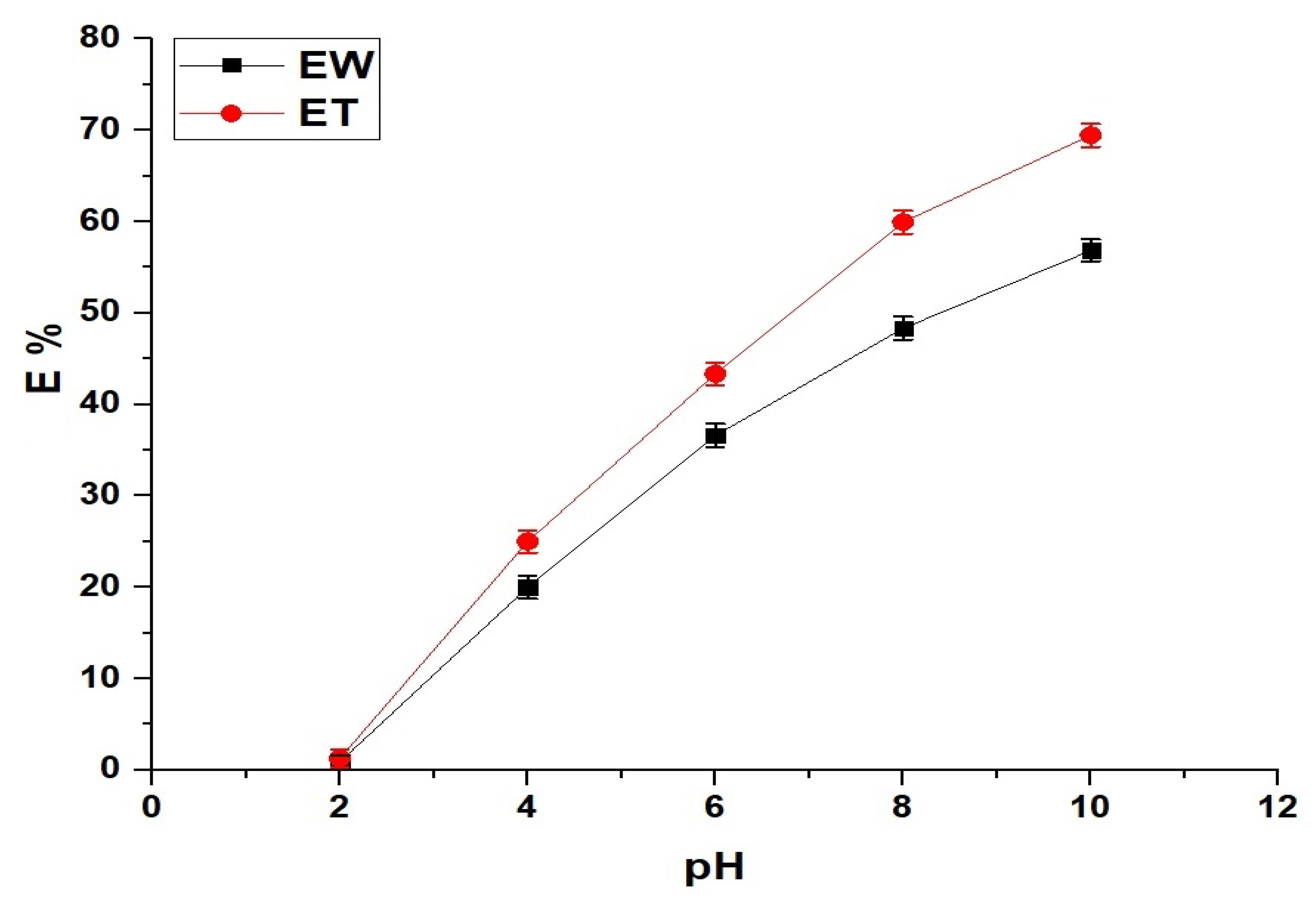
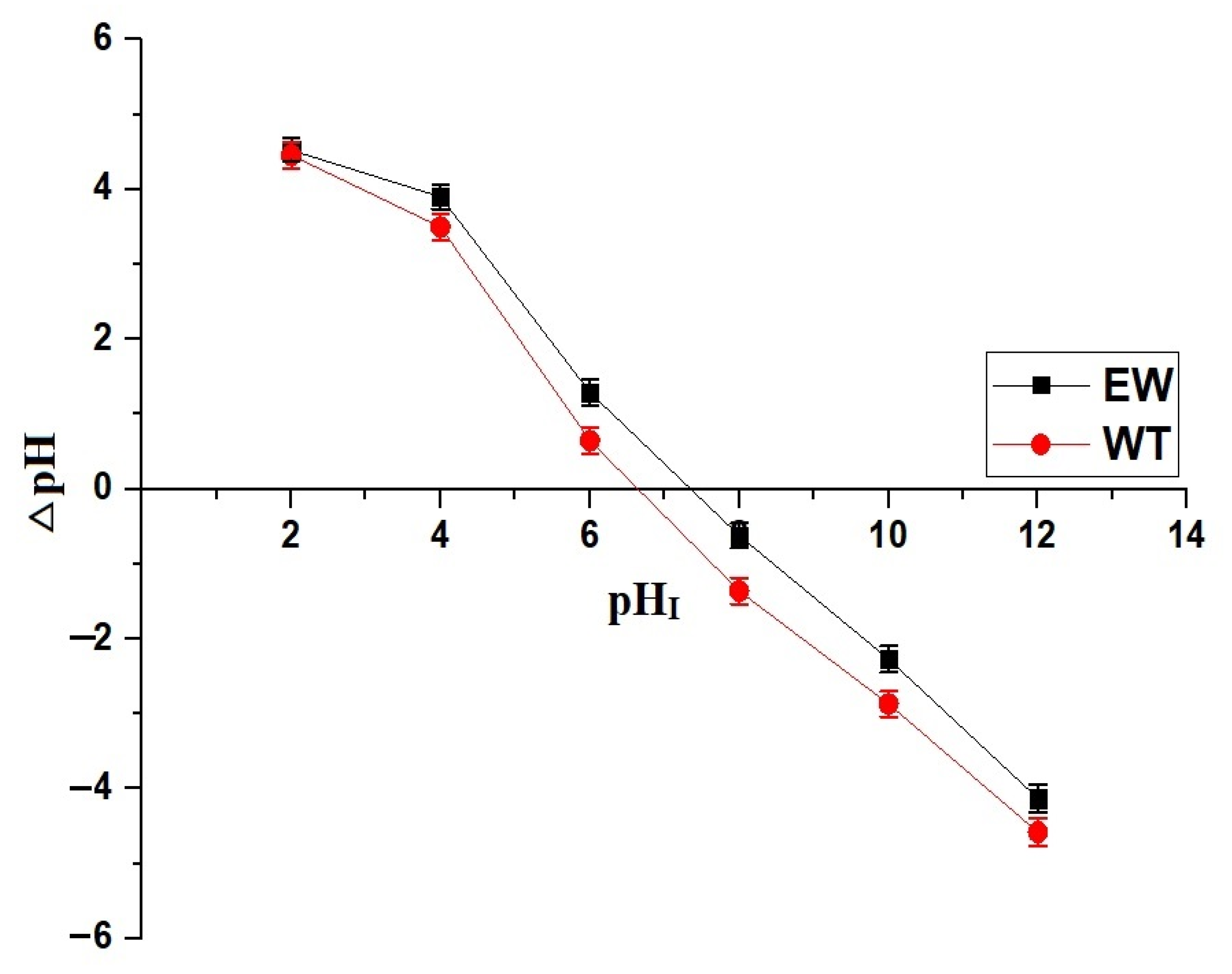
2.2.1. Effect of pH
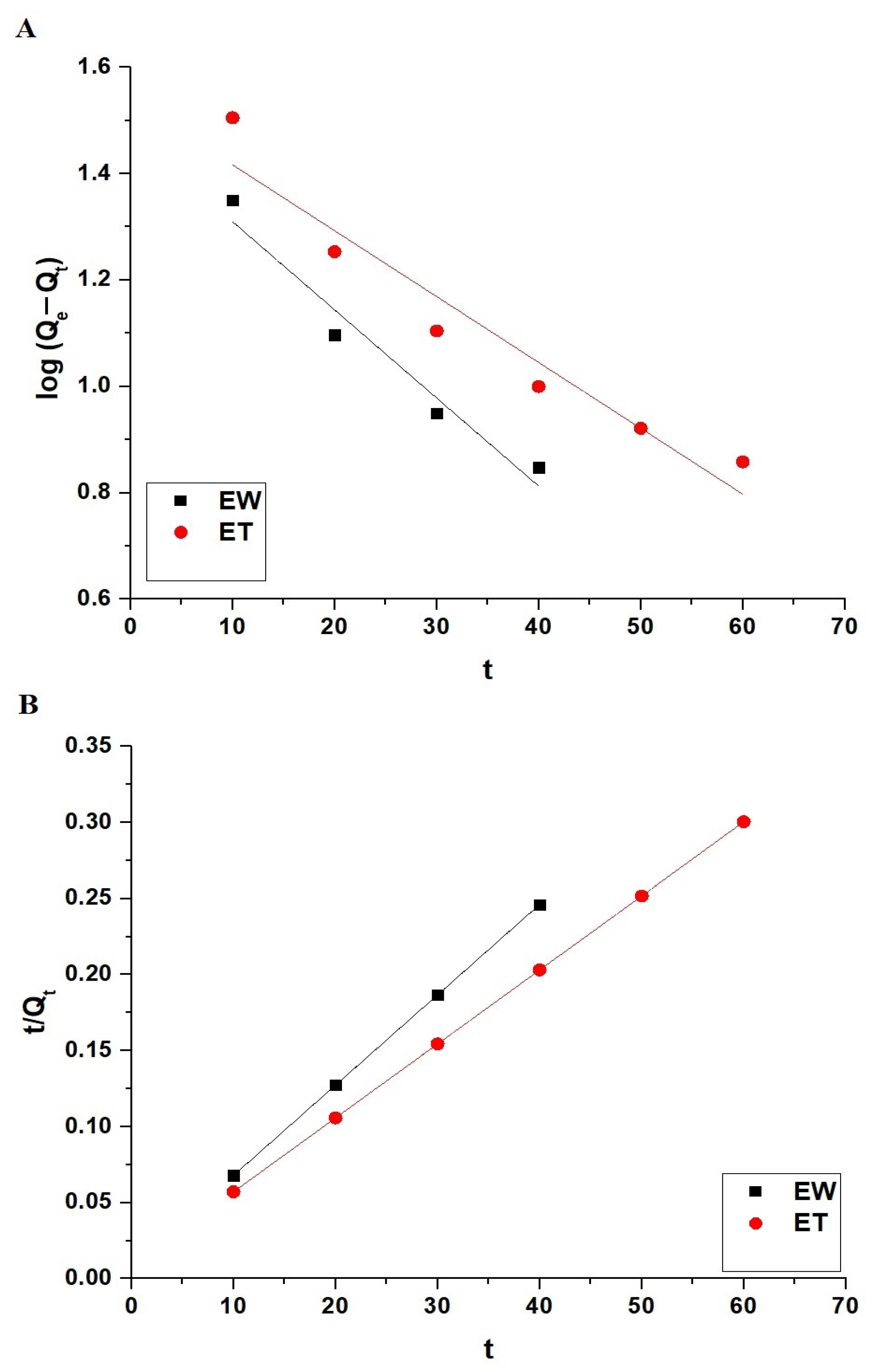
2.2.2. Effect of Contact Time
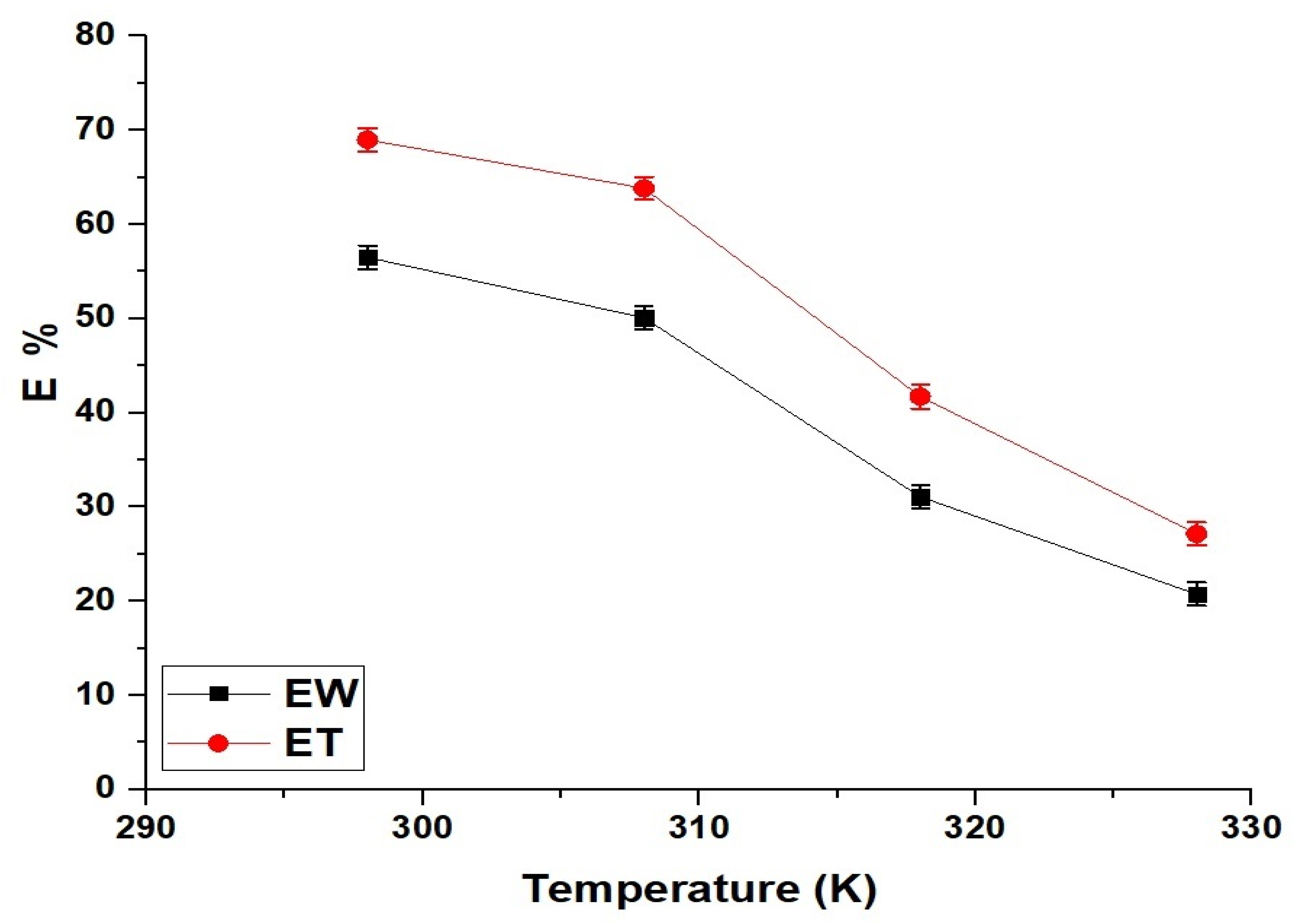
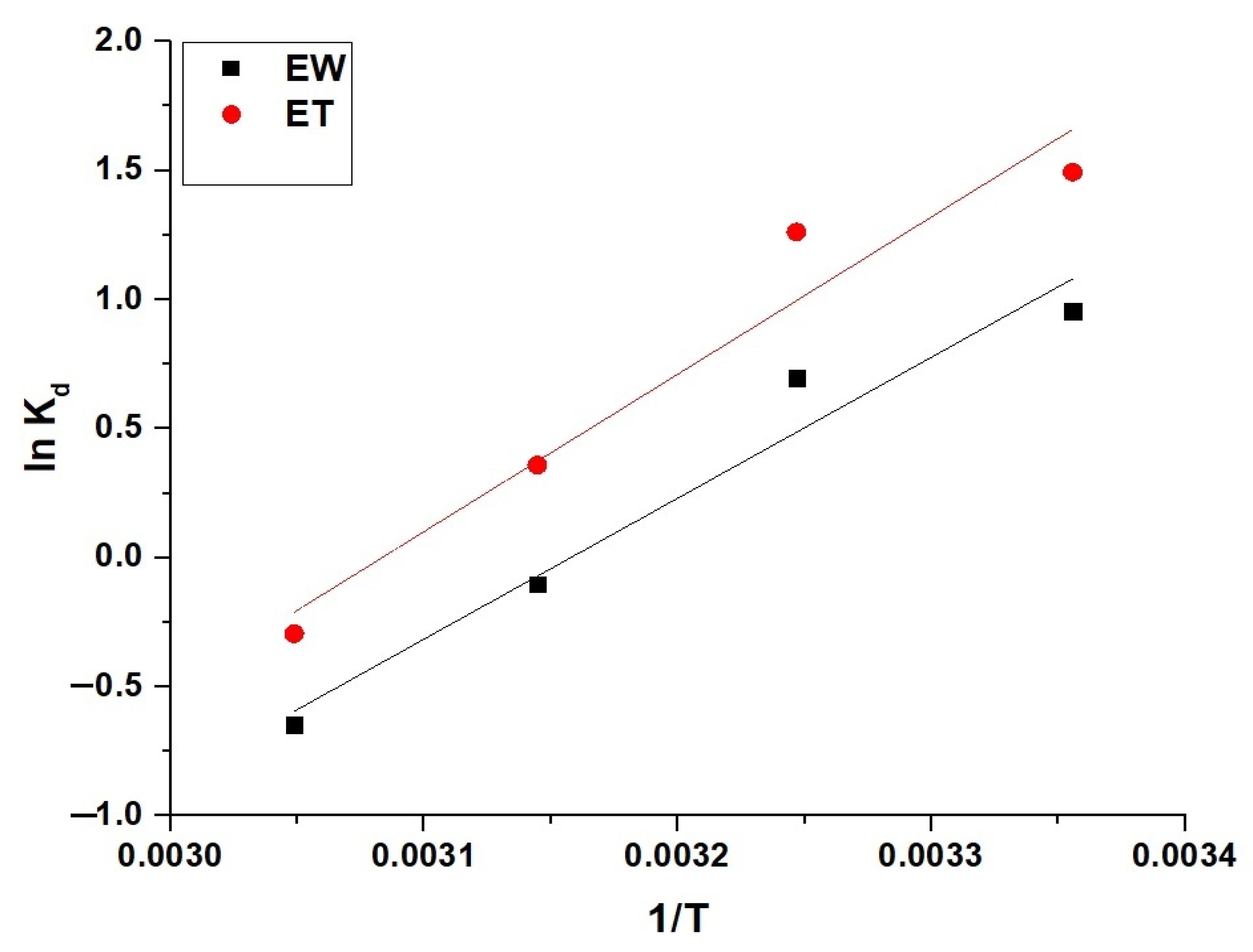
2.2.3. Effect of Temperature
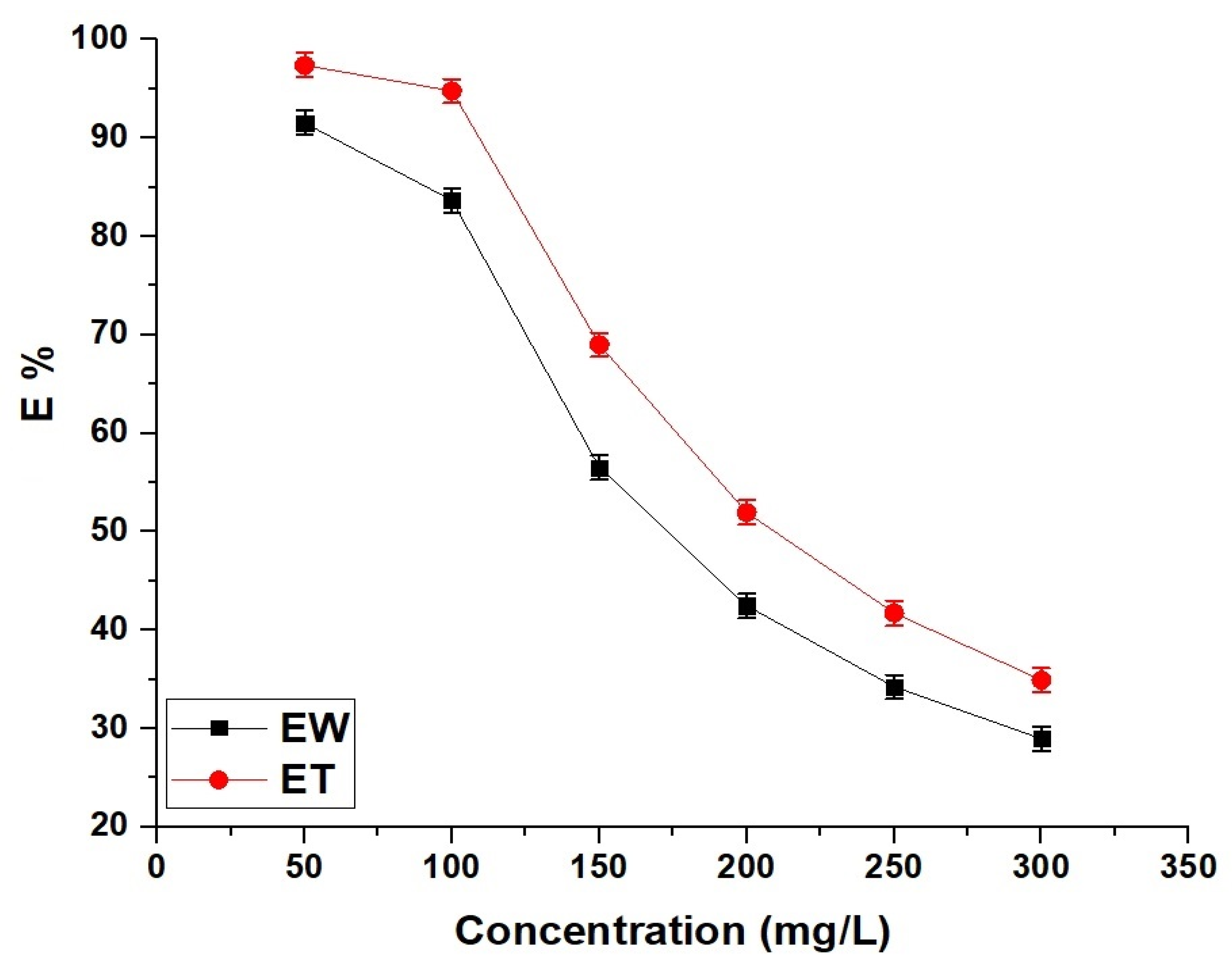
2.2.4. Effect of Concentration
3. Experimental Methodology
3.1. Materials
3.2. Synthesis of Analcime@Nickel Orthosilicate Nanocomposites
3.3. Instrumentation
3.4. Procedure of Rhodamine B Dye Adsorption from Aqueous Solutions
3.5. pHPZC of Analcime@Nickel Orthosilicate Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadore, V.; Mishra, S.R.; Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Advances in Zeolite-Based Materials for Dye Removal: Current Trends and Future Prospects. Inorg. Chem. Commun. 2024, 166, 112606. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Surana, M.; Kumar, A.; Singh, D.; Pal, D. Graphitic Carbon Nitride(g-C3N4)-Based Photocatalysts for Dye Removal: Current Status. Sustain. Chem. Environ. 2024, 7, 100141. [Google Scholar] [CrossRef]
- Fattahi, N.; Fattahi, T.; Kashif, M.; Ramazani, A.; Jung, W.K. Lignin: A Valuable and Promising Bio-Based Absorbent for Dye Removal Applications. Int. J. Biol. Macromol. 2024, 276, 133763. [Google Scholar] [CrossRef]
- Lee, S.Y.; Tan, Y.H.; Lau, S.Y.; Mubarak, N.M.; Tan, Y.Y.; Tan, I.S.; Lee, Y.H.; Ibrahim, M.L.; Karri, R.R.; Khalid, M.; et al. A State-of-the-Art Review of Metal Oxide Nanoflowers for Wastewater Treatment: Dye Removal. Environ. Res. 2024, 259, 119448. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Inyinbor, A.A.; Atunwa, B.T.; Gonuguntla, S.; Bello, O.S.; Adekola, F.A.; Pal, U. Agrowaste-Carbon and Carbon-Based Nanocomposites for Endocrine Disruptive Cationic Dyes Removal: A Critical Review. Biotechnol. Rep. 2024, 44, e00860. [Google Scholar] [CrossRef] [PubMed]
- Deivayanai, V.C.; Karishma, S.; Thamarai, P.; Saravanan, A.; Yaashikaa, P.R. Efficient Red Azo Dye Removal from Wastewater Using Magnetic Nanoparticle Impregnated Prosopis Juliflora Biomass: ANN Modeling Approach. Desalin. Water Treat. 2024, 320, 100746. [Google Scholar] [CrossRef]
- Loura, N.; Rathee, K.; Dhull, R.; Singh, M.; Dhull, V. Carbon Nanotubes for Dye Removal: A Comprehensive Study of Batch and Fixed-Bed Adsorption, Toxicity, and Functionalization Approaches. J. Water Process Eng. 2024, 67, 106193. [Google Scholar] [CrossRef]
- Kayani, K.F. Bimetallic Metal-Organic Frameworks (BMOFs) for Dye Removal: A Review. RSC Adv. 2024, 14, 31777–31796. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A Review of Eco-Sustainable Techniques for the Removal of Rhodamine B Dye Utilizing Biomass Residue Adsorbents. Phys. Chem. Earth 2022, 128, 103267. [Google Scholar] [CrossRef]
- Ta, M.; An, Y.; Yang, H.; Bai, C.; Wang, T.; Zhang, T.; Cai, H.; Zheng, H. Attraction Behavior Induces the Aggregation and Precipitation of Organic Dyes: Improving Efficiency through Co-Treatment Strategy. J. Mol. Liq. 2024, 415, 126331. [Google Scholar] [CrossRef]
- Amanda, S.; Amanda, T.; Rifqah, N.; Eka, S.; Putra, M.; Khairurrijal, K.; Arif, M.F.; Taher, T.; Rianjanu, A. Complex Mixture Dye Removal Using Natural Zeolite Modified Polyacrylonitrile/Polyvinylidene Fluoride (Ze-PAN/PVDF) Composite Nanofiber Membrane via Vacuum Filtration Technique. Mater. Today Commun. 2025, 42, 111357. [Google Scholar] [CrossRef]
- Cui, F.; Hua, D.; Cheng, X.; Luo, C.; He, J.; Zhou, Y.; Wen, X.; Zhan, G. Design of Ternary GO@AgNPs@g-C3N4 Membranes for Efficient Dye Removals in Integrated Photocatalysis-Filtration Reactors. Sep. Purif. Technol. 2025, 356, 129930. [Google Scholar] [CrossRef]
- Lima, A.M.; de Oliveira, A.M.; de Sousa, T.G.; Pereira, A.C.; de Carvalho, R.B.; Pinheiro, W.A. Filtration Membranes of Reduced Graphene Oxide for Dye Removal—Production and Characterization. Desalin. Water Treat. 2022, 278, 217–225. [Google Scholar] [CrossRef]
- Sugha, A.; Bhatti, M.S. Optimization of Electrocoagulation Removal of a Mixture of Three Azo Dyes: Spectrophotometric Colour Characteristics for Best Operating Conditions. RSC Adv. 2025, 15, 6492–6505. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Y.; Fu, H.; Zhong, L.; Qi, B.; Chen, D.; Yu, J.; Zhang, P.; Lee, S.S. Simultaneous Removal of Phosphorus, Chromium (VI), and Antimony (V) from Textile Dye Wastewater by Electrocoagulation. J. Hazard. Mater. 2025, 488, 137409. [Google Scholar] [CrossRef]
- Pratap Singh, V.; Godara, P.; Srivastava, A. Sustainable Microalgal Bioremediation of Heavy Metals and Dyes from Synthetic Wastewater: Progressing towards United Nations Sustainable Development Goals. Waste Manag. Bull. 2024, 2, 123–135. [Google Scholar] [CrossRef]
- Goswami, D.; Mukherjee, J.; Mondal, C.; Bhunia, B. Bioremediation of Azo Dye: A Review on Strategies, Toxicity Assessment, Mechanisms, Bottlenecks and Prospects. Sci. Total Environ. 2024, 954, 176426. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Raashid, M.; Akram, A.; Kazmi, M.; Farman, S. Removal of Methylene Blue Dye from Aqueous Solutions by Adsorption in Combination with Ozonation on Iron Loaded Sodium Zeolite: Role of Adsorption. Desalin. Water Treat. 2021, 237, 302–306. [Google Scholar] [CrossRef]
- Al-Hazmi, G.H.; Refat, M.S.; El-Desouky, M.G.; El-Bindary, A.A. Effective Adsorption and Removal of Industrial Dye from Aqueous Solution Using Mesoporous Zinc Oxide Nanoparticles via Metal Organic Frame Work: Equilibrium, Kinetics and Thermodynamic Studies. Desalin. Water Treat. 2022, 272, 277–289. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, Z.U.H.; Sabahat, S.; Sun, J.; Shah, N.S.; Khan, Z.U.; Muhammad, N.; Mir, S.; Rahim, A.; Nadeem, M.; et al. Innovations in Metal Oxides-Biochar Nanoparticles for Dye Removal. Nano-Struct. Nano-Objects 2024, 39, 101269. [Google Scholar] [CrossRef]
- Luo, B.; Zhang, M.; Zhou, N.; Fu, Y.; Ma, X.; Dai, C. Mechanism Study of a Novel Modified Mesoporous Silicon-Based NiT50/MCM-41 Adsorbent for the Selective Adsorption of Methylene Blue from Water. RSC Adv. 2025, 15, 5737–5749. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.R.; Zhu, W.; Shi, Z.; Wang, D. Combustion Synthesis-Aqueous Hybridization of Nanostructured Graphene-Coated Silicon and Its Dye Removal Performance. Mater. Chem. Phys. 2022, 277, 125565. [Google Scholar] [CrossRef]
- Jia, Z.; Han, C.; Wu, L.; Zhang, D.; Li, M. Biotemplated Synthesis of Hollow Nickel Silicate Fiber for Organic Dye Contaminants and Its Selective Adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129219. [Google Scholar] [CrossRef]
- Chen, C.; Ma, J.; Yi, Z.; Wang, S.; Gao, H.; Wang, Y.; Yang, H. Highly-Efficient and Recyclable Bi2O2CO3 Adsorbent Achieved by Surfactant Modification and Its Application in Pollutant Removal. Mater. Res. Bull. 2023, 159, 112091. [Google Scholar] [CrossRef]
- Ritter, M.T.; Lobo-Recio, M.Á.; Padilla, I.; Nagel-Hassemer, M.E.; Romero, M.; López-Delgado, A. Adsorption of Safranine-T Dye Using a Waste-Based Zeolite: Optimization, Kinetic and Isothermal Study. J. Ind. Eng. Chem. 2024, 136, 177–187. [Google Scholar] [CrossRef]
- Panic, V.V.; Velickovic, S.J. Removal of Model Cationic Dye by Adsorption onto Poly(Methacrylic Acid)/Zeolite Hydrogel Composites: Kinetics, Equilibrium Study and Image Analysis. Sep. Purif. Technol. 2014, 122, 384–394. [Google Scholar] [CrossRef]
- Rianjanu, A.; Taher, T.; Desriani, F.; Delmita, R.O.; Sianturi, A.G.N.; Amanda, S.; Ariwahjoedi, B.; Istiqomah, N.; Rastri, D.; Arif, M.F. Examining the Influence of Sintering Temperatures on the Efficiency of 3D-Printed Natural Zeolite for Methylene Blue Dye Adsorption. Results Surf. Interfaces 2024, 17, 100337. [Google Scholar] [CrossRef]
- Chumee, J.; Javadi, B.; Peungsamran, N.; Kumpun, S.; Seekakee, J.; Hoonsuwan, T.; Ohama, P. Synthesis of Zeolite P-Metal Organic Composite Beads for Superior Cationic Dye Removal. Inorg. Chem. Commun. 2025, 177, 114344. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, X.; Xu, W.; Xing, H.; Zhai, N. Porous Analcime Composite Synthesized from Solid Waste: A Cost-Effective and Superb Adsorbent for Efficient Removal of Cu(II) and Cationic Dye. Chem. Eng. Res. Des. 2023, 189, 474–484. [Google Scholar] [CrossRef]
- Kishore, K.; Kaur, J.; Saddeek, Y.B.; Sharma, M.; Singh, M.; Thakur, P.; Walia, Y.K.; Lal, M.; Suman, R.; Reddy, A.S.; et al. Efficient Removal of Toxic Dyes from Water Using Mn3O4 Nanoparticles: Synthesis, Characterization, and Adsorption Mechanisms. J. Mol. Struct. 2025, 1333, 141756. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Haouti, R.; El Guerdaoui, A.; Haounati, R.; Amaterz, E.; Addi, A.A.; Akbal, F.; Taha, M.L. Experimental and Molecular Dynamics Simulation Study on the Adsorption of Rhodamine B Dye on Magnetic Montmorillonite Composite γ-Fe2O3@Mt. J. Mol. Liq. 2020, 309, 113142. [Google Scholar] [CrossRef]
- Rubab, R.; Ali, S.; Rehman, A.U.; Khan, S.A.; Khan, A.M. Templated Synthesis of NiO/SiO2 Nanocomposite for Dye Removal Applications: Adsorption Kinetics and Thermodynamic Properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126253. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.Y.; Zhao, J.; Yuan, H. Microplastics Affect the Removal of Dye in Textile Wastewater: Adsorption Capacity and Its Effect on Coagulation Behavior. Sep. Purif. Technol. 2025, 359, 130505. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Abdelrahman, E.A.; Alamro, F.S.; Saad, F.A.; Al-Raimi, D.S. Innovative nanocomposite comprising of ZrO2, MnCO3, and CdCO3 for superior crystal violet dye adsorption: Synthesis, characterization, and regeneration insights. Sci Rep. 2025, 15, 5525. [Google Scholar] [CrossRef]
- Fathi, A.G.; Abdelrahman, E.A.; Abou-Krisha, M.M.; Shah, R.; Saad, F.A.; El Rayes, S.M. Facile Synthesis of Novel Nanocomposite Consists of β-FeOOH, Chitosan, and Salicylaldehyde for Efficient Removal of Zn (II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2025, in press. [Google Scholar] [CrossRef]
- Saheed, I.O.; Zairuddin, N.I.; Nizar, S.A.; Hanafiah, M.A.K.M.; Latip, A.F.A.; Suah, F.B.M. Adsorption Potential of CuO-Embedded Chitosan Bead for the Removal of Acid Blue 25 Dye. Ain Shams Eng. J. 2024, 15, 103125. [Google Scholar] [CrossRef]
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Efficient Removal of Rhodamine B Dye Using Biochar as an Adsorbent: Study the Performance, Kinetics, Thermodynamics, Adsorption Isotherms and Its Reusability. Chemosphere 2024, 354, 141702. [Google Scholar] [CrossRef]
- Venkatesan, A.; Srividhya, B.; Alajmi, A.; Sadeq, A.M.; Chava, R.K.; Habila, M.A.; Senthil Kumar, D.; Guganathan, L.; Ragupathy, S. High Adsorption Capacities of Rhodamine B Dye by Activated Carbon Synthesized from Cotton Stalks Agricultural Waste by Chemical Activation. Ceram. Int. 2025, in press. [Google Scholar] [CrossRef]
- Damasceno, B.S.; da Silva, V.C.; Rodrigues, A.R.; Falcão, E.H.L.; Vaz de Araújo, A.C. Use of Magnetic Nanoparticles of Iron Oxide and Their Derivatives in the Adsorption of Rhodamine 6G and Rhodamine B Dyes. J. Alloys Compd. 2024, 1005, 175907. [Google Scholar] [CrossRef]
- Jinendra, U.; Bilehal, D.; Nagabhushana, B.M.; Kumar, A.P. Adsorptive Removal of Rhodamine B Dye from Aqueous Solution by Using Graphene—Based Nickel Nanocomposite. Heliyon 2021, 7, e06851. [Google Scholar] [CrossRef]
- Konicki, W.; Siber, D.; Narkiewicz, U. Removal of Rhodamine B from Aqueous Solution by ZnFe2O4 Nanocomposite with Magnetic Separation Performance. Pol. J. Chem. Technol. 2017, 19, 65–74. [Google Scholar] [CrossRef]
- Alwan, S.H.; Alshamsi, H.A.H.; Jasim, L.S. Rhodamine B Removal on A-RGO/Cobalt Oxide Nanoparticles Composite by Adsorption from Contaminated Water. J. Mol. Struct. 2018, 1161, 356–365. [Google Scholar] [CrossRef]
- El-Shafey, S.S.; Sayed Ahmed, S.A.; Aboelenin, R.M.; Fathy, N.A. Synthesis of SBA-16 Mesoporous Silica from Rice Husk Ash for Removal of Rhodamine B Cationic Dye: Effect of Hydrothermal Treatment Time. Desalin. Water Treat. 2024, 317, 100179. [Google Scholar] [CrossRef]
- Saigl, Z.M.; Aljuaid, O.A. Adsorption of Rhodamine B Dye onto Iodo-Polyurethane Foam: Kinetics and Thermodynamic Study. Desalin. Water Treat. 2023, 315, 227–240. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Zhang, D.; Han, G.; Cao, Y.; Liu, J. Fabrication and Characterization of Dinickel Orthosilicate Nanosheets as High Performance Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2019, 785, 80–88. [Google Scholar] [CrossRef]
- Fotouh, A.E.; Al-Farraj, E.S.; Kotp, Y.H.; El Rayes, S.M.; Elfalleh, W.; Khezami, L. Facile Synthesis of Analcime (NaSi2AlO6·H2O) Nanoparticles Using Polyethylene Glycol 400 as an Organic Template for Effective Removal of Zn(II) and Cd(II) Ions from Aqueous Solutions. Silicon 2024, 16, 2671–2687. [Google Scholar] [CrossRef]
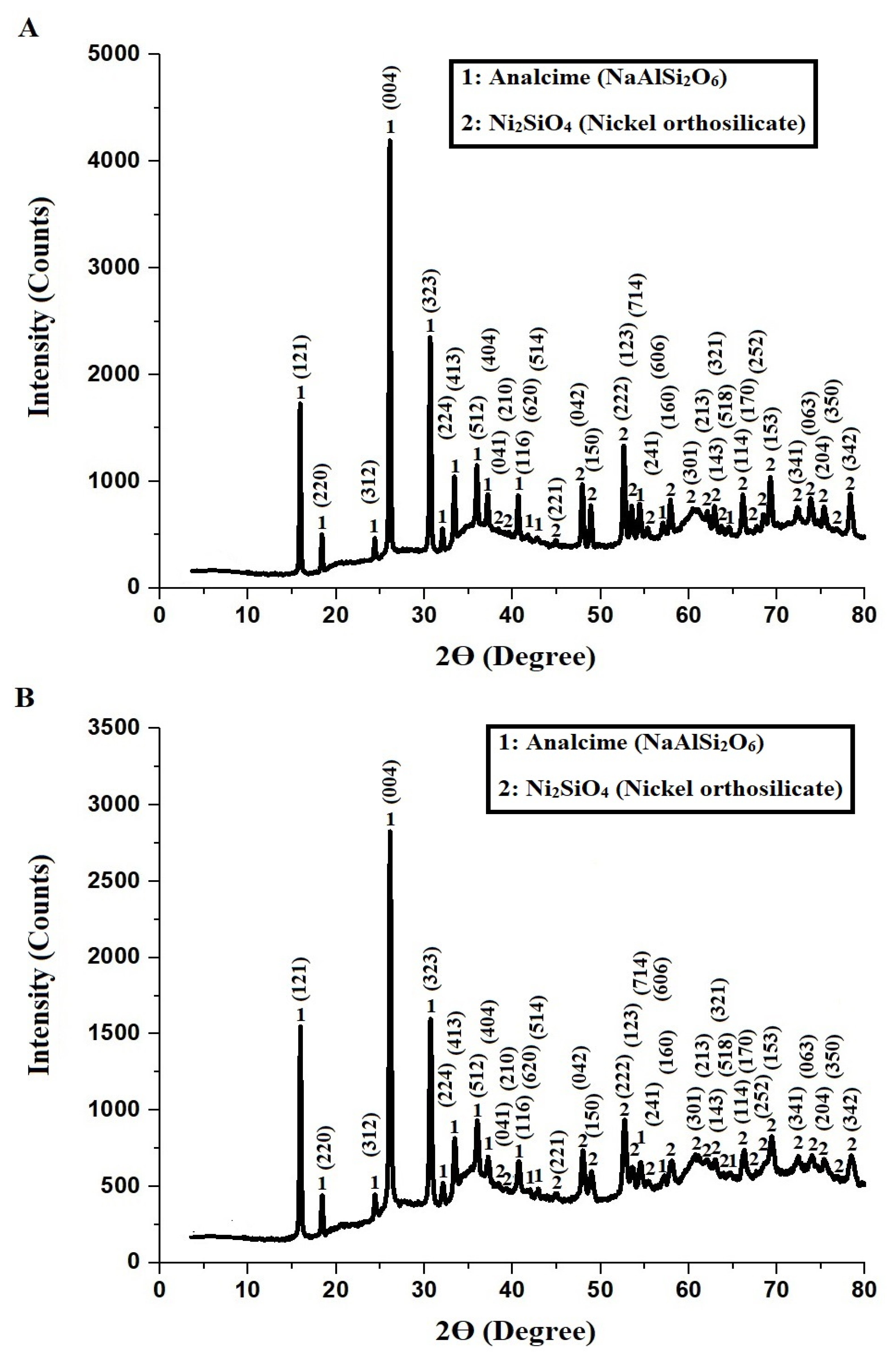

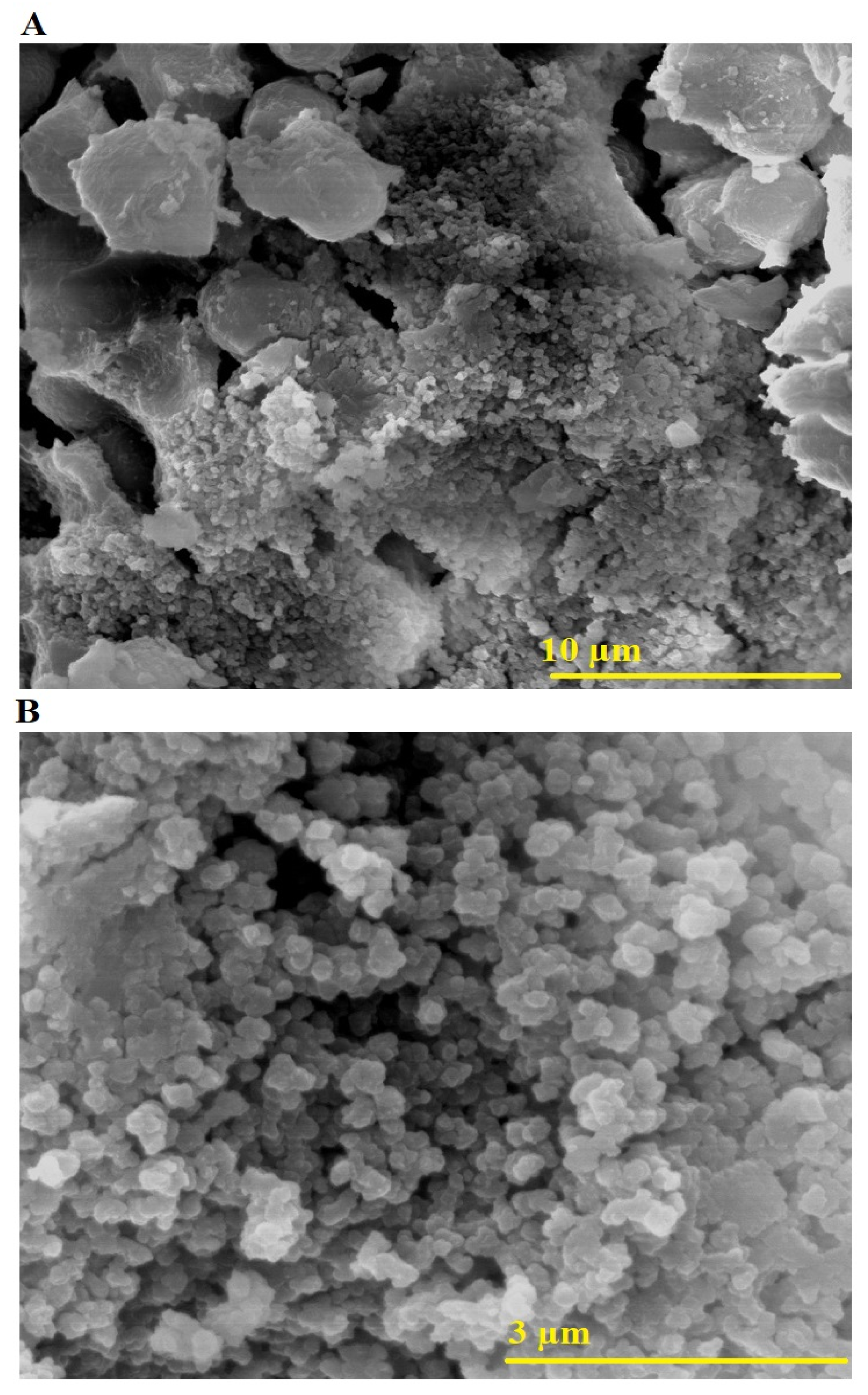





| Sample | Atomic Percentages | ||||||
|---|---|---|---|---|---|---|---|
| % O | % Na | % Al | % Si | % Ni | Na–Al Ratio | Analcime–Nickel Orthosilicate Ratio | |
| EW | 54.1 | 9.4 | 6.1 | 22.1 | 8.3 | 1.54 | 3:2 |
| ET | 56.0 | 8.2 | 5.5 | 21.7 | 8.6 | 1.49 | 4:3 |
| Sample | QExp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| K1 (1/min) | R2 | Qe (mg/g) | K2 (g/mg·min) | R2 | Qe (mg/g) | ||
| EW | 169.52 | 0.0382 | 0.9377 | 29.87 | 0.00411 | 0.9999 | 168.35 |
| ET | 206.96 | 0.0285 | 0.9162 | 34.70 | 0.00278 | 0.9999 | 205.76 |
| Sample | ΔS° (kJ/mol.K) | ΔH° (kJ/mol) | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| EW | 0.1441 | −45.62 | −88.55 | −89.99 | −91.43 | −92.87 |
| ET | 0.1569 | −50.92 | −97.70 | −99.27 | −100.84 | −102.41 |
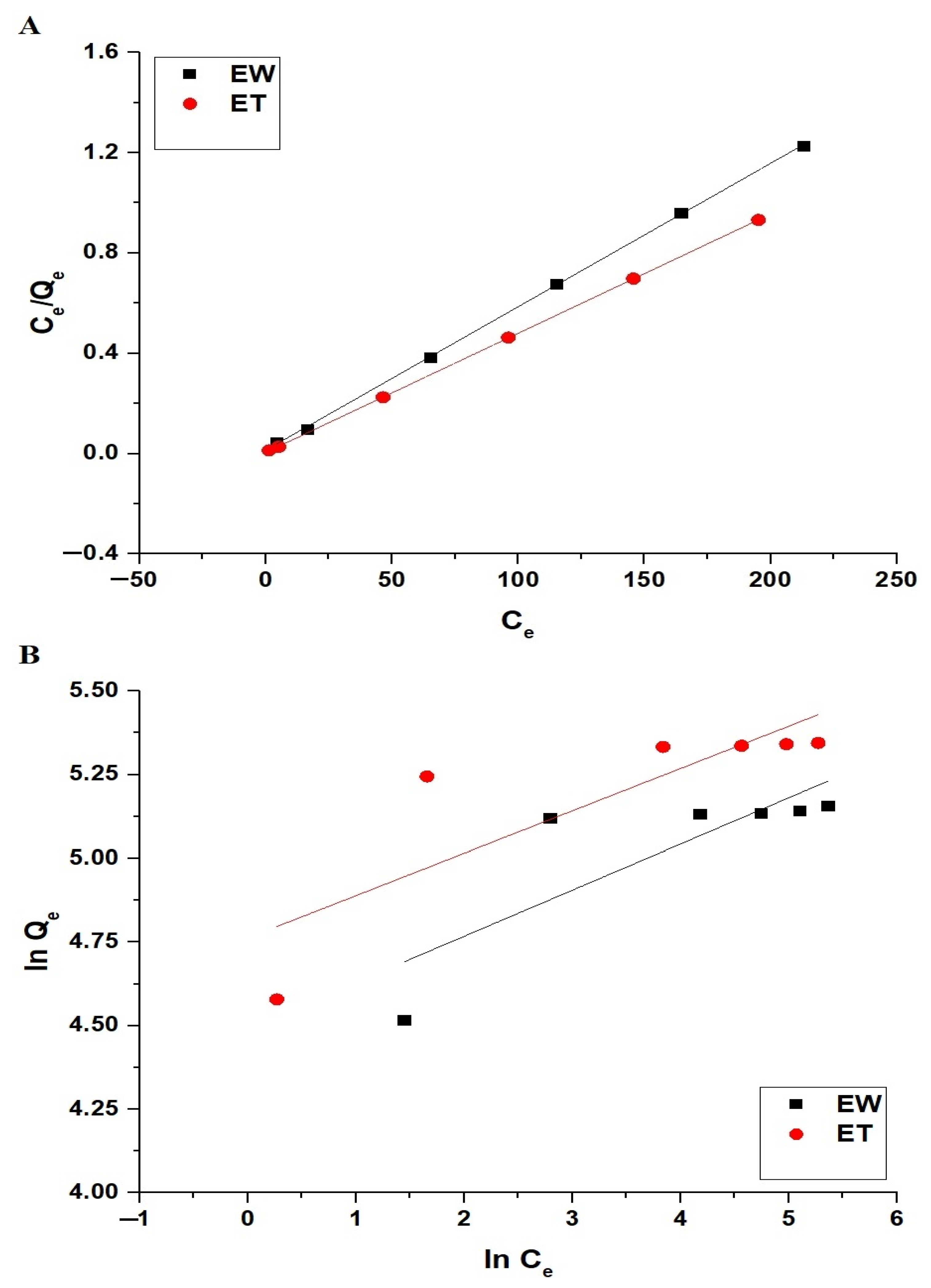
| Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | R2 | K3 (L/mg) | K4 (mg/g)(L/mg)1/n | Qmax (mg/g) | 1/n | R2 | |
| EW | 174.83 | 0.9997 | 0.3958 | 89.29 | 185.21 | 0.1377 | 0.6048 |
| ET | 210.53 | 0.9999 | 0.9654 | 117.02 | 228.69 | 0.1265 | 0.6268 |
| Adsorbent | Qmax (mg/g) | Ref. |
|---|---|---|
| Biochar | 169.50 | [37] |
| Activated carbon | 144.36 | [38] |
| Fe3O4 nanoparticles | 138.40 | [39] |
| CoFe2O4 nanoparticles | 145.10 | [39] |
| Graphene-based nickel nanocomposite | 65.31 | [40] |
| Magnetic ZnFe2O4 nanocomposite | 9.83 | [41] |
| NiO/SiO2 nanocomposite | 68.00 | [32] |
| Co3O4@reduced graphene oxide nanocomposite | 102.90 | [42] |
| SBA-16 mesoporous silica | 166.70 | [43] |
| Iodo-polyurethane foam | 22.03 | [44] |
| EW | 174.83 | This study |
| ET | 210.53 | This study |
| Sample | BET Surface Area (m2/g) | Average Pore Size (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| EW | 66 | 4.52 | 0.1162 |
| ET | 108 | 10.06 | 0.3812 |
| Effect | V (L) | Co (mg/L) | W (mg) | T (K) | t (min) | pH |
|---|---|---|---|---|---|---|
| pH | 0.1 | 150 | 50 | 298 | 240 | 2–10 |
| Time | 0.1 | 150 | 50 | 298 | 10–100 | 10 |
| Temperature | 0.1 | 150 | 50 | 298–328 | 50 (ET) 70 (EW) | 10 |
| Concentration of rhodamine B dye | 0.1 | 50–300 | 50 | 298 | 50 (ET) 70 (EW) | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, E.A.; Saad, F.A.; Abou-Krisha, M.M.; Khedr, A.M.; Alqahtani, Z. Straightforward Synthesis and Characterization of Analcime@Nickel Orthosilicate Novel Nanocomposite for Efficient Removal of Rhodamine B Dye from Aqueous Media. Inorganics 2025, 13, 120. https://doi.org/10.3390/inorganics13040120
Abdelrahman EA, Saad FA, Abou-Krisha MM, Khedr AM, Alqahtani Z. Straightforward Synthesis and Characterization of Analcime@Nickel Orthosilicate Novel Nanocomposite for Efficient Removal of Rhodamine B Dye from Aqueous Media. Inorganics. 2025; 13(4):120. https://doi.org/10.3390/inorganics13040120
Chicago/Turabian StyleAbdelrahman, Ehab A., Fawaz A. Saad, Mortaga M. Abou-Krisha, Abdalla M. Khedr, and Zahrah Alqahtani. 2025. "Straightforward Synthesis and Characterization of Analcime@Nickel Orthosilicate Novel Nanocomposite for Efficient Removal of Rhodamine B Dye from Aqueous Media" Inorganics 13, no. 4: 120. https://doi.org/10.3390/inorganics13040120
APA StyleAbdelrahman, E. A., Saad, F. A., Abou-Krisha, M. M., Khedr, A. M., & Alqahtani, Z. (2025). Straightforward Synthesis and Characterization of Analcime@Nickel Orthosilicate Novel Nanocomposite for Efficient Removal of Rhodamine B Dye from Aqueous Media. Inorganics, 13(4), 120. https://doi.org/10.3390/inorganics13040120






