Exploring Metal- and Porphyrin-Modified TiO2-Based Photocatalysts for Efficient and Sustainable Hydrogen Production
Abstract
1. Introduction
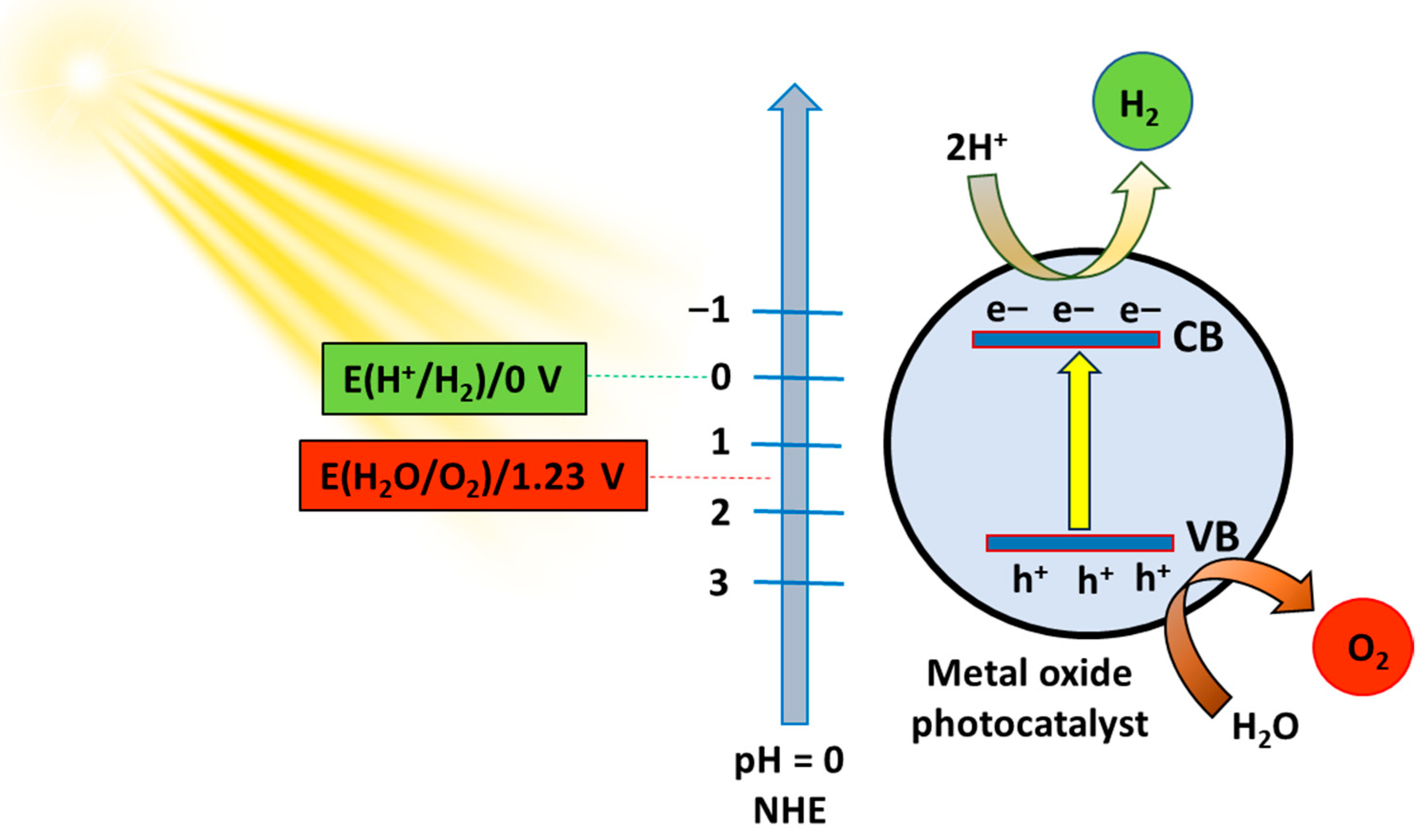
2. Semiconductor Photocatalysis
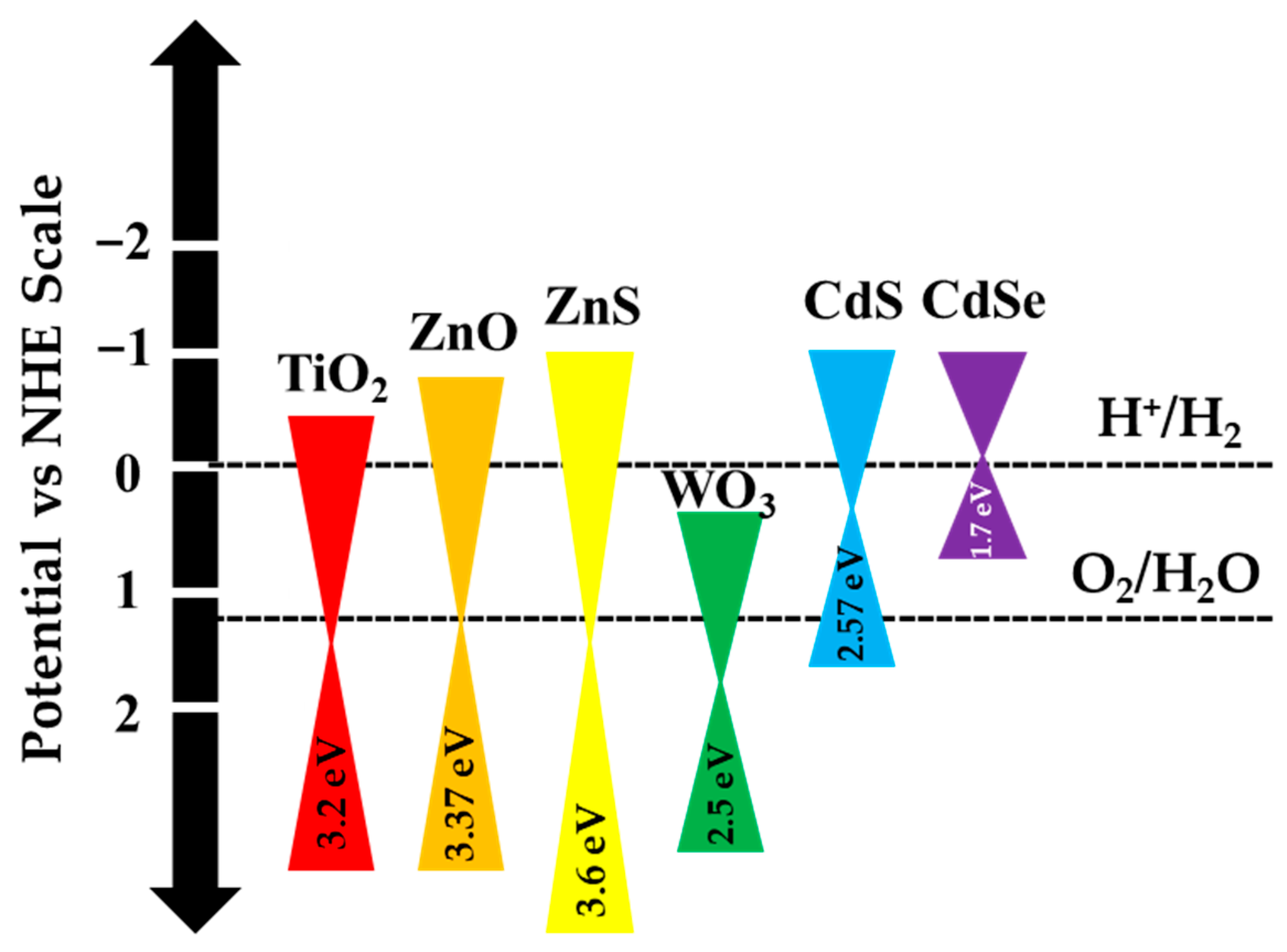
3. Titanium Dioxide as Semiconductor in Photocatalysis
3.1. The Importance of TiO2 Modification
- i.
- Extend light absorption into the visible region.
- ii.
- Efficiently separate charge carriers and promote their transfer to surface reactants.
- iii.
- Incorporate suitable cocatalysts to boost photocatalytic activity.
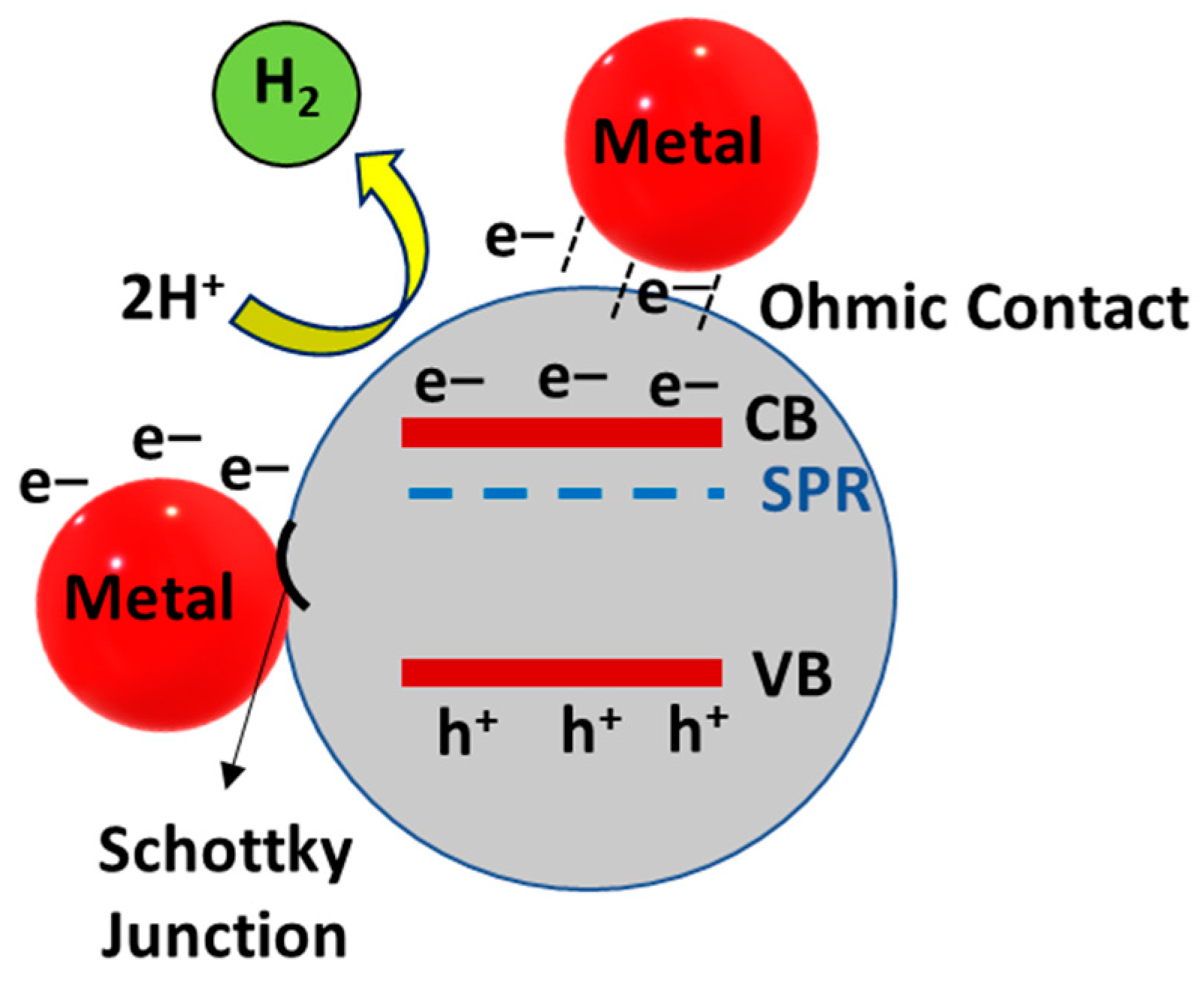
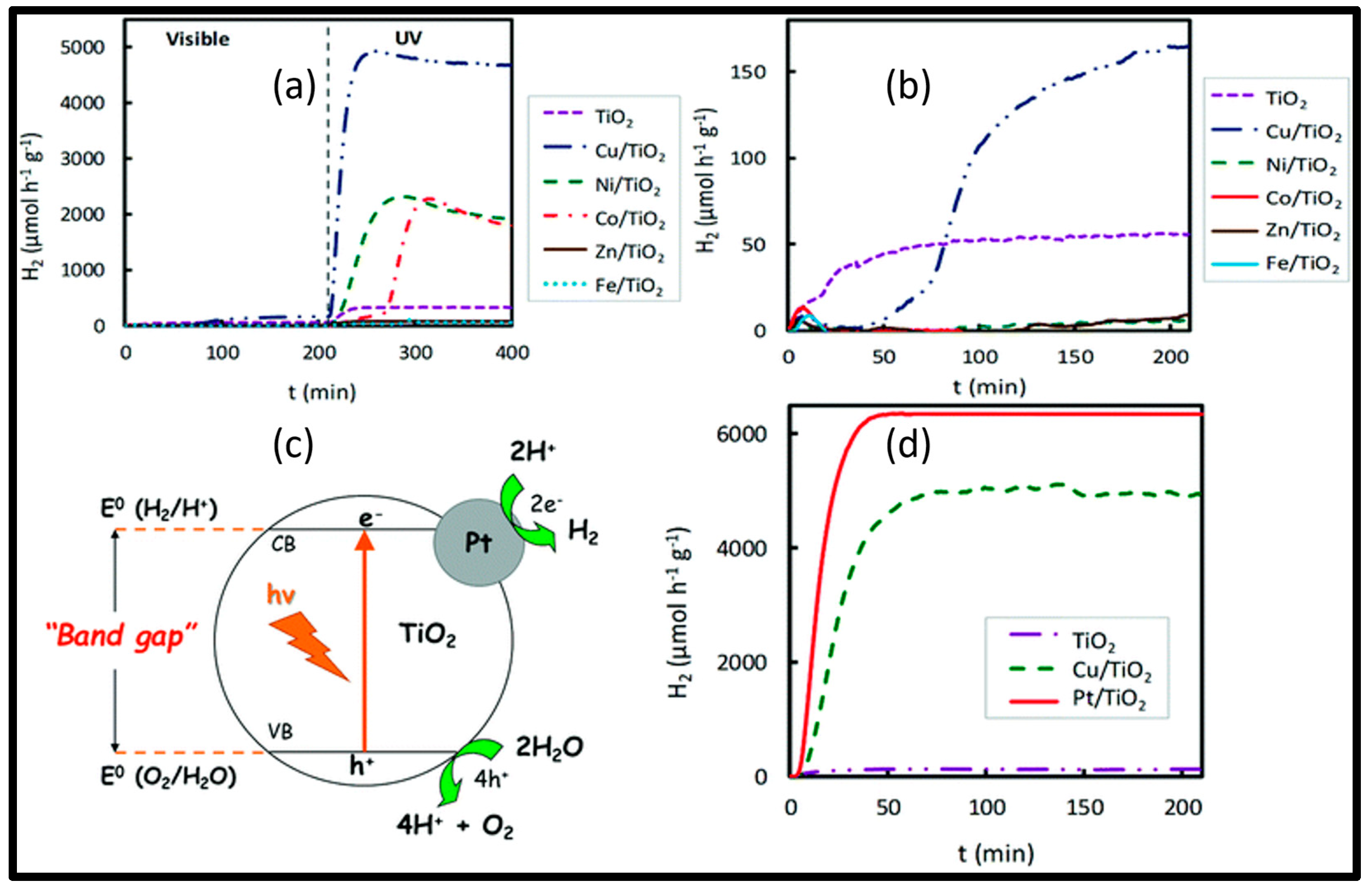
3.2. Metal Modification of TiO2
4. Porphyrin-Doped TiO2 for Photocatalytic Hydrogen Production
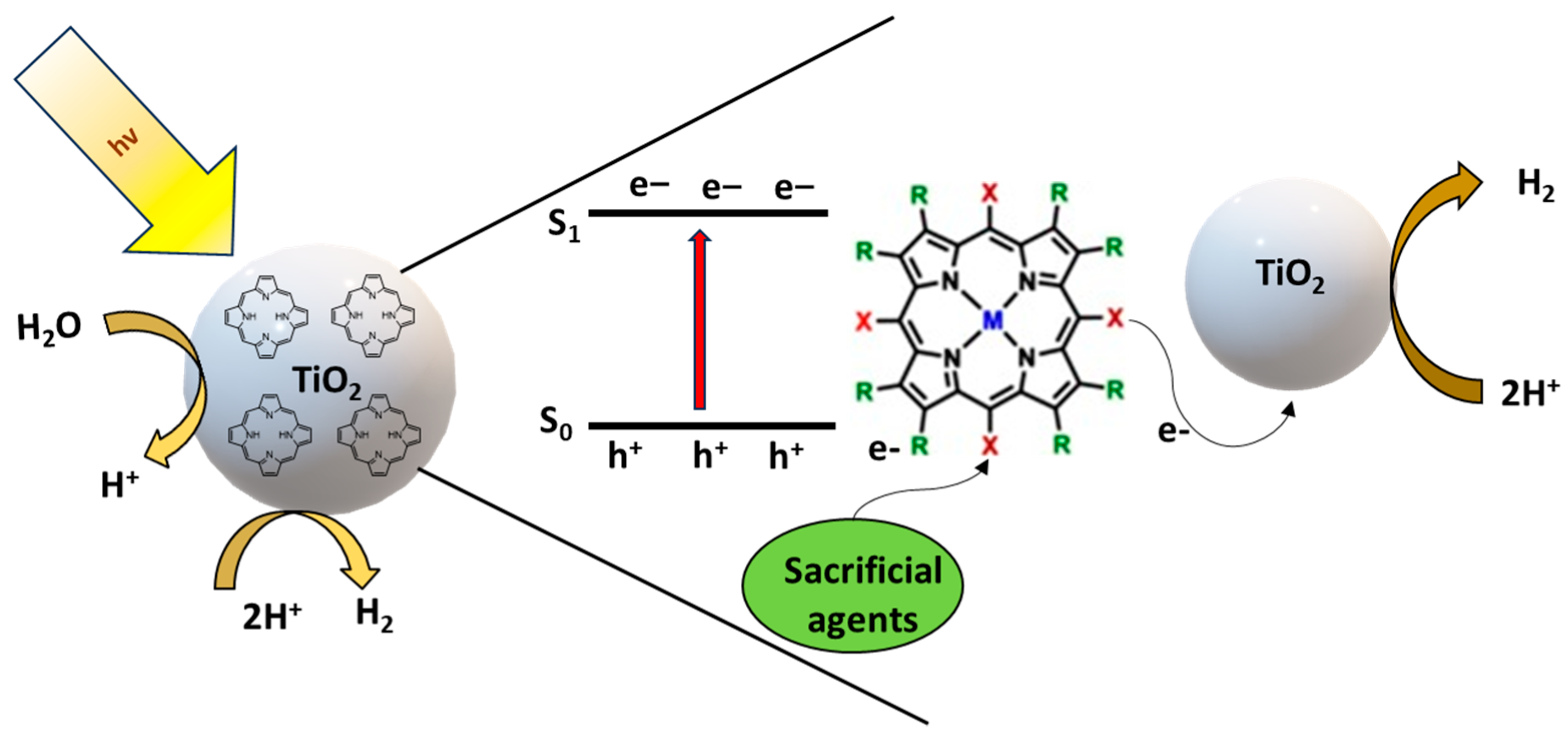
4.1. Mechanism of Hydrogen Production in Porphyrin-Doped TiO2 Systems
4.2. Types of Porphyrins and Their Suitability
4.3. Recent Examples of Porphyrin-Doped TiO2 Systems for Hydrogen Production
4.4. Drawbacks and Challenges of Porphyrin-Doped TiO2 Systems for Hydrogen Production
5. Conclusions
6. Future Perspectives and Environmental Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Global Energy Crisis. Available online: https://www.iea.org/topics/global-energy-crisis (accessed on 14 January 2025).
- Zayer Kabeh, K.; Teimouri, A.; Changizian, S.; Ahmadi, P. Techno-economic assessment of small-scale gas to liquid technology to reduce waste flare gas in a refinery plant. Sustain. Energy Technol. Assess. 2023, 55, 102955. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Teets, T.S.; Nocera, D.G. Photocatalytic hydrogen production. Chem. Commun. 2011, 47, 9268–9274. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Wei, D.; Shi, X.; Qu, R.; Junge, K.; Junge, H.; Beller, M. Toward a Hydrogen Economy: Development of Heterogeneous Catalysts for Chemical Hydrogen Storage and Release Reactions. ACS Energy Lett. 2022, 7, 3734–3752. [Google Scholar] [CrossRef]
- Love, J.G.; O’Mullane, A.P.; Boulaire, F.A.; Mackinnon, I.D.R. Impact of fuel cells on hydrogen energy pathways in a sustainable energy economy. Sustain. Energy Fuels 2022, 6, 4008–4023. [Google Scholar] [CrossRef]
- van der Spek, M.; Banet, C.; Bauer, C.; Gabrielli, P.; Goldthorpe, W.; Mazzotti, M.; Munkejord, S.T.; Røkke, N.A.; Shah, N.; Sunny, N.; et al. Perspective on the hydrogen economy as a pathway to reach net-zero CO2 emissions in Europe. Energy Environ. Sci. 2022, 15, 1034–1077. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Orfanos, E.; Ladomenou, K.; Angaridis, P.A.; Papadopoulos, T.; Charalambidis, G.; Vasilopoulou, M.; Coutsolelos, A.G. A stable platinum porphyrin based photocatalyst for hydrogen production under visible light in water. Sustain. Energy Fuels 2022, 6, 5072–5076. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Huang, J.-F.; Lei, Y.; Qin, S.; Liu, J.-M. Porous Hybrid Materials Based on Mesotetrakis(Hydroxyphenyl) Porphyrins and TiO2 for Efficient Visible-Light-Driven Hydrogen Production. Catalysts 2020, 10, 656. [Google Scholar] [CrossRef]
- Ladomenou, K.; Natali, M.; Iengo, E.; Charalampidis, G.; Scandola, F.; Coutsolelos, A.G. Photochemical hydrogen generation with porphyrin-based systems. Coord. Chem. Rev. 2015, 304–305, 38–54. [Google Scholar] [CrossRef]
- Hausmann, J.N.; Schlögl, R.; Menezes, P.W.; Driess, M. Is direct seawater splitting economically meaningful? Energy Environ. Sci. 2021, 14, 3679–3685. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Checchetti, A.; Fantini, A. Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric Effect. World J. Chem. Educ. 2015, 3, 87–92. [Google Scholar] [CrossRef]
- Jaffe, H.H.; Miller, A.L. The fates of electronic excitation energy. J. Chem. Educ. 1966, 43, 469. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Gómez-Avilés, A.; Peñas-Garzón, M.; Rodriguez, J.J. Chapter 22—Semiconductor Photocatalysis for Water Purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–651. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Meng, L.; Tian, W.; Li, L. Recent Progress on Semiconductor Heterojunction-Based Photoanodes for Photoelectrochemical Water Splitting. Small Sci. 2022, 2, 2100112. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge Carrier Dynamics at TiO2 Particles: Reactivity of Free and Trapped Holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Zhao, D.; Zhuang, Z.; Cao, X.; Zhang, C.; Peng, Q.; Chen, C.; Li, Y. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 2020, 49, 2215–2264. [Google Scholar] [CrossRef]
- Goddard, W.A. Electrocatalytic Water Splitting (H2O→H2 + ½ O2). In Computational Materials, Chemistry, and Biochemistry: From Bold Initiatives to the Last Mile: In Honor of William A. Goddard’s Contributions to Science and Engineering; Shankar, S., Muller, R., Dunning, T., Chen, G.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1257–1264. [Google Scholar] [CrossRef]
- Zhang, W.L.; Wei, W.; Liu, W.; Guan, T.; Tian, Y.; Zeng, H. Engineering the morphology of TiO2/carbon hybrids via oxidized Ti3C2Tx MXene and associated electrorheological activities. Chem. Eng. J. 2019, 378, 122170. [Google Scholar] [CrossRef]
- Wang, H.-X.; Li, X.-X.; Tang, L. Effects of surfactants on the morphology and properties of TiO2. Appl. Phys. A 2020, 126, 448. [Google Scholar] [CrossRef]
- Najafi, M.; Kermanpur, A.; Rahimipour, M.R.; Najafizadeh, A. Effect of TiO2 morphology on structure of TiO2-graphene oxide nanocomposite synthesized via a one-step hydrothermal method. J. Alloys Compd. 2017, 722, 272–277. [Google Scholar] [CrossRef]
- Nasir, J.A.; Rehman, Z.u.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent developments and perspectives in CdS-based photocatalysts for water splitting. J. Mater. Chem. A 2020, 8, 20752–20780. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Yang, D.-X.; Qu, D.; Miao, X.; Jiang, W.-S.; An, L.; Wen, Y.-J.; Wu, D.-D.; Sun, Z.-C. TiO2 sensitized by red-, green-, blue-emissive carbon dots for enhanced H2 production. Rare Met. 2019, 38, 404–412. [Google Scholar] [CrossRef]
- Rajaambal, S.; Sivaranjani, K.; Gopinath, C.S. Recent developments in solar H2 generation from water splitting. J. Chem. Sci. 2015, 127, 33–47. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Mbuyazi, T.B.; Oluwalana, A.E. Lead Sulphide Nanoparticles as Photocatalyst for the Degradation of Methylene Blue: Effects of pH, Time, Adsorption Kinetics and Recyclability Studies. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2197–2208. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium Dioxide (Anatase and Rutile): Surface Chemistry, Liquid–Solid Interface Chemistry, and Scientific Synthesis of Supported Catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Sultana, M.; Mondal, A.; Islam, S.; Khatun, M.A.; Rahaman, M.H.; Chakraborty, A.K.; Rahman, M.S.; Rahman, M.M.; Nur, A.S.M. Strategic development of metal doped TiO2 photocatalysts for enhanced dye degradation activity under UV–Vis irradiation: A review. Curr. Res. Green Sustain. Chem. 2023, 7, 100383. [Google Scholar] [CrossRef]
- Cai, Y.; Feng, Y.P. Review on charge transfer and chemical activity of TiO2: Mechanism and applications. Prog. Surf. Sci. 2016, 91, 183–202. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.; Henglein, A.; Lilie, J.; Spanhel, L. Flash photolysis observation of the absorption spectra of trapped positive holes and electrons in colloidal titanium dioxide. J. Phys. Chem. 1984, 88, 709–711. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, F.; Xu, S.-Q.; Zhu, S.; Zhang, Y.; Liu, H.; Zong, P.; Ma, L.; Hong, K.; Wang, Y. Boosting Photocatalytic Hydrogen Evolution by a Light Coupling and Charge Carrier Confinement Strategy. Adv. Funct. Mater. 2025, 35, 2417553. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.; Zhang, S.; Zhang, X.; Wang, Z.; Qin, W.; Wang, J.; Zhou, W.; Liu, H.; Sang, Y. Suppressing Photoinduced Charge Recombination via the Lorentz Force in a Photocatalytic System. Adv. Sci. 2019, 6, 1901244. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Augustin, A.; Ganguly, P.; Shenoy, S.; Chuaicham, C.; Pillai, S.C.; Sasaki, K.; Lee, A.F.; Sekar, K. Impact of Hole Scavengers on Efficient Photocatalytic Hydrogen Production. Adv. Sustain. Syst. 2024, 8, 2400321. [Google Scholar] [CrossRef]
- Rozman, N.; Nadrah, P.; Cornut, R.; Jousselme, B.; Bele, M.; Dražić, G.; Gaberšček, M.; Kunej, Š.; Škapin, A.S. TiO2 photocatalyst with single and dual noble metal co-catalysts for efficient water splitting and organic compound removal. Int. J. Hydrogen Energy 2021, 46, 32871–32881. [Google Scholar] [CrossRef]
- Ding, D.; Liu, K.; He, S.; Gao, C.; Yin, Y. Ligand-Exchange Assisted Formation of Au/TiO2 Schottky Contact for Visible-Light Photocatalysis. Nano Lett. 2014, 14, 6731–6736. [Google Scholar] [CrossRef]
- Yu, S.; Han, B.; Lou, Y.; Liu, Z.; Qian, G.; Wang, Z. Rational design and fabrication of TiO2 nano heterostructure with multi-junctions for efficient photocatalysis. Int. J. Hydrogen Energy 2020, 45, 28640–28650. [Google Scholar] [CrossRef]
- Brady, M.D.; Sampaio, R.N.; Wang, D.; Meyer, T.J.; Meyer, G.J. Dye-Sensitized Hydrobromic Acid Splitting for Hydrogen Solar Fuel Production. J. Am. Chem. Soc. 2017, 139, 15612–15615. [Google Scholar] [CrossRef]
- Jaafar, S.N.H.; Minggu, L.J.; Arifin, K.; Kassim, M.B.; Wan, W.R.D. Natural dyes as TIO2 sensitizers with membranes for photoelectrochemical water splitting: An overview. Renew. Sustain. Energy Rev. 2017, 78, 698–709. [Google Scholar] [CrossRef]
- Nikolaou, V.; Charalambidis, G.; Ladomenou, K.; Nikoloudakis, E.; Drivas, C.; Vamvasakis, I.; Panagiotakis, S.; Landrou, G.; Agapaki, E.; Stangel, C.; et al. Controlling Solar Hydrogen Production by Organizing Porphyrins. ChemSusChem 2021, 14, 961–970. [Google Scholar] [CrossRef]
- Mulewa, W.; Tahir, M.; Amin, N.A.S. MMT-supported Ni/TiO2 nanocomposite for low temperature ethanol steam reforming toward hydrogen production. Chem. Eng. J. 2017, 326, 956–969. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, M.; Hou, L.; Xiao, L.; Li, Q.; Yang, J. Z-scheme BCN-TiO2 nanocomposites with oxygen vacancy for high efficiency visible light driven hydrogen production. Int. J. Hydrogen Energy 2017, 42, 28434–28444. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Kubacka, A.; Luque, R.; Fernández-García, M. Sunlight-Driven Hydrogen Production Using an Annular Flow Photoreactor and g-C3N4-Based Catalysts. ChemPhotoChem 2018, 2, 870–877. [Google Scholar] [CrossRef]
- Sun, S. N-Doped TiO2 Nanobelts with Coexposed (001) and (101) Facets and Their Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. ACS Appl. Mater. 2016, 8, 18126–18131. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hiltunen, J.; Sápi, A.; Avila, A.; Larsson, W.; Liao, H.-C.; Huuhtanen, M.; Tóth, G.; Shchukarev, A.; Laufer, N.; et al. Nitrogen-Doped Anatase Nanofibers Decorated with Noble Metal Nanoparticles for Photocatalytic Production of Hydrogen. ACS Nano 2011, 5, 5025–5030. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Banerjee, B.; Amoli, V.; Maurya, A.; Sinha, A.K.; Bhaumik, A. Green synthesis of Pt-doped TiO2 nanocrystals with exposed (001) facets and mesoscopic void space for photo-splitting of water under solar irradiation. Nanoscale 2015, 7, 10504–10512. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Rodríguez-Castellón, E.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. Measuring and interpreting quantum efficiency for hydrogen photo-production using Pt-titania catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]
- Fang, J.; Cao, S.-W.; Wang, Z.; Shahjamali, M.M.; Loo, S.C.J.; Barber, J.; Xue, C. Mesoporous plasmonic Au–TiO2 nanocomposites for efficient visible-light-driven photocatalytic water reduction. Int. J. Hydrogen Energy 2012, 37, 17853–17861. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Ouyang, W.; Muñoz-Batista, M.J.; Kubacka, A.; Luque, R.; Fernández-García, M. Enhancing photocatalytic performance of TiO2 in H2 evolution via Ru co-catalyst deposition. Appl. Catal. B Environ. 2018, 238, 434–443. [Google Scholar] [CrossRef]
- Díaz, L.; Rodríguez, V.D.; González-Rodríguez, M.; Rodríguez-Castellón, E.; Algarra, M.; Núñez, P.; Moretti, E. M/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts for photocatalytic hydrogen production under UV and visible light irradiation. Inorg. Chem. Front. 2021, 8, 3491–3500. [Google Scholar] [CrossRef]
- David, T.M.; Dev, P.R.; Wilson, P.; Sagayaraj, P.; Mathews, T. A critical review on the variations in anodization parameters toward microstructural formation of TiO2 nanotubes. Electrochem. Sci. Adv. 2022, 2, e202100083. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Tudu, B.; Nalajala, N.; Prabhakar Reddy, K.; Saikia, P.; Gopinath, C.S. Rationally Designed, Efficient, and Earth-Abundant Ni–Fe Cocatalysts for Solar Hydrogen Generation. ACS Sustain. Chem. Eng. 2021, 9, 13915–13925. [Google Scholar] [CrossRef]
- Pinna, M.; Wei, A.W.W.; Spanu, D.; Will, J.; Yokosawa, T.; Spiecker, E.; Recchia, S.; Schmuki, P.; Altomare, M. Amorphous NiCu Thin Films Sputtered on TiO2 Nanotube Arrays: A Noble-Metal Free Photocatalyst for Hydrogen Evolution. ChemCatChem 2022, 14, e202201052. [Google Scholar] [CrossRef]
- Gao, D.; Liu, W.; Xu, Y.; Wang, P.; Fan, J.; Yu, H. Core-shell Ag@Ni cocatalyst on the TiO2 photocatalyst: One-step photoinduced deposition and its improved H2-evolution activity. Appl. Catal. B Environ. 2020, 260, 118190. [Google Scholar] [CrossRef]
- Ngo, N.M.; Nguyen, M.D.; Tran, H.-V.; Medhi, R.; Lee, J.M.; Lee, T.R. Photocatalytic Hydrogen Generation by Monodisperse TiO2 Nanoparticles Singly and Dually Doped with Niobium and Tantalum. ACS Appl. Nano Mater. 2025, 8, 4841–4851. [Google Scholar] [CrossRef]
- Afrin, M.F.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Uzzaman, M.; Kaneco, S. Enhanced Photocatalytic Hydrogen Generation from Methanol Solutions via In Situ Ni/Pt Co-Deposition on TiO2. J. Compos. Sci. 2025, 9, 68. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A critical review of metal-doped TiO2 and its structure–physical properties–photocatalytic activity relationship in hydrogen production. Int. J. Hydrogen Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Charisiadis, A.; Nikolaou, V.; Nikoloudakis, E.; Ladomenou, K.; Charalambidis, G.; Coutsolelos, A.G. Metalloporphyrins in bio-inspired photocatalytic conversions. Chem. Commun. 2025, 61, 4630–4646. [Google Scholar] [CrossRef]
- Landrou, G.; Panagiotopoulos, A.A.; Ladomenou, K.; Coutsolelos, A.G. Photochemical hydrogen evolution using Sn-porphyrin as photosensitizer and a series of Cobaloximes as catalysts. J. Porphyr. Phthalocyanines 2016, 20, 534–541. [Google Scholar] [CrossRef]
- Xing, X.; Zhu, H.; Zhang, M.; Hou, L.; Li, Q.; Yang, J. Interfacial oxygen vacancy layer of a Z-scheme BCN–TiO2 heterostructure accelerating charge carrier transfer for visible light photocatalytic H2 evolution. Catal. Sci. Technol. 2018, 8, 3629–3637. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Qin, L.; Kang, S.-Z.; Li, G. A highly active nano-micro hybrid derived from Cu-bridged TiO2/porphyrin for enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2019, 243, 1–9. [Google Scholar] [CrossRef]
- Gonuguntla, S.; Tiwari, A.; Madanaboina, S.; Lingamallu, G.; Pal, U. Revealing high hydrogen evolution activity in zinc porphyrin sensitized hierarchical porous TiO2 photocatalysts. Int. J. Hydrogen Energy 2020, 45, 7508–7516. [Google Scholar] [CrossRef]
- Chanda, N.; Koteshwar, D.; Gonuguntla, S.; Bojja, S.; Pal, U.; Giribabu, L. Efficient visible-light-driven hydrogen production by Zn–porphyrin based photocatalyst with engineered active donor–acceptor sites. Mater. Adv. 2021, 2, 4762–4771. [Google Scholar] [CrossRef]
- Gkika, D.A.; Ladomenou, K.; Bououdina, M.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption and photocatalytic applications of porphyrin-based materials for environmental separation processes: A review. Sci. Total Environ. 2024, 908, 168293. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; López-Duarte, I.; Charalambidis, G.; Ladomenou, K.; Ince, M.; Coutsolelos, A.G. Porphyrins and phthalocyanines as biomimetic tools for photocatalytic H2 production and CO2 reduction. Chem. Soc. Rev. 2022, 51, 6965–7045. [Google Scholar] [CrossRef]
- Nikolaou, V.; Agapaki, E.; Nikoloudakis, E.; Achilleos, K.; Ladomenou, K.; Charalambidis, G.; Triantafyllou, E.; Coutsolelos, A.G. Highly efficient light-driven hydrogen evolution utilizing porphyrin-based nanoparticles. Chem. Commun. 2023, 59, 11256–11259. [Google Scholar] [CrossRef]
- Agapaki, E.; Ladomenou, K.; Nikolaou, V.; Coutsolelos, A.G. Efficient solar hydrogen production of zinc trimesityl porphyrin-based photocatalysts. J. Porphyr. Phthalocyanines 2023, 27, 479–489. [Google Scholar] [CrossRef]
- Wang, D.; Niu, L.; Qiao, Z.-Y.; Cheng, D.-B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, Y.; Ma, Q.; Gao, D.; Zhong, J.; Li, J.; Wang, F.; He, Y.; Wang, R. A flower-like TiO2 with photocatalytic hydrogen evolution activity modified by Zn(II) porphyrin photocatalysts. J. Mater. Sci. Mater. Electron. 2017, 28, 2123–2127. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, W.; Kang, S.-Z.; Qin, L.; Zhang, T.; Li, X. Facile interfacial modification in a novel TiO2/porphyrin nano-micro hybrid for dual functions of boosting photocatalytic H2 production and methylene blue degradation. Appl. Surf. Sci. 2024, 644, 158783. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Zhong, Y.; Wang, G.; Liu, S.; Chen, S.; Qi, C.; He, M.; Shangguan, P.; Luo, Z.; et al. Self-Assembly Regulated Photocatalysis of Porphyrin-TiO2 Nanocomposites. Molecules 2024, 29, 3872. [Google Scholar] [CrossRef]
- Wang, S.; Hu, G.; Dou, Y.; Li, S.; Li, M.; Feng, H.; Feng, Y.-S. Z-scheme promoted interfacial charge transfer on Cu/In-porphyrin MOFs/CdIn2S4 heterostructure for efficient photocatalytic H2 evolution. Sep. Purif. Technol. 2025, 354, 129220. [Google Scholar] [CrossRef]
- Myltykbayeva, Z.; López Nieto, J.M.; Moreno-Torralbo, B.M.; Concepción, P.; Abylaikhan, A.; Koca, A. Optimizing the electrocatalytic hydrogen production with vanadyl porphyrin impregnated on mesoporous SiO2 nanoparticles. Electrochim. Acta 2025, 514, 145686. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Q.; Wang, X.; Li, B.; Li, X.; Yang, X.; Li, W.; Zhang, H. Integration of platinum nanoparticles and Pd-porphyrin photosensitiser into a metal–organic framework for effective photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2025, 685, 165–172. [Google Scholar] [CrossRef]
- Van Tran, C.; Dang, T.-D.; Lai, V.D.; Thi Nguyen, G.; Thi Hoa, B.; Nguyen, H.T.; Thi, H.P.N.; Nguyen, T.T.; La, D.D. Self-assembly of porphyrin nanofiber on the surface CuFe2O4 nanoparticles: A novel photoanode for enhanced photo-electrochemical water splitting. Fuel 2025, 380, 133196. [Google Scholar] [CrossRef]

| Entry | Photocatalyst | Metal Concentration | Light Source | H2 Production Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | Pt/TiO2 | 1.0 wt% Pt | AM 1.5 G sunlight | 11.2 mmol h−1 g−1 | [59] |
| 2 | Pt/TiO2 | 5.0 wt% Pt | UV (280–400 nm) | 27.6 mmol h−1 g−1 | [60] |
| 3 | Au/TiO2 | 2.0 wt% Au | Vis (500 ± 20 nm) | 0.05702 mmol h−1 g−1 | [61] |
| 4 | Ag/TiO2 | 1.5 wt% Ag | UV (365 nm) | 23.5 mmol h−1 g−1 | [62] |
| 5 | Ru/TiO2 | 3.0 wt% Ru | UV (280–400 nm) | 4.7 mmol h−1 g−1 | [63] |
| 6 | Cu/TiO2 | 2.0 wt% Cu | UV (365 nm) | 5.0 mmol h−1 g−1 | [64] |
| 7 | Ni/TiO2 | 2.0 wt% Ni | UV (365 nm) | 2.3 mmol h−1 g−1 | [64] |
| 8 | Ni/TiO2 | 5.0 wt% Ni | UV (365 nm) | 1.608 mmol h−1g−1 | [65] |
| 9 | Co/TiO2 | 2.0 wt% Co | UV (365 nm) | 2.25 mmol h−1 g−1 | [64] |
| 10 | Co/TiO2 | 1.0 wt% Co | Sunlight | 0.22 mmol h−1 g−1 | [66] |
| 11 | Fe/TiO2 | 1.1 wt% Fe | UV (250 nm) to visible (750 nm) | 0.0155 mmol h−1 g−1 | [67] |
| 12 | Ni-Fe/TiO2 | 1 wt% Ni–Fe (1:3) | Sunlight | 8.27 mmol h−1 g−1 | [68] |
| 13 | Ni-Cu/TiO2 | 1 wt% Ni–Cu (1:1) | UV (365 nm) | 186 μL h−1 cm−2 | [69] |
| 14 | Ag-Ni/TiO2 | Ag-Ni (1.5:1.5) | UV (365 nm) | 2.9339 mmol h−1 g−1 | [70] |
| 15 | Nb-Ta/TiO2 (NTTO) | 2 wt% Nb and 4 wt% Ta | Simulated solar illumination | 1.168 mmol h−1 g−1 | [71] |
| 16 | Ni-Pt/TiO2 | 0.01 wt% Ni and 1.0 wt% Pt | 15 W black lamp (352 nm) | 3.983 mmol g−1 h−1 | [72] |
| Entry | Photocatalyst | Porphyrin | Light Source | H2 Production Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | Pt/TiO2-Pt-Tc3CP | Pt-Tc3CP | 40 W white LED | 707 mmol g−1 h−1 | [82] |
| 2 | Pt-TiO2 NPs-Zn-TM(pCOOH)P | ZnTM(pCOOH)P | 40 W white LED | 1959 mmol g−1 h−1 | [83] |
| 3 | PdTCP and PtTCP-TiO2 | PdTCP and PtTCP | visible light irradiation (λ > 420 nm) | 30,200 μmol g−1 | [52] |
| 4 | Pt/TiO2-THPP-Pd | THPP-Pd |
visible light irradiation
(λ > 420 nm) | 2025.4 µmol g−1 h −1 | [13] |
| 5 | TiO2 MS-Cu-TCPP | TCPP-Cu | 300 W Xenon | 1.3 mmol g−1 h−1 | [77] |
| 6 | Pt/TiO2-(LG-5) | Zinc–porphyrin dye (LG-5) | 450 W Xenon | 4196 mmol g−1 h−1 | [78] |
| 7 | Pt–TiO2-PCT-LG-23 | Zinc-PCT-LG-23 | 300 W Xenon | 9793.5 μmol g−1 h−1 | [79] |
| 8 | TiO2/ZnTmMpHPP + 0.5%Pt | ZnTmMpHPP | 300 W Xenon | 326.3 μmol h−1 | [85] |
| 9 | THPP-TiO2-H NPs | THPP | UV cutoff filter > 400 nm | 4.80 mmol g−1 | [87] |
| 10 | TiO2/Co/PA | TCPP | 300 W xenon lamp | 4318.4 μmol g−1 h−1 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitsos, D.R.; Salepis, A.; Orfanos, E.; Coutsolelos, A.G.; Kosheleva, R.I.; Mitropoulos, A.C.; Ladomenou, K. Exploring Metal- and Porphyrin-Modified TiO2-Based Photocatalysts for Efficient and Sustainable Hydrogen Production. Inorganics 2025, 13, 121. https://doi.org/10.3390/inorganics13040121
Bitsos DR, Salepis A, Orfanos E, Coutsolelos AG, Kosheleva RI, Mitropoulos AC, Ladomenou K. Exploring Metal- and Porphyrin-Modified TiO2-Based Photocatalysts for Efficient and Sustainable Hydrogen Production. Inorganics. 2025; 13(4):121. https://doi.org/10.3390/inorganics13040121
Chicago/Turabian StyleBitsos, Dimitrios Rafail, Apostolos Salepis, Emmanouil Orfanos, Athanassios G. Coutsolelos, Ramonna I. Kosheleva, Athanassios C. Mitropoulos, and Kalliopi Ladomenou. 2025. "Exploring Metal- and Porphyrin-Modified TiO2-Based Photocatalysts for Efficient and Sustainable Hydrogen Production" Inorganics 13, no. 4: 121. https://doi.org/10.3390/inorganics13040121
APA StyleBitsos, D. R., Salepis, A., Orfanos, E., Coutsolelos, A. G., Kosheleva, R. I., Mitropoulos, A. C., & Ladomenou, K. (2025). Exploring Metal- and Porphyrin-Modified TiO2-Based Photocatalysts for Efficient and Sustainable Hydrogen Production. Inorganics, 13(4), 121. https://doi.org/10.3390/inorganics13040121













