Boosting C-C Coupling for Electrochemical CO2 Reduction over Novel Cu-Cubic Catalysts with an Amorphous Shell
Abstract
:1. Introduction
2. Results
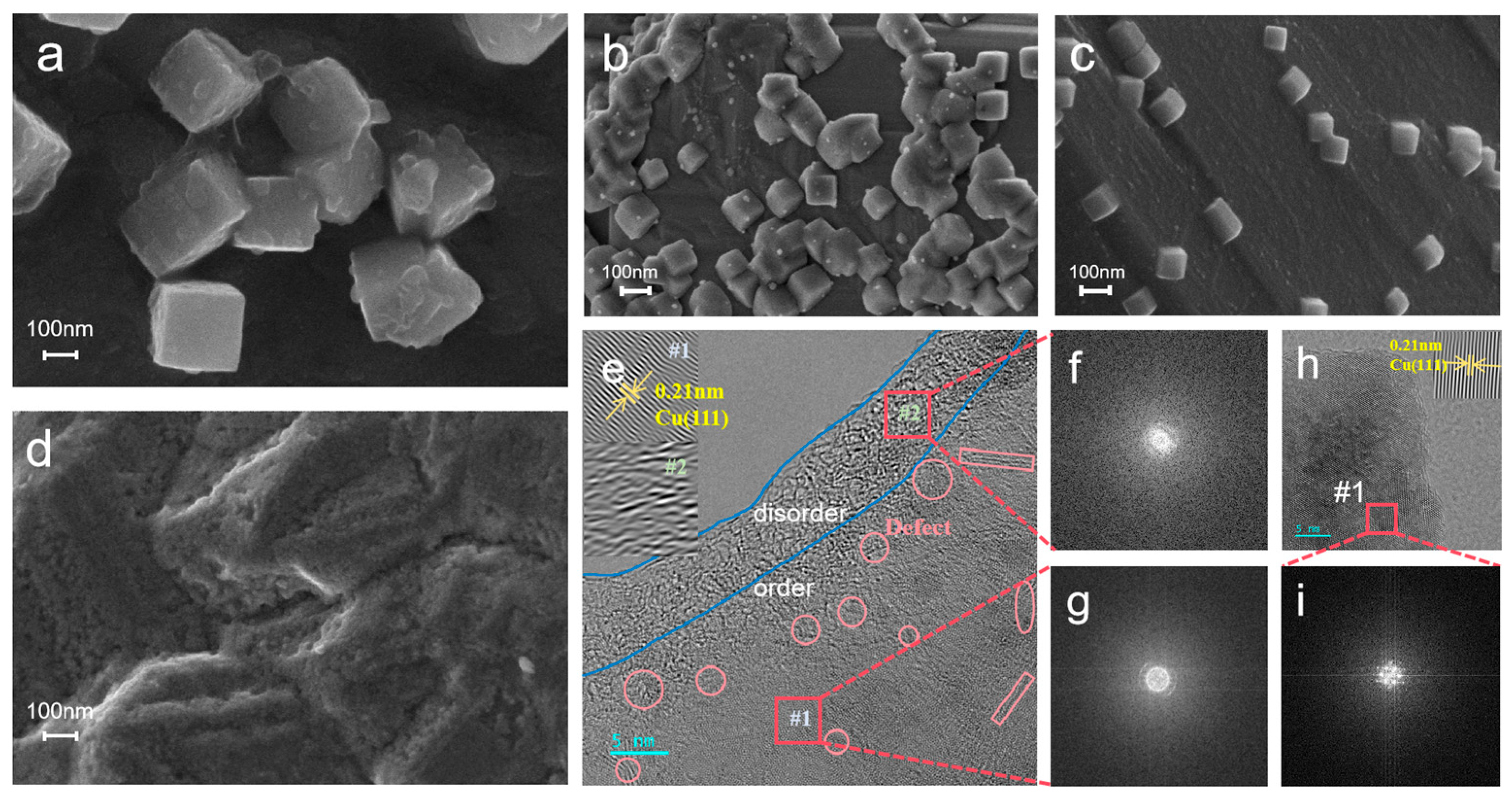
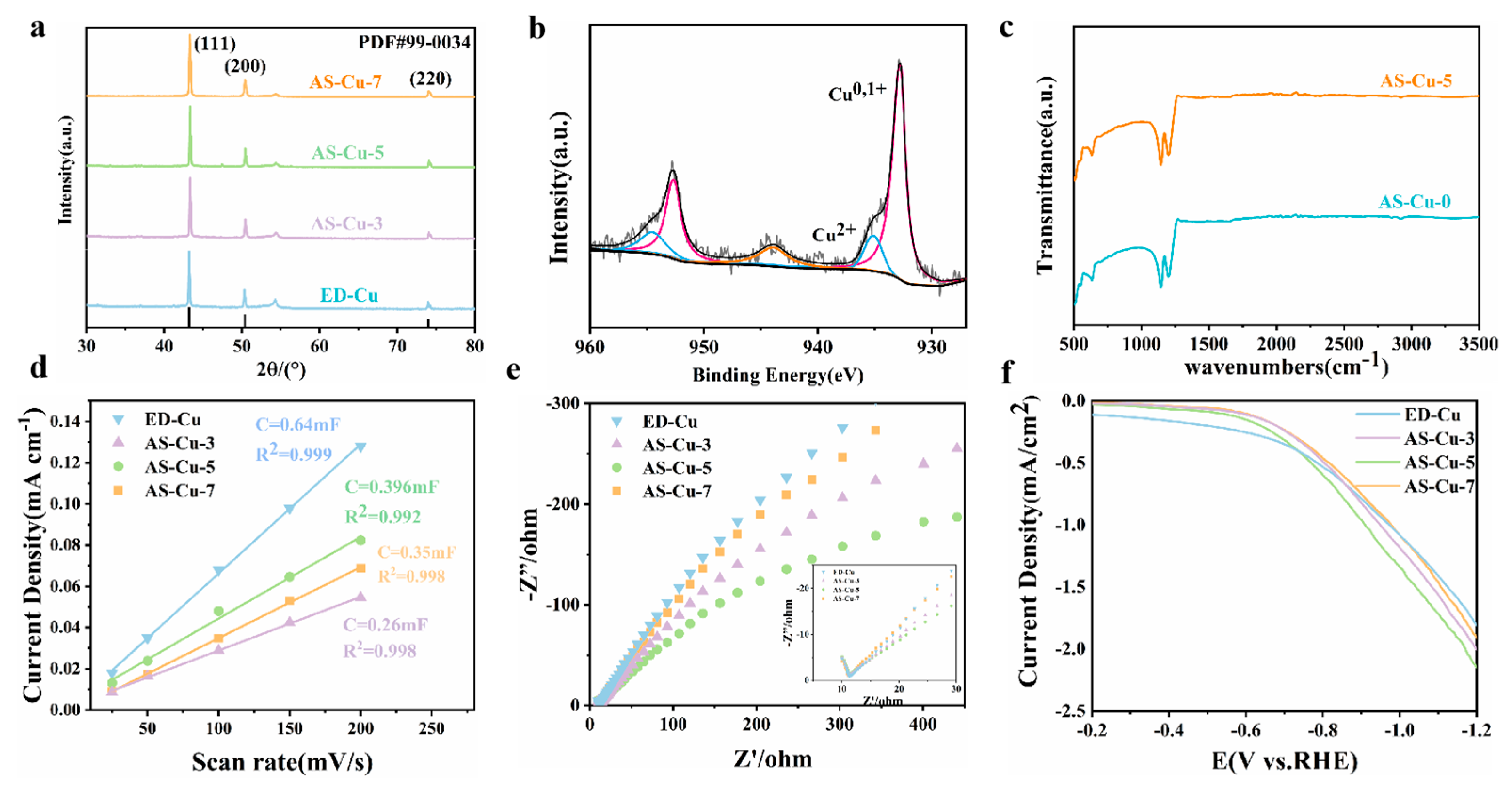
2.1. Structure and Morphology
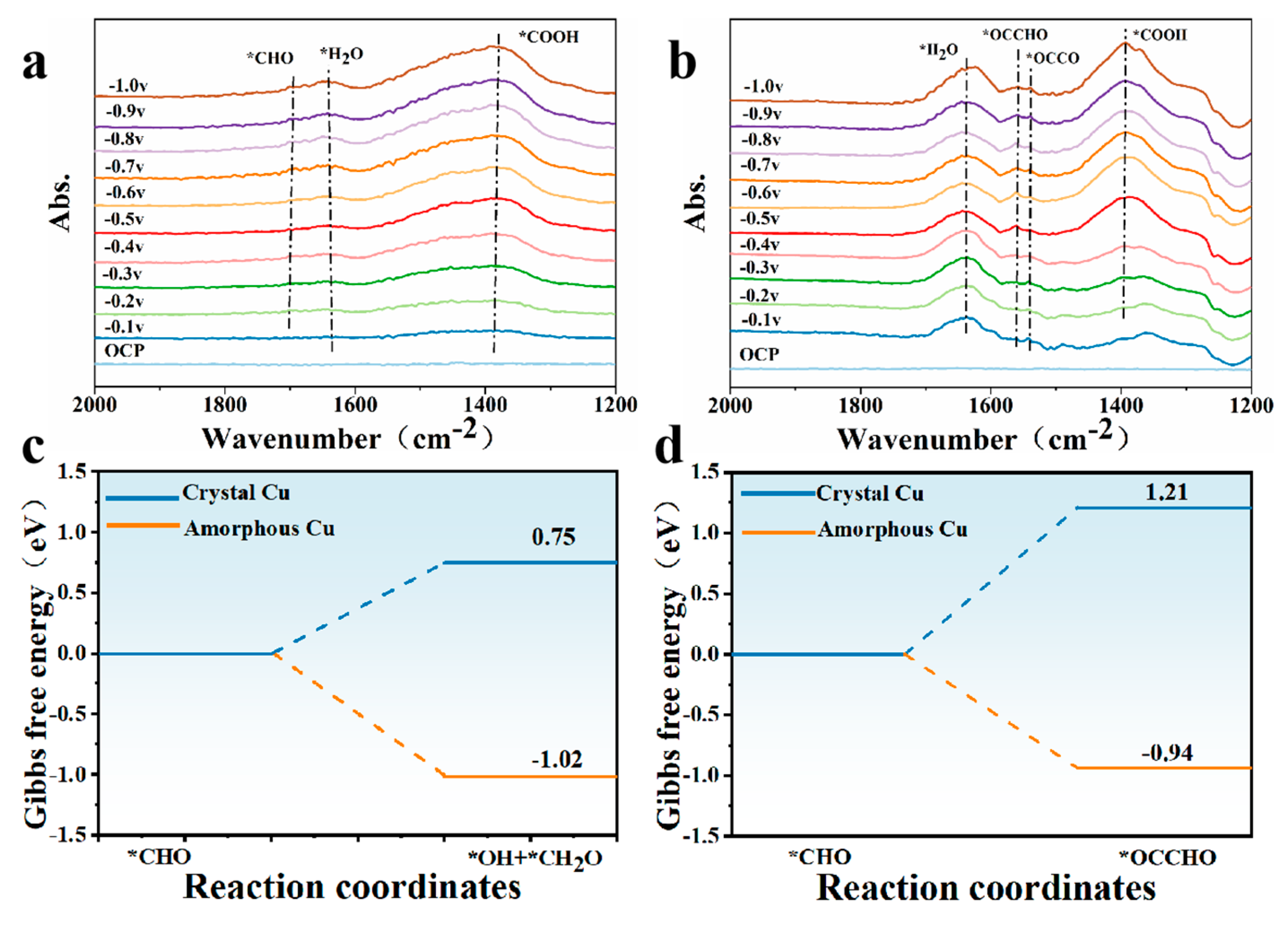
2.2. Activity for CO2 Reduction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Electrode
4.3. Electrochemical Measurements
4.4. In Situ ATR-SEIRAS Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gattrell, M.; Gupta, N.; Co, A. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007, 48, 1255–1265. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural transformation of heterogeneous materials for electrocatalytic oxygen evolution reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Gao, D.; Sinev, I.; Scholten, F.; Arán-Ais, R.M.; Divins, N.J.; Kvashnina, K.; Timoshenko, J.; Roldan Cuenya, B. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. Angew. Chem. Int. Ed. 2019, 58, 17047–17053. [Google Scholar] [CrossRef]
- Yang, Y.; He, A.; Yang, M.; Zou, Q.; Li, H.; Liu, Z.; Tao, C.; Du, J. Selective electroreduction of CO2 to ethanol over a highly stable catalyst derived from polyaniline/CuBi2O4. Catal. Sci. Technol. 2021, 11, 5908–5916. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Wang, T.; Liang, J.; Duan, S.; Xie, L.; Han, J.; Li, Q. Promoting C2+ production from electrochemical CO2 reduction on shape-controlled cuprous oxide nanocrystals with high-index facets. ACS Sustain. Chem. Eng. 2020, 8, 15223–15229. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Yang, P.-P.; Zhang, X.-L.; Gao, F.-Y.; Zheng, Y.-R.; Niu, Z.-Z.; Yu, X.; Liu, R.; Wu, Z.-Z.; Qin, S.; Chi, L.-P.; et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ Fuels. J. Am. Chem. Soc. 2020, 142, 6400–6408. [Google Scholar] [CrossRef]
- Abdinejad, M.; Farzi, A.; Möller-Gulland, R.; Mulder, F.; Liu, C.; Shao, J.; Biemolt, J.; Robert, M.; Seifitokaldani, A.; Burdyny, T. Eliminating redox-mediated electron transfer mechanisms on a supported molecular catalyst enables CO2 conversion to ethanol. Nat. Catal. 2024, 7, 1109–1119. [Google Scholar] [CrossRef]
- Tan, M.; Huang, B.; Su, L.; Jiao, X.; Feng, F.; Gao, Y.; Huang, Q.; Huang, Z.; Ge, Y. Amorphous nanomaterials: Emerging catalysts for electrochemical carbon dioxide reduction. Adv. Energy Mater. 2024, 14, 2402424. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, L.; Ge, Y.; Huang, Z.; Shi, Z.; Liu, J.; Zhai, W.; Liang, J.; Zhang, H. Wet-chemical synthesis of two-dimensional metal nanomaterials for electrocatalysis. Natl. Sci. Rev. 2022, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, W.; Shi, F.; Yang, X.; Li, X.; Wu, J.; Yin, Y.; Jin, M. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 2021, 598, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, W.; Luo, Q.; Yin, R.; Wang, B.; Weissenrieder, J.; Soldemo, M.; Yan, H.; Lin, Y.; Sun, Z.; et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 2019, 565, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.-Y.; Hu, S.-J.; Zhang, X.-L.; Zheng, Y.-R.; Wang, H.-J.; Niu, Z.-Z.; Yang, P.-P.; Bao, R.-C.; Ma, T.; Dang, Z.; et al. High-curvature transition-metal chalcogenide nanostructures with a pronounced proximity effect enable fast and selective CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 8706–8712. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, P.; Yun, Q.; Ge, Y.; Zhang, X.; Chen, Y.; Zhang, H. Phase engineering of metal nanocatalysts for electrochemical CO2 reduction. eScience 2022, 2, 467–485. [Google Scholar] [CrossRef]
- Feijóo, J.; Yang, Y.; Fonseca Guzman, M.V.; Vargas, A.; Chen, C.; Pollock, C.J.; Yang, P. Operando high-energy-resolution X-ray spectroscopy of evolving Cu nanoparticle electrocatalysts for CO2 reduction. J. Am. Chem. Soc. 2023, 145, 20208–20213. [Google Scholar] [CrossRef]
- Ahmad, T.; Liu, S.; Sajid, M.; Li, K.; Ali, M.; Liu, L.; Chen, W. Electrochemical CO2 reduction to C2+ products using Cu-based electrocatalysts: A review. Nano Res. Energy 2022, 1, 9120021. [Google Scholar] [CrossRef]
- Tan, Z.; Peng, T.; Tan, X.; Wang, W.; Wang, X.; Yang, Z.; Ning, H.; Zhao, Q.; Wu, M. Controllable synthesis of leaf-like CuO nanosheets for selective CO2 electroreduction to ethylene. ChemElectroChem 2020, 7, 2020–2025. [Google Scholar] [CrossRef]
- Luo, H.; Li, B.; Ma, J.-G.; Cheng, P. Surface modification of nano-Cu2O for controlling CO2 electrochemical reduction to ethylene and syngas. Angew. Chem. Int. Ed. 2022, 61, e202116736. [Google Scholar] [CrossRef]
- Keerthiga, G.; Chetty, R. Electrochemical reduction of CO2 on flame annealed Cu. ChemElectroChem 2021, 6, 2887–2892. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Selective formation of C2 compounds from electrochemical reduction of CO2 at a series of copper single crystal electrodes. J. Phys. Chem. B 2002, 106, 15–17. [Google Scholar] [CrossRef]
- Jiao, J.; Lin, R.; Liu, S.; Cheong, W.-C.; Zhang, C.; Chen, Z.; Pan, Y.; Tang, J.; Wu, K.; Hung, S.-F.; et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem. 2019, 11, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, Y.; Quan, X.; Chen, S.; Yu, H. CO2 electroreduction at low overpotential on oxide-derived Cu/carbons fabricated from metal organic framework. Acs Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.; Chen, Z.; Quan, Y.; Peng, C.; Wang, Z.; Sham, T.-K.; Yang, C.; Ji, Y.; Qian, L.; Xu, X.; et al. Boosting CO2 electroreduction to CH4 via tuning neighboring single-Copper sites. ACS Energy Lett. 2020, 5, 1044–1053. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Liu, B.; Cheng, D.; Hu, C.; Zhang, G.; Zhu, W.; Wang, H.; Zhao, Z.-J.; Gong, J. Grain-boundary-rich copper for efficient solar-driven electrochemical CO2 reduction to ethylene and ethanol. J. Am. Chem. Soc. 2020, 142, 6878–6883. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Fan, L.; Chen, J.; Zhang, T.; Chen, J.; Xi, S.; Chen, B.; Wang, L. Additive-assisted electrodeposition of Cu on gas diffusion electrodes enables selective CO2 reduction to multicarbon products. ACS Catal. 2023, 13, 11934–11944. [Google Scholar] [CrossRef]
- Li, S.-J.; Bao, D.; Shi, M.-M.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Amorphizing of Au Nanoparticles by CeO–RGO Hybrid Support towards Highly Efficient Electrocatalyst for N2 Reduction under Ambient Conditions. Adv. Mater. 2017, 29, 1700001. [Google Scholar] [CrossRef]
- Chen, C.; Yan, X.; Wu, Y.; Zhang, X.; Liu, S.; Zhang, F.; Sun, X.; Zhu, Q.; Zheng, L.; Zhang, J.; et al. Oxidation of metallic Cu by supercritical CO2 and control synthesis of amorphous nano-metal catalysts for CO2 electroreduction. Nat. Commun. 2023, 14, 1092. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Q.; Wei, S.; Guo, X.; Chimtali, P.J.; Xu, W.; Chen, S.; Cao, Y.; Zhang, P.; Zhu, K.; et al. Anion additive integrated electric double layer and solvation shell for aqueous zinc ion battery. Small Methods 2024, 8, 2301115. [Google Scholar] [CrossRef]
- Lim, C.F.C.; Harrington, D.A.; Marshall, A.T. Effects of mass transfer on the electrocatalytic CO2 reduction on Cu. Electrochim. Acta 2017, 238, 56–63. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, S.; Cheng, D.; Li, L.; Zhu, W.; Zhong, D.; Zhao, Z.-J.; Li, J.; Wang, T.; Gong, J. Controllable Cu0-Cu+ sites for electrocatalytic reduction of carbon dioxide. Angew. Chem. Int. Ed. 2021, 60, 15344–15347. [Google Scholar] [CrossRef] [PubMed]
- Vennekoetter, J.-B.; Sengpiel, R.; Wessling, M. Beyond the catalyst: How electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 2019, 364, 89–101. [Google Scholar] [CrossRef]
- Geng, Z.; Kong, X.; Chen, W.; Su, H.; Liu, Y.; Cai, F.; Wang, G.; Zeng, J. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO. Angew. Chem. Int. Ed. 2018, 57, 6054–6059. [Google Scholar] [CrossRef]
- Jia, S.; Zhu, Q.; Wu, H.; Chu, M.; Han, S.; Feng, R.; Tu, J.; Zhai, J.; Han, B. Efficient electrocatalytic reduction of carbon dioxide to ethylene on copper–antimony bimetallic alloy catalyst. Chin. J. Catal. 2020, 41, 1091–1098. [Google Scholar] [CrossRef]
- Wu, T.; Han, M.Y.; Xu, Z.J. Size effects of electrocatalysts: More than a variation of surface area. ACS Nano 2022, 16, 8531–8539. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Ma, S.; Gold, J.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis. ACS Catal. 2017, 7, 3313–3321. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Li, Z.; Xia, S.; Cai, R.; Ma, L.; Zhang, T.; Ackley, J.; Yang, S.; Wu, Y.; et al. Grain boundary-derived Cu+/Cu0 interfaces in CuO nanosheets for low overpotential carbon dioxide electroreduction to ethylene. Adv. Sci. 2022, 9, e2200454. [Google Scholar] [CrossRef]
- Chou, T.-C.; Chang, C.-C.; Yu, H.-L.; Yu, W.-Y.; Dong, C.-L.; Velasco-Vélez, J.-J.; Chuang, C.-H.; Chen, L.-C.; Lee, J.-F.; Chen, J.-M.; et al. Controlling the oxidation state of the Cu electrode and reaction intermediates for electrochemical CO2 reduction to ethylene. J. Am. Chem. Soc. 2020, 142, 2857–2867. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Yue, K.; Qin, Y.; Huang, H.; Lv, Z.; Cai, M.; Su, Y.; Huang, F.; Yan, Y. Stabilized Cu0-Cu1+ dual sites in a cyanamide framework for selective CO2 electroreduction to ethylene. Nat. Commun. 2024, 15, 7820. [Google Scholar] [CrossRef]
- Deng, B.; Huang, M.; Li, K.; Zhao, X.; Geng, Q.; Chen, S.; Xie, H.; Dong, X.; Wang, H.; Dong, F. The Crystal plane is not the key factor for CO2-to-methane electrosynthesis on reconstructed Cu2O microparticles. Angew. Chem. Int. Ed. 2021, 134, e202114080. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Wan, H.; Li, C.; An, W.; Sheng, X.; Liang, X.; Wang, X.; Ren, Y.; Zheng, X.; et al. Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction. Chin. Chem. Lett. 2022, 33, 2259–2269. [Google Scholar] [CrossRef]
- Chhetri, M.; Wan, M.; Jin, Z.; Yeager, J.; Sandor, C.; Rapp, C.; Wang, H.; Lee, S.; Bodenschatz, C.J.; Zachman, M.J.; et al. Dual-site catalysts featuring platinum-group-metal atoms on copper shapes boost hydrocarbon formations in electrocatalytic CO2 reduction. Nat. Commun. 2023, 14, 3075. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y.; Jin, S.; Yang, Y.; Zhu, J.; Li, X.; Lv, X.; Fu, J.; Hong, Z.; Su, Y.; et al. Customizing CO2 Electroreduction by Pulse-Induced Anion Enrichment. J. Am. Chem. Soc. 2023, 145, 26213–26221. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y.; Huang, Y.; Wang, H.; Murphy, E.; Delafontaine, L.; Chen, J.L.; Zenyuk, I.V.; Atanassov, P. Promoting electrolysis of carbon monoxide toward acetate and 1-propanol in flow electrolyzer. ACS Energy Lett. 2023, 8, 935–942. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, T.; Chen, W.; Wu, J.; Fan, Y.; Liu, Y.; Cao, L.; Chen, Z. A surface strategy boosting the ethylene selectivity for CO2 reduction and in situ mechanistic insights. Nat. Commun. 2024, 15, 1257. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, P.; Yuan, T.; Cheng, D.; Zhen, S.; Gao, H.; Wang, T.; Zhao, Z.-J.; Gong, J. Modulation of *CHxO adsorption to facilitate electrocatalytic reduction of CO2 to CH4 over Cu-based catalysts. J. Am. Chem. Soc. 2023, 145, 6622–6627. [Google Scholar] [CrossRef]
- Huang, F.; Chen, X.; Sun, H.; Zeng, Q.; Ma, J.; Wei, D.; Zhu, J.; Chen, Z.; Liang, T.; Yin, X.; et al. Atmosphere induces tunable oxygen vacancies to stabilize single-atom copper in ceria for robust electrocatalytic CO2 reduction to CH4. Angew. Chem. Int. Ed. 2024, 64, e202415642. [Google Scholar] [CrossRef]
- Hung, S.-F.; Xu, A.; Wang, X.; Li, F.; Hsu, S.-H.; Li, Y.; Wicks, J.; Cervantes, E.G.; Rasouli, A.S.; Li, Y.C.; et al. A metal-supported single-atom catalytic site enables carbon dioxide hydrogenation. Nat. Commun. 2022, 13, 819. [Google Scholar] [CrossRef]
- Zhi, X.; Vasileff, A.; Zheng, Y.; Jiao, Y.; Qiao, S.-Z. Role of oxygen-bound reaction intermediates in selective electrochemical CO2 reduction. Energ. Environ. Sci. 2021, 14, 3912–3930. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, X.; Zhu, P.; Li, Y.; Zhuang, Z.; Wu, H.; Zhu, J.; Xiao, C.; Chen, M.; Wang, P.; et al. Copper atom pairs stabilize *OCCO dipole toward highly selective CO2 electroreduction to C2H4. Angew. Chem. Int. Ed. 2024, 63, e202411591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Zhang, X.L.; Wu, Z.Z.; Niu, Z.Z.; Chi, L.P.; Gao, F.Y.; Yang, P.P.; Wang, Y.H.; Yu, P.C.; Duanmu, J.W.; et al. Facet-switching of rate-determining step on copper in CO2-to-ethylene electroreduction. Proc. Natl. Acad. Sci. USA 2024, 121, e2400546121. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.; Li, A.; Wei, B.; Si, K.; Wei, Y.; Wang, X.; Zhu, G.; Chen, Q.; Gu, X.; et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 2022, 13, 1877. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mensi, M.D.; Oveisi, E.; Mantella, V.; Buonsanti, R. Structural Sensitivities in Bimetallic Catalysts for Electrochemical CO2 Reduction Revealed by Ag-Cu Nanodimers. J. Am. Chem. Soc. 2019, 141, 2490–2499. [Google Scholar] [CrossRef]
- Hou, L.; Han, J.; Wang, C.; Zhang, Y.; Wang, Y.; Bai, Z.; Gu, Y.; Gao, Y.; Yan, X. Ag nanoparticle embedded Cu nanoporous hybrid arrays for the selective electrocatalytic reduction of CO2 towards ethylene. Inorg. Chem. Front. 2020, 7, 2097–2106. [Google Scholar] [CrossRef]
- Ning, H.; Mao, Q.; Wang, W.; Yang, Z.; Wang, X.; Zhao, Q.; Song, Y.; Wu, M. N-doped reduced graphene oxide supported Cu2O nanocubes as high active catalyst for CO2 electroreduction to C2H4. J. Alloys Compd. 2019, 785, 7–12. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.; Wang, J.; Kulinich, S.A.; Du, X. Laser-Prepared CuZn Alloy Catalyst for Selective Electrochemical Reduction of CO2 to Ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef]
- Mao, M.-J.; Zhang, M.-D.; Meng, D.-L.; Chen, J.-X.; He, C.; Huang, Y.-B.; Cao, R. Imidazolium-Functionalized Cationic Covalent Triazine Frameworks Stabilized Copper Nanoparticles for Enhanced CO2 Electroreduction. Chemcatchem 2020, 12, 3530–3536. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Wang, J.; Ling, C.; Niu, W.; Huang, Z.; Liu, G.; Chen, B.; Lai, Z.; Liu, X.; et al. Ethylene Selectivity in Electrocatalytic CO2 Reduction on Cu Nanomaterials: A Crystal Phase-Dependent Study. J. Am. Chem. Soc. 2020, 142, 12760–12766. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, T.; Dong, G.; Zhang, L.; Pan, F.; Zhu, Y. Boosting C-C Coupling for Electrochemical CO2 Reduction over Novel Cu-Cubic Catalysts with an Amorphous Shell. Inorganics 2025, 13, 130. https://doi.org/10.3390/inorganics13050130
Wang H, Wang T, Dong G, Zhang L, Pan F, Zhu Y. Boosting C-C Coupling for Electrochemical CO2 Reduction over Novel Cu-Cubic Catalysts with an Amorphous Shell. Inorganics. 2025; 13(5):130. https://doi.org/10.3390/inorganics13050130
Chicago/Turabian StyleWang, Hanlin, Tian Wang, Gaigai Dong, Linbo Zhang, Fan Pan, and Yunqing Zhu. 2025. "Boosting C-C Coupling for Electrochemical CO2 Reduction over Novel Cu-Cubic Catalysts with an Amorphous Shell" Inorganics 13, no. 5: 130. https://doi.org/10.3390/inorganics13050130
APA StyleWang, H., Wang, T., Dong, G., Zhang, L., Pan, F., & Zhu, Y. (2025). Boosting C-C Coupling for Electrochemical CO2 Reduction over Novel Cu-Cubic Catalysts with an Amorphous Shell. Inorganics, 13(5), 130. https://doi.org/10.3390/inorganics13050130





