Mg2(Co1/3Fe1/3Ni1/3), Mg2(Cu1/3Fe1/3Ni1/3), Mg2(Co1/3Cu1/3Fe1/3), Mg2(Co1/3Cu1/3Ni1/3), and Mg2(Co1/4Cu1/4Fe1/4Ni1/4) Materials for Hydrogen Storage
Abstract
1. Introduction
2. Results
2.1. Hydriding and Dehydriding Reactions
2.2. Characterization of the As-Milled and Hydrided Materials
3. Discussion
4. Materials and Methods
4.1. Mixtures Preparation
4.2. Hydriding and Dehydriding Reactions
4.3. Physicochemical Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DOE Technical targets. Available online: https://www.energy.gov/eere/fuelcells/articles/target-explanation-document-onboard-hydrogen-storage-light-duty-fuel-cell (accessed on 9 April 2025).
- Paul, A.R.; Mehla, S.; Bhargava, S. Intermetallic Compounds for Hydrogen Storage: Current Status and Future Perspectives. Small 2024, 21, 2408889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chabane, D.; Elkedim, O. Intermetallic Compounds Synthesized by Mechanical Alloying for Solid-State Hydrogen Storage: A Review. Energies 2021, 14, 5758. [Google Scholar] [CrossRef]
- de Jesús Vega-Soria, J.; Ruiz-Santacruz, E.D.; Torres-García, N.L.; Suárez-Alcántara, K. Systematic study of first-row transition metals chlorides as reaction accelerators for Mg/MgH2 for hydrogen storage application. Int. J. Hydrogen Energy, 2024. in press. [CrossRef]
- Bogdanović, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg–Fe–H system and its potential for thermochemical thermal energy storage. J. Alloys Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Sai Raman, S.S.; Davidson, D.J.; Bobet, J.L.; Srivastava, O.N. Investigations on the synthesis, structural and microstructural characterizations of Mg-based K2PtCl6 type (Mg2FeH6) hydrogen storage material prepared by mechanical alloying. J. Alloys Compd. 2002, 333, 282–290. [Google Scholar] [CrossRef]
- Khan, D.; Panda, S.; Ma, Z.; Ding, W.; Zou, J. Formation and hydrogen storage behavior of nanostructured Mg2FeH6 in a compressed 2MgH2–Fe composite. Int. J. Hydrogen Energy 2020, 45, 21676–21686. [Google Scholar] [CrossRef]
- Chaudhary, A.-L.; Dietzel, S.; Li, H.-W.; Akiba, E.; Bergemann, N.; Pistidda, C.; Klassen, T.; Dornheim, M. Synthesis of Mg2 FeD6 under low pressure conditions for Mg2FeH6 hydrogen storage studies. Int. J. Hydrogen Energy 2017, 42, 11422–11428. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Qu, J.; Zhao, W.; Xie, L.; Tian, W.; Li, X. The synthesis and hydrogen storage properties of pure nanostructured Mg2FeH6. Nanotechnology 2010, 21, 095706. [Google Scholar] [CrossRef]
- Niaz, N.A.; Ahmad, I.; Khalid, N.R.; Ahmed, E.; Abbas, S.M.; Jabeen, N. Preparation of Mg2FeH6 Nanoparticles for Hydrogen Storage Properties. J. Nanomater. 2013, 2013, 095706. [Google Scholar] [CrossRef]
- Leiva, D.R.; Huot, J.; Ishikawa, T.T.; Bolfarini, C.; Kiminami, C.S.; Jorge, A.M.; Botta Filho, W.J. Hydrogen Activation Behavior of Commercial Magnesium Processed by Different Severe Plastic Deformation Routes. Mater. Sci. Forum 2010, 667–669, 1047–1051. [Google Scholar] [CrossRef]
- Didisheim, J.J.; Zolliker, P.; Yvon, K.; Fischer, P.; Schefer, J.; Gubelmann, M.; Williams, A.F. Dimagnesium iron(II) hydride, Mg2FeH6, containing octahedral FeH64- anions. Inorg. Chem. 1984, 23, 1953–1957. [Google Scholar] [CrossRef]
- Bashir, A.I.; Arif, H.; Azam, S.; Irfan, M.; Khan, N. First-principles quantum computations to investigate prospects of Mg2FeH6 for optoelectronics and hydrogen-storage applications. Int. J. Hydrogen Energy 2023, 48, 23930–23942. [Google Scholar] [CrossRef]
- Zhang, J.; Cuevas, F.; Zaïdi, W.; Bonnet, J.-P.; Aymard, L.; Bobet, J.-L.; Latroche, M. Highlighting of a Single Reaction Path during Reactive Ball Milling of Mg and TM by Quantitative H2 Gas Sorption Analysis to Form Ternary Complex Hydrides (TM = Fe, Co, Ni). J. Phys. Chem. C 2011, 115, 4971–4979. [Google Scholar] [CrossRef]
- Zolliker, P.; Yvon, K.; Fischer, P.; Schefer, J. Dimagnesium cobalt(I) pentahydride, Mg2CoH5, containing square-pyramidal pentahydrocobaltate(4-) (CoH54-) anions. Inorg. Chem. 1985, 24, 4177–4180. [Google Scholar] [CrossRef]
- Norek, M.; Nielsen, T.K.; Polanski, M.; Kunce, I.; Płociński, T.; Jaroszewicz, L.R.; Cerenius, Y.; Jensen, T.R.; Bystrzycki, J. Synthesis and decomposition mechanisms of ternary Mg2CoH5 studied using in situ synchrotron X-ray diffraction. Int. J. Hydrogen Energy 2011, 36, 10760–10770. [Google Scholar] [CrossRef]
- Černý, R.; Bonhomme, F.; Yvon, K.; Fischer, P.; Zolliker, P.; Cox, D.E.; Hewat, A. Hexamagnesium dicobalt undecadeuteride Mg6Co2D11: Containing [CoD4]5− and [CoD5]4− complex anions conforming to the 18-electron rule. J. Alloys Compd. 1992, 187, 233–241. [Google Scholar] [CrossRef]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Co-Mg (Cobalt-Magnesium) system. Bull. Alloy Phase Diagrams 1987, 8, 352–355. [Google Scholar] [CrossRef]
- Matheus, F.H.; Zepon, G.; Oliveira, V.B.; Leiva, D.R. Highly reactive hydrogen storage Mg2Ni alloy prepared by mechanochemistry and H-cycling. Int. J. Hydrogen Energy 2023, 51, 320–328. [Google Scholar] [CrossRef]
- Lyu, J.; Lider, A.; Kudiiarov, V. Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview. Metals 2019, 9, 768. [Google Scholar] [CrossRef]
- Janot, R.; Aymard, L.; Rougier, A.; Nazri, G.A.; Tarascon, J.M. Fast hydrogen sorption kinetics for ball-milled Mg2Ni alloys. J. Phys. Chem. Solids 2004, 65, 529–534. [Google Scholar] [CrossRef]
- Ebrahimi-Purkani, A.; Kashani-Bozorg, S.F. Nanocrystalline Mg2Ni-based powders produced by high-energy ball milling and subsequent annealing. J. Alloys Compd. 2008, 456, 211–215. [Google Scholar] [CrossRef]
- Baraban, A.P.; Dmitriev, V.A.; Gabis, I.E.; Voyt, A.P.; Klyamkin, S.N.; Shikin, I.V. Direct synthesis of Mg2NiH4 from MgH2 and Ni. Phys. Complex Syst. 2023, 4, 94–102. [Google Scholar] [CrossRef]
- Zolliker, P.; Yvon, K.; Jorgensen, J.D.; Rotella, F.J. Structural studies of the hydrogen storage material magnesium nickel hydride (Mg2NiH4). 2. Monoclinic low-temperature structure. Inorg. Chem. 1986, 25, 3590–3593. [Google Scholar] [CrossRef]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Bowman, R.C.; Fultz, B. Altering hydrogen storage properties by hydride destabilization through alloy formation: LiH and MgH2 destabilized with Si. J. Phys. Chem. B 2004, 108, 13977–13983. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and copper. Inorg. Chem. 1967, 6, 2220–2223. [Google Scholar] [CrossRef]
- Andreasen, A.; Sørensen, M.B.; Burkarl, R.; Møller, B.; Molenbroek, A.M.; Pedersen, A.S.; Vegge, T.; Jensen, T.R. Dehydrogenation kinetics of air-exposed MgH2/Mg2Cu and MgH2/MgCu2 studied with in situ X-ray powder diffraction. Appl. Phys. A Mater. Sci. Process. 2006, 82, 515–521. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, B.; Zhang, J.; Yang, Q.; Jiang, Z.; Dong, H.; Pan, F. Diffusion Kinetics in Mg-Cu Binary System. J. Phase Equilibria Diffus. 2015, 36, 613–619. [Google Scholar] [CrossRef]
- Jurczyk, M.; Okonska, I.; Iwasieczko, W.; Jankowska, E.; Drulis, H. Thermodynamic and electrochemical properties of nanocrystalline Mg2Cu-type hydrogen storage materials. J. Alloys Compd. 2007, 429, 316–320. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Cui, M. Study on the synthesis and hydrogen storage properties of Mg2CuH3. Int. J. Hydrogen Energy 2016, 41, 13152–13155. [Google Scholar] [CrossRef]
- Verbovytskyy, Y.; Zhang, J.; Cuevas, F.; Paul-Boncour, V.; Zavaliy, I. Synthesis and properties of the Mg2Ni0.5Co0.5H4.4 hydride. J. Alloys Compd. 2015, 645, S408–S411. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Fu, J.; Guo, Q.; Pan, F.; Deng, S.; Li, J.; Zhao, G. The synthesis and hydrogen storage properties of Mg2Ni substituted with Cu, Co. J. Mater. Res. 2009, 24, 1311–1316. [Google Scholar] [CrossRef]
- Saita, I.; Saito, K.; Akiyama, T. Hydriding combustion synthesis of Mg2Ni1−xFex hydride. J. Alloys Compd. 2005, 390, 265–269. [Google Scholar] [CrossRef]
- Mendoza-Zélis, L.; Meyer, M.; Baum, L. Complex quaternary hydrides Mg2(Fe,Co)Hy for hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 600–605. [Google Scholar] [CrossRef]
- Polanski, M.; Płociński, T.; Kunce, I.; Bystrzycki, J. Dynamic synthesis of ternary Mg2FeH6. Int. J. Hydrogen Energy 2010, 35, 1257–1266. [Google Scholar] [CrossRef]
- Fernández, I.G.; Meyer, G.O.; Gennari, F.C. Hydriding/dehydriding behavior of Mg2CoH5 produced by reactive mechanical milling. J. Alloys Compd. 2008, 464, 111–117. [Google Scholar] [CrossRef]
- Chen, J.; Takeshita, H.T.; Chartouni, D.; Kuriyama, N.; Sakai, T. Synthesis and characterization of nanocrystaline Mg2CoH5 obtained by mechanical alloying. J. Mater. Sci. 2001, 36, 5829–5834. [Google Scholar] [CrossRef]
- Fernández, I.G.; Meyer, G.O.; Gennari, F.C. Reversible hydrogen storage in Mg2CoH5 prepared by a combined milling-sintering procedure. J. Alloys Compd. 2007, 446–447, 106–109. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Zheng, J.; Yu, X. Construction of carbon covered Mg2NiH4 nanocrystalline for hydrogen storage. J. Alloys Compd. 2022, 905, 164169. [Google Scholar] [CrossRef]
- Shao, H.; Xu, H.; Wang, Y.; Li, X. Preparation and hydrogen storage properties of Mg2Ni intermetallic nanoparticles. Nanotechnology 2004, 15, 269–274. [Google Scholar] [CrossRef]
- Hsu, F.-K.; Hsu, C.-W.; Chang, J.-K.; Lin, C.-K.; Lee, S.-L.; Jiang, C.-E. Structure and hydrogen storage properties of Mg2Cu1−xNix (x=0–1) alloys. Int. J. Hydrogen Energy 2010, 35, 13247–13254. [Google Scholar] [CrossRef]
- Darnaudery, J.; Darriet, B.; Pezat, M. The Mg2Ni0.75M0.25 alloys (M = 3d element): Their application to hydrogen storage. Int. J. Hydrogen Energy 1983, 8, 705–708. [Google Scholar] [CrossRef]
- Deledda, S.; Hauback, B.C. The formation mechanism and structural characterization of the mixed transition-metal complex hydride Mg2 (FeH6)0.5(CoH5)0.5 obtained by reactive milling. Nanotechnology 2009, 20, 204010. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Webbook NIST. Available online: https://webbook.nist.gov/chemistry/ (accessed on 9 April 2025).
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Zareii, S.M.; Sarhaddi, R. Structural, electronic properties and heat of formation of Mg2FeH6 complex hydride: An ab initio study. Phys. Scr. 2012, 86, 015701. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Shi, F.G.; Sommer, F.; Chen, L.-Q.; Liu, Z.-K.; Napolitano, R.E. Modeling of Thermodynamic Properties and Phase Equilibria for the Cu-Mg Binary System. J. Phase Equilibria Diffus. 2007, 28, 158–166. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Mostafa, A.O.; Medraj, M. Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data. J. Mater. 2014, 2014, 704283. [Google Scholar] [CrossRef]
- Babenko, A.; Ghasali, E.; Raza, S.; Baghchesaraee, K.; Cheng, Y.; Hayat, A.; Liu, P.; Zhao, S.; Orooji, Y. Comprehensive insights into recent innovations: Magnesium-inclusive high-entropy alloys. J. Magnes. Alloy. 2024, 12, 1311–1345. [Google Scholar] [CrossRef]
- Ruiz-Santacruz, E.D.; Vega-Soria, J.d.J.; Cruz-Jiménez, A.K.; Caudillo-Flores, U.; Torres-García, N.L.; Suárez-Alcántara, K. High-load Mg2Ni nanoparticle-carbon nanofiber composites for hydrogen storage. Nanoscale 2024, 16, 17908–17925. [Google Scholar] [CrossRef]
- Baum, L.; Meyer, M.; Mendozazelis, L. Complex Mg-based hydrides obtained by mechanosynthesis: Characterization and formation kinetics. Int. J. Hydrogen Energy 2008, 33, 3442–3446. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Lu, X.; Wu, F.; Sun, X.; Zhao, H.; Li, Q. Surprising cocktail effect in high entropy alloys on catalyzing magnesium hydride for solid-state hydrogen storage. Chem. Eng. J. 2023, 465, 142766. [Google Scholar] [CrossRef]
- Wan, H.; Yang, X.; Zhou, S.; Ran, L.; Lu, Y.; Chen, Y.; Wang, J.; Pan, F. Enhancing hydrogen storage properties of MgH2 using FeCoNiCrMn high entropy alloy catalysts. J. Mater. Sci. Technol. 2023, 149, 88–98. [Google Scholar] [CrossRef]
- Gupta, A.; Faisal, M.; Flamina, A.; Raghavendra, R.M.; Hassan, F.; Kundan, N.; Kumar, N. Enhanced hydrogen storage in Mg catalysed by Cu–Ni–Co–Fe quaternary multi-component alloy. Int. J. Hydrogen Energy 2024, 50, 932–945. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y. The CALPHAD approach for HEAs: Challenges and opportunities. MRS Bull. 2022, 47, 158–167. [Google Scholar] [CrossRef]
- Carrillo-Bucio, J.L.; Tena-Garcia, J.R.; Armenta-Garcia, E.P.; Hernandez-Silva, O.; Cabañas-Moreno, J.G.; Suárez-Alcántara, K. Low-cost Sieverts-type apparatus for the study of hydriding/dehydriding reactions. HardwareX 2018, 4, e00036. [Google Scholar] [CrossRef]

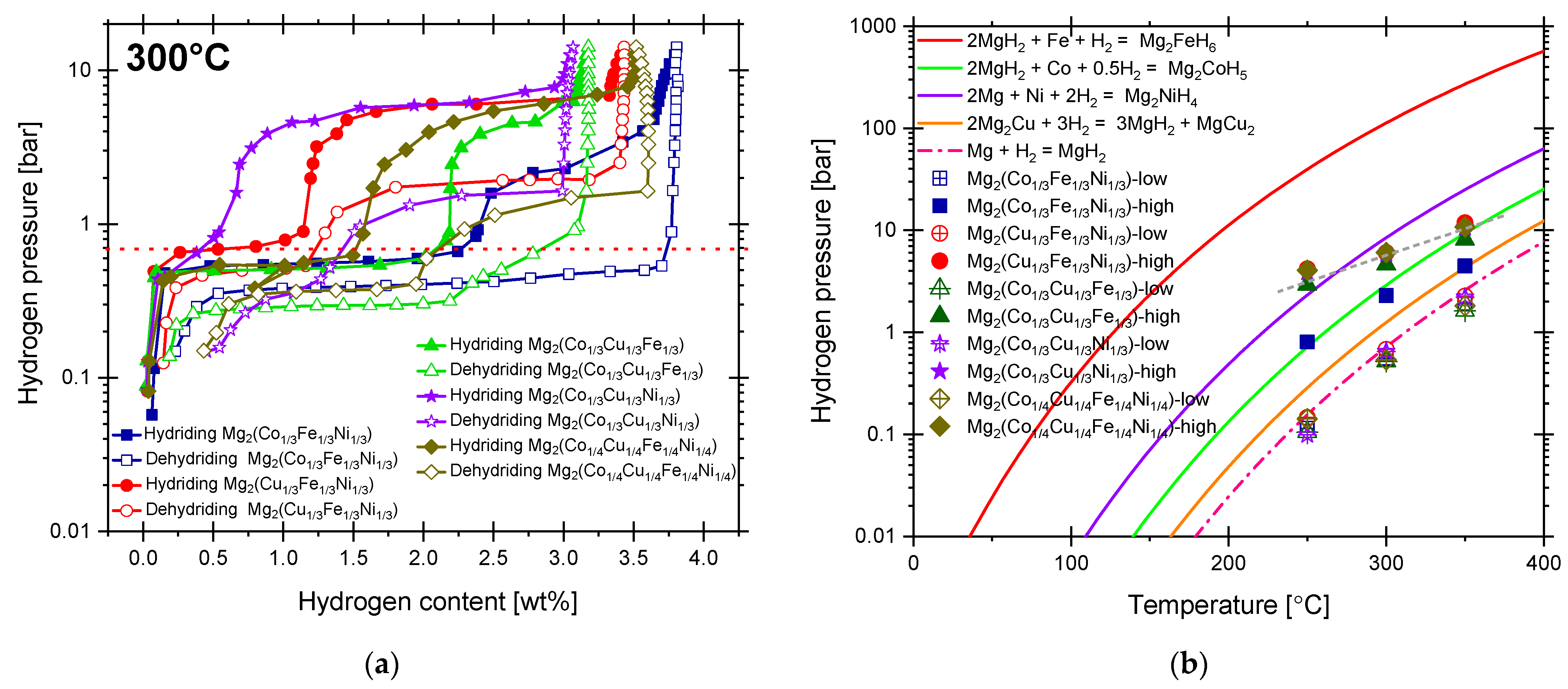
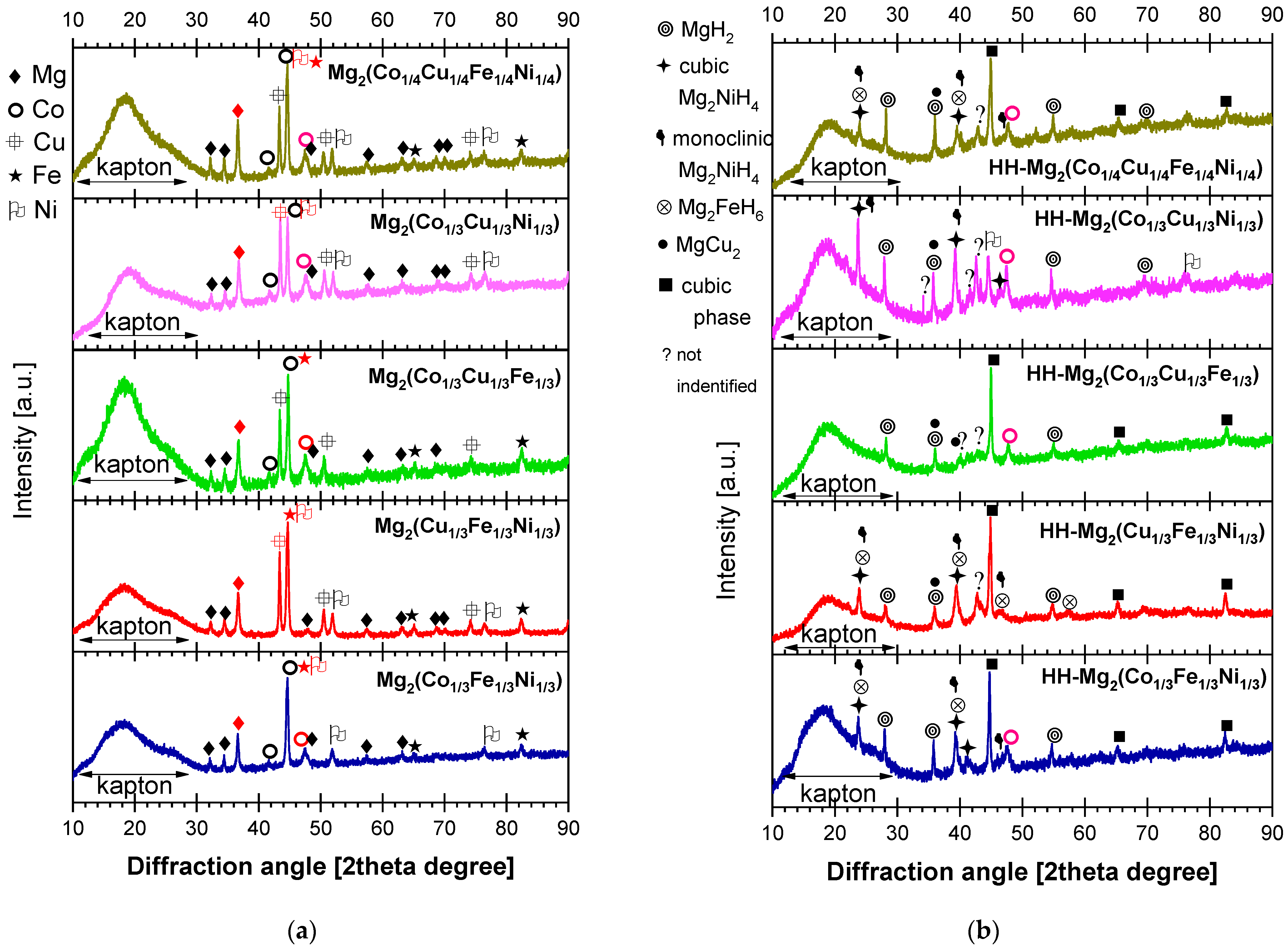
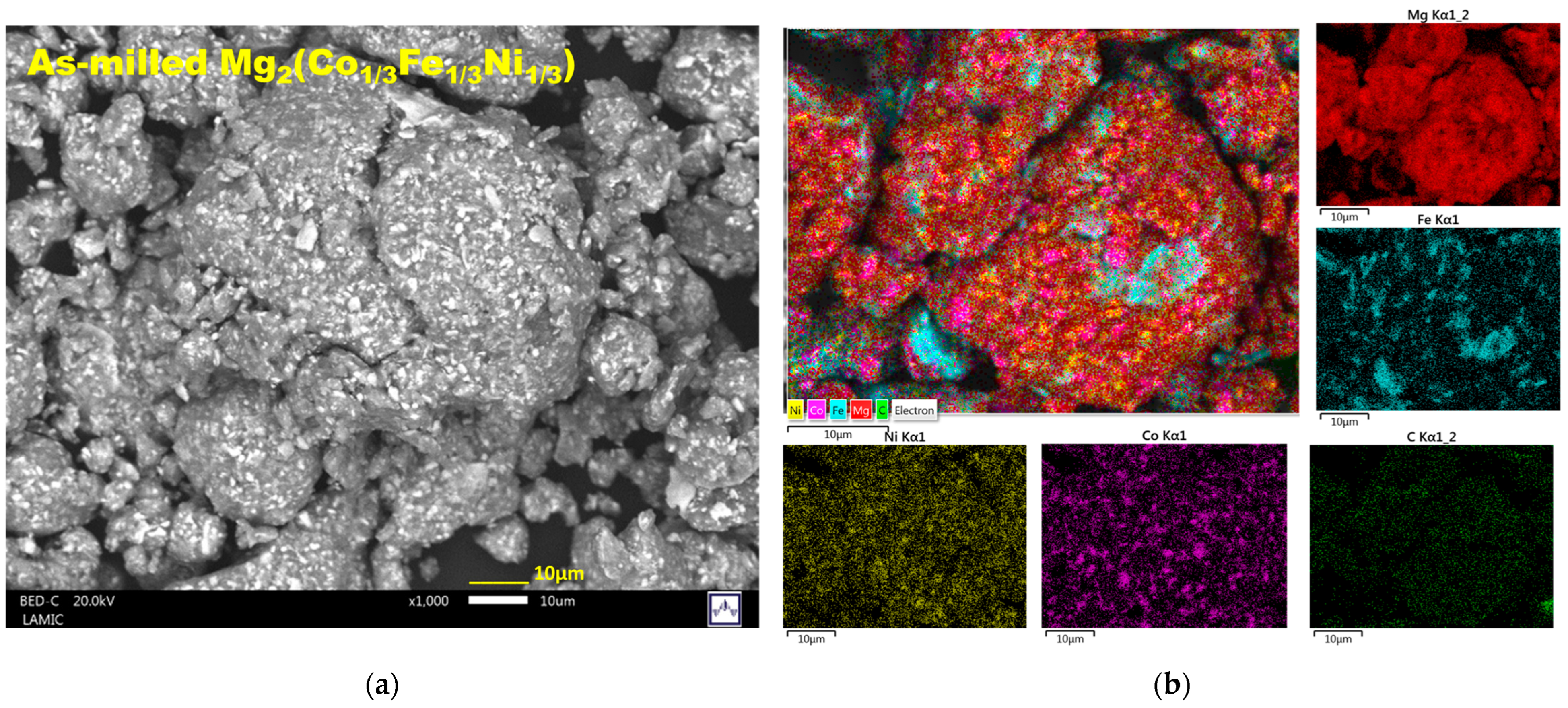

| Material (Not Hydrided) | Temperature [°C], Pressure [bar] | Hydrogen Storage Capacity [wt. %] | Reversibility and Technique | Number of Equilibrium Plateaus | Raw Materials/Producing Technique/Comments | Reference |
|---|---|---|---|---|---|---|
| Mg2Fe | 300, 100 (20) ϰ | 4.95 | Yes, PCI | 1 | Nanoparticles from reduction of organometallic precursors | [10] |
| 350, 100 (5) ϰ | 5.03 | Yes PCI * | 1 | Sintering of the elements | [9] | |
| 50 to 500, 90–70 | 4.55 | Peaks at 315 °C or 338 °C, DSC | 2 | Mechanical milling of the elements | [35] | |
| 450–520, 20–120 (32) ϰ | 4.6 | Yes, PCI * | 2 | Mixed and pressing of elements, hydriding of the pellets for up to 10 days | [12] | |
| 350, 10 | 5.4 | Yes, PCI * | 2 | Mixing MgH2 + Fe manually, further 50 hydriding/dehydriding cycles | [5] | |
| 325, 20 | 4.7 | Yes, PCI | 1 | 2MgH2 + Fe, mixing, ball milled and dehydrided | [7] | |
| ***, 75 | 4.34 | Yes, TPD | 1 | Reactive ball milling of the elements | [14] | |
| Mg2Fe + D2 | RT-500, *** | 6.5 ** | Yes, TGA * | 1 | 2MgD2 + Fe + D2, reactive milling at 10 bar D2 pressure | [8] |
| Mg2Co | 300, 4.5 | 1.8 | Yes, PCI | 2 | Mechanical milling of the elements | [36] |
| 300, 10 | 1.6 | Yes, PCI | 2 | Reactive mechanical milling of 2MgH2 + Co | [37] | |
| 400, 50 (30) ϰ | 3.0 | Yes, PCI | 2 | Mechanical milling of the elements | [38] | |
| ***, 75 | 3.80 | Yes, TPD | 1 | Mechanical milling of the elements | [14] | |
| Mg2Ni | 400, 30 | 3.5 | Yes, PCI * | 1 | Mechanical milling of the elements, then annealing at high temperature | [39] |
| 250, 10 | 2.77 | Yes, PCI | 1 | Compressed (pellet) of the elements | [40] | |
| ***, 75 | 3.03 | Yes, TPD | 1 | Mechanical milling of the elements | [14] | |
| Mg2Cu | 300, 25 (5) ϰ | 2.6 | Yes, PCI | 1 | Mechanical milling of the elements | [29] |
| Mg2Ni0.8Cu0.2 | 300, 40 (25) ϰ | 3.5 | Yes, PCI | 2 | Melting of the elements. The study covers a range of variations of compositions. | [41] |
| Mg2Ni0.75Fe0.25 | 350, 30 | 2.8 | Yes, kinetic (wt. % vs. time) | 1 | Melting of the elements at 900 °C, pressed into rods, and reheated at 550 °C for several days | [42] |
| Mg2Ni0.75Co0.25 | 350, 30 | 3.1 | Yes, kinetic (wt. % vs. time) | 1 | Melting of the elements at 900 °C, pressed into rods, and reheated at 550 °C for several days. | [42] |
| Mg2Fe0.75Co0.25 | ***, 3 | 3.24 | **** | 1 | Reactive ball milling, automatically refilling of H2 as consumed during milling. | [34] |
| Mg2Fe0.5Co0.5 | ***, 50 | 4.7 | **** | 2 | Reactive ball milling | [43] |
| Material | Maximum Expected Hydrogen Storage [wt. %] | Hydrogen Uptake at 15 bar and 300 °C | Onset Hydriding Temperature at 15 bar, +0.1 wt. % [°C] | Temperature Reaching 80% Hydrogen Storage [°C] | Onset Dehydriding Temperature at 0.015 bar, −0.1 wt. % [°C] | Hydrogen Uptake in PCI Experiments [wt. %] | Equilibrium Pressure at 300 °C, First and Second Hydriding Plateaus [bar] |
|---|---|---|---|---|---|---|---|
| Mg2(Co1/3Fe1/3Ni1/3) | 4.48 | 3.18 | 55 | 220 | 243 | 3.80 | 0.55, 2.29 |
| Mg2(Cu1/3Fe1/3Ni1/3) | 3.55 | 2.66 | 87 | 293 | 263 | 3.43 | 0.68, 6.02 |
| Mg2(Co1/3Cu1/3Fe1/3) | 3.78 | 2.64 | 58 | 265 | 262 | 3.17 | 0.52, 4.62 |
| Mg2(Co1/3Cu1/3Ni1/3) | 3.57 | 2.81 | 72 | 268 | 264 | 3.06 | 0.65, 5.92 |
| Mg2(Co1/4Cu1/4Fe1/4Ni1/4) | 3.75 | 3.19 | 77 | 279 | 245 | 3.51 | 0.53, 6.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Santacruz, E.D.; Núñez, P.d.C.C.; García, N.L.T.; Suárez-Alcántara, K. Mg2(Co1/3Fe1/3Ni1/3), Mg2(Cu1/3Fe1/3Ni1/3), Mg2(Co1/3Cu1/3Fe1/3), Mg2(Co1/3Cu1/3Ni1/3), and Mg2(Co1/4Cu1/4Fe1/4Ni1/4) Materials for Hydrogen Storage. Inorganics 2025, 13, 135. https://doi.org/10.3390/inorganics13050135
Ruiz-Santacruz ED, Núñez PdCC, García NLT, Suárez-Alcántara K. Mg2(Co1/3Fe1/3Ni1/3), Mg2(Cu1/3Fe1/3Ni1/3), Mg2(Co1/3Cu1/3Fe1/3), Mg2(Co1/3Cu1/3Ni1/3), and Mg2(Co1/4Cu1/4Fe1/4Ni1/4) Materials for Hydrogen Storage. Inorganics. 2025; 13(5):135. https://doi.org/10.3390/inorganics13050135
Chicago/Turabian StyleRuiz-Santacruz, Eduardo David, Paula del Carmen Cintrón Núñez, Nidia Libia Torres García, and Karina Suárez-Alcántara. 2025. "Mg2(Co1/3Fe1/3Ni1/3), Mg2(Cu1/3Fe1/3Ni1/3), Mg2(Co1/3Cu1/3Fe1/3), Mg2(Co1/3Cu1/3Ni1/3), and Mg2(Co1/4Cu1/4Fe1/4Ni1/4) Materials for Hydrogen Storage" Inorganics 13, no. 5: 135. https://doi.org/10.3390/inorganics13050135
APA StyleRuiz-Santacruz, E. D., Núñez, P. d. C. C., García, N. L. T., & Suárez-Alcántara, K. (2025). Mg2(Co1/3Fe1/3Ni1/3), Mg2(Cu1/3Fe1/3Ni1/3), Mg2(Co1/3Cu1/3Fe1/3), Mg2(Co1/3Cu1/3Ni1/3), and Mg2(Co1/4Cu1/4Fe1/4Ni1/4) Materials for Hydrogen Storage. Inorganics, 13(5), 135. https://doi.org/10.3390/inorganics13050135









