Cyclometalated Iridium(III) Complexes Containing Benzoxazole Derivatives and Different Ancillary Ligands for Catalytic Oxidation of Toluene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
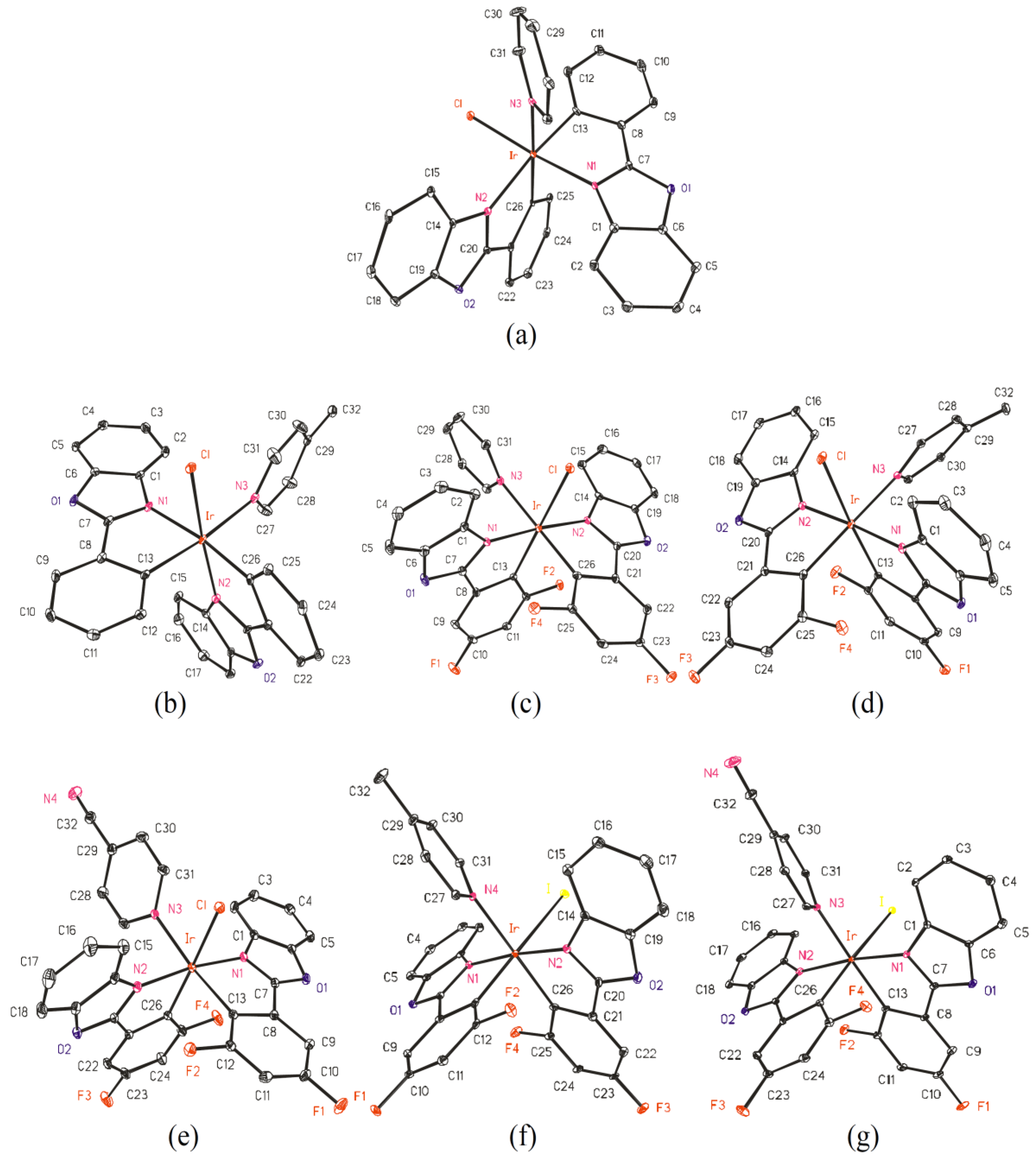
2.2. Crystal Structures
2.3. Oxidation of Toluene with Dioxygen
2.4. Chemical Kinetics
2.5. Performance on Catalytic Oxidation
3. Experimental Section
3.1. Materials and Methods
3.2. Synthetic Procedures
3.2.1. Synthesis of Ligands and Cyclometalated Ir(III) Chloro-Bridged Dimers (D1 and D2)
3.2.2. Synthesis of Cyclometalated Ir(III) Chloro-Bridged Dimer [(dfpbo)2Ir(μ-I)2)Ir(dfpbo)2] (D3)
3.2.3. Synthesis of Bis(2-phenylbenzoxazolato-N,C2′)-pyridinato Chloroiridium(III) [Ir(pbo)2(Cl)(py)] (1)
3.2.4. Synthesis of Bis(2-phenylbenzoxazolato-N,C2′)-(4-methylpyridinato) Chloroiridium(III) [Ir(pbo)2(Cl)(4mpy)] (2)
3.2.5. Synthesis of Bis(2-phenylbenzoxazolato-N,C2′)-(4-cyanopyridinato) Chloroiridium(III) [Ir(pbo)2(Cl)(4cnpy)] (3)
3.2.6. Synthesis of Bis(2-(3,5-difluorophenyl)benzoxazolato-N,C2′)-pyridinato Chloroiridium(III) [Ir(dfpbo)2(Cl)(py)] (4)
3.2.7. Synthesis of Bis(2-(3,5-difluorophenyl)benzoxazolato-N,C2′)-(4-methylpyridinato) Chloroiridium(III) [Ir(dfpbo)2(Cl)(4mpy)] (5)
3.2.8. Synthesis of Bis(2-(3,5-difluorophenyl)benzoxazolato-N,C2′)-(4-cyanopyridinato) Chloroiridium(III) [Ir(dfpbo)2(Cl)(4cnpy)] (6)
3.2.9. Synthesis of Bis(2-(3,5-difluorophenyl)benzoxazolato-N,C2′)-pyridinato Iodoiridium(III) [Ir(dfpbo)2(I)(py)] (7)
3.2.10. Synthesis of Bis(2-(3,5-difluorophenyl)benzoxazolato-N,C2′)-(4-methylpyridinato) Iodoiridium(III) [Ir(dfpbo)2(I)(4mpy)] (8)
3.2.11. Synthesis of Bis(2-(3,5-difluorophenyl)benzoxazolato-N,C2′)-(4-cyanopyridinato) Iodoiridium(III) [Ir(dfpbo)2(I)(4cnpy)] (9)
3.3. X-ray Absorption Near-Edge Spectroscopy (XANES)
3.4. Thermogravimetric Analysis (TGA)
3.5. Electron Paramagnetic Resonance (EPR)
3.6. Single Crystal X-ray Diffraction
3.7. Catalytic Activity of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.-J.; Wang, J.-S.; Jin, F.-Z.; Liu, M.-Y.; Zhao, C.-W.; Li, Y.-A.; Dong, Y.-B. Pd@Cu(II)-MOF-Catalyzed Aerobic Oxidation of Benzylic Alcohols in Air with High Conversion and Selectivity. Inorg. Chem. 2016, 55, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Backvall, J.E. Modern Oxidation Reactions, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Fernandez, M.I.; Tojo, G. Oxidation of Alcohols to Aldehydes and Ketones. In A Guide to Current Common Practice; Springer: New York, NY, USA, 2006. [Google Scholar]
- Long, R.; Huang, H.; Li, Y.P.; Song, L.; Xiong, Y. Palladium-Based Nanomaterials: A Platform to Produce Reactive Oxygen Species for Catalyzing Oxidation Reactions. Adv. Mater. 2015, 27, 7025–7042. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-R.; Lee, H.-P.; Chen, J.-D.; Chen, K.H.-C. An 18+δ iridium dimer releasing metalloradicals spontaneously. Dalton Trans. 2010, 39, 9458–9461. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-R.; Liu, P.-C.; Lee, H.-P.; Wu, F.-S.; Chen, K.H.-C. Cyclometalated Iridium(III) Complexes with Ligand Effects on the Catalytic C–H Bond Activation of Toluene. Eur. J. Inorg. Chem. 2017, 13, 2023–2031. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Miedziak, P.J.; Morad, M.; Sankar, M.; Waldron, C.; Cattaneo, S.; Cao, E.; Pattisson, S.; Morgan, D.; Bethell, D.; et al. Deactivation Behavior of Supported Gold Palladium Nanoalloy Catalysts during the Selective Oxidation of Benzyl Alcohol in a Micropacked Bed Reactor. Ind. Eng. Chem. Res. 2017, 56, 12984–12993. [Google Scholar] [CrossRef]
- Jiang, N.; Ragauskas, A.J. Cu(II)-Catalyzed Selective Aerobic Oxidation of Alcohols under Mild Conditions. J. Org. Chem. 2006, 71, 7087–7090. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, B.C.; Lu, H.; Li, R.; Wang, L.; Landfester, K.; Zhang, K.A.I. Visible-Light-Promoted Selective Oxidation of Alcohols Using a Covalent Triazine Framework. ACS Catal. 2017, 7, 5438–5442. [Google Scholar] [CrossRef]
- Graves, C.R.; Zeng, B.-S.; Nguyen, S.T. Efficient and Selective Al-Catalyzed Alcohol Oxidation via Oppenauer Chemistry. J. Am. Chem. Soc. 2006, 128, 12596–12597. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, F.; Yang, Z.; Cui, X.; Wang, J.; Zhang, L. New Reaction Pathway Induced by Plasmon for Selective Benzyl Alcohol Oxidation on BiOCl Possessing Oxygen Vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Khenkin, A.M.; Neumann, R. Oxygen Transfer from Sulfoxides: Selective Oxidation of Alcohols Catalyzed by Polyoxomolybdates. J. Org. Chem. 2002, 67, 7075–7079. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.M.A.; Emmanuvel, L.; Sudalai, A. NaIO4-Mediated Selective Oxidation of Alkylarenes and Benzylic Bromides/Alcohols to Carbonyl Derivatives Using Water as Solvent. J. Org. Chem. 2006, 71, 5043–5046. [Google Scholar] [CrossRef] [PubMed]
- Chorghade, R.; Battilocchio, C.; Hawkins, J.M.; Ley, S.V. Sustainable Flow Oppenauer Oxidation of Secondary Benzylic Alcohols with a Heterogeneous Zirconia Catalyst. Org. Lett. 2013, 15, 5698–5701. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Selective Oxidation of Alcohols Using Photoactive VO@g-C3N4. ACS Sustain. Chem. Eng. 2016, 4, 1094–1098. [Google Scholar] [CrossRef]
- Peterson, K.P.; Larock, R.C. Palladium-Catalyzed Oxidation of Primary and Secondary Allylic and Benzylic Alcohols. J. Org. Chem. 1998, 63, 3185–3189. [Google Scholar] [CrossRef]
- Sharma, M.; Das, B.; Sharma, M.; Deka, B.K.; Park, Y.-B.; Bhargava, S.K.; Bania, K.K. Pd/Cu-Oxide Nanoconjugate at Zeolite-Y Crystallite Crafting the Mesoporous Channels for Selective Oxidation of Benzyl-Alcohols. ACS Appl. Mater. Interfaces 2017, 9, 35453–35462. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Takemura, M.; Togo, H. Direct and Selective Benzylic Oxidation of Alkylarenes via C–H Abstraction Using Alkali Metal Bromides. Org. Lett. 2012, 14, 2414–2417. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Wu, R.; Silks, L.P. Mild and Selective Vanadium-Catalyzed Oxidation of Benzylic, Allylic, and Propargylic Alcohols Using Air. Org. Lett. 2011, 13, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Xie, X.; Xu, J.; Gu, Q.; Chen, L.; Wang, X. Nitrogen-Doped Graphene Nanosheets as Metal-Free Catalysts for Aerobic Selective Oxidation of Benzylic Alcohols. ACS Catal. 2012, 2, 622–631. [Google Scholar] [CrossRef]
- Xu, J.; Luo, L.; Xiao, G.; Zhang, Z.; Lin, H.; Wang, X.; Long, J. Layered C3N3S3 Polymer/Graphene Hybrids as Metal-Free Catalysts for Selective Photocatalytic Oxidation of Benzylic Alcohols under Visible Light. ACS Catal. 2014, 4, 3302–3306. [Google Scholar] [CrossRef]
- Opembe, N.N.; Guild, C.; King’ondu, C.; Nelson, N.C.; Slowing, I.I.; Suib, S.L. Vapor-Phase Oxidation of Benzyl Alcohol Using Manganese Oxide Octahedral Molecular Sieves (OMS-2). Ind. Eng. Chem. Res. 2014, 53, 19044–19051. [Google Scholar] [CrossRef] [Green Version]
- Sundar, J.V.; Subramanian, V. Novel Chemistry for the Selective Oxidation of Benzyl Alcohol by Graphene Oxide and N-Doped Graphene. Org. Lett. 2013, 15, 5920–5923. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Tian, B.; Zhang, J. Core–Shell Structural CdS@SnO2 Nanorods with Excellent Visible-Light Photocatalytic Activity for the Selective Oxidation of Benzyl Alcohol to Benzaldehyde. ACS Appl. Mater. Interfaces 2015, 7, 13849–13858. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yuan, H.; Janssen, K.P.F.; Solís-Fernández, G.; Wang, Y.; Tan, C.Y.X.; Jonckheere, D.; Debroye, E.; Long, J.; Hendrix, J.; et al. Efficient and Selective Photocatalytic Oxidation of Benzylic Alcohols with Hybrid Organic–Inorganic Perovskite Materials. ACS Energy Lett. 2018, 3, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Lahtinen, P.; Korpi, H.; Haavisto, E.; Leskela, M.; Repo, T. Parallel Screening of Homogeneous Copper Catalysts for the Oxidation of Benzylic Alcohols with Molecular Oxygen in Aqueous Solutions. J. Comb. Chem. 2004, 6, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Dun, R.; Wang, X.; Tan, M.; Huang, Z.; Huang, X.; Ding, W.; Lu, X. Quantitative Aerobic Oxidation of Primary Benzylic Alcohols to Aldehydes Catalyzed by Highly Efficient and Recyclable P123-Stabilized Pd Nanoclusters in Acidic Aqueous Solution. ACS Catal. 2013, 3, 3063–3066. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Aerobic Oxidation of Benzylic Alcohols Catalyzed by Metal-Organic Frameworks Assisted by TEMPO. ACS Catal. 2011, 1, 48–53. [Google Scholar] [CrossRef]
- Chen, T.-R. Synthesis and characterization of cyclometalated iridium(III) complexes containing benzoxazole derivatives and different ancillary ligands. J. Organomet. Chem. 2008, 693, 3117–3130. [Google Scholar] [CrossRef]
- Lee, H.-P.; Hsu, Y.-F.; Chen, T.-R.; Chen, J.-D.; Chen, K.H.-C.; Wang, J.-C. A Novel Cyclometalated Dimeric Iridium Complex, [(dfpbo)2Ir]2 [dfpbo = 2-(3,5-Difluorophenyl)benzoxazolato-N,C2′], Containing an Unsupported IrII–IrII Bond. Inorg. Chem. 2009, 48, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-R.; Lee, H.-P.; Chen, J.-D. Water Attack Umpolung Aromatic Systems to Release Hydrogen. Inorg. Chem. 2011, 50, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Hoff, C.D. Thermodynamic and kinetic studies of stable low valent transition metal radical complexes. Coord. Chem. Rev. 2000, 206, 451–467. [Google Scholar] [CrossRef]
- Lehman, M.C.; Pahls, D.R.; Meredith, J.M.; Sommer, R.D.; Heinekey, D.M.; Cundari, T.R.; Ison, E.A. Oxyfunctionalization with CpIrIII(NHC)(Me)(Cl) with O2: Identification of a Rare Bimetallic IrIV μ-Oxo Intermediate. J. Am. Chem. Soc. 2015, 137, 3574–3584. [Google Scholar] [CrossRef] [PubMed]
- Rendina, L.M.; Puddephatt, R.J. Oxidative Addition Reactions of Organoplatinum(II) Complexes with Nitrogen-Donor Ligands. Chem. Rev. 1997, 97, 1735–1754. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.G.; Mikhaylov, A.A.; Churakov, A.V.; Vener, M.V.; Tripol’skaya, T.A.; Cohen, S.; Lev, O.; Prikhodchenko, P.V. Potassium, Cesium, and Ammonium Peroxogermanates with Inorganic Hexanuclear Peroxo Bridged Germanium Anion Isolated from Aqueous Solution. Inorg. Chem. 2015, 54, 8058–8065. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Wang, X.; Antonietti, M. Solvent-Free and Metal-Free Oxidation of Toluene Using O2 and g-C3N4 with Nanopores: Nanostructure Boosts the Catalytic Selectivity. ACS Catal. 2012, 2, 2082–2086. [Google Scholar] [CrossRef]
- Gunduz, G.; Akpolat, O. Catalytic vapor-phase oxidation of toluene to benzaldehyde. Ind. Eng. Chem. Res. 1990, 29, 45–48. [Google Scholar] [CrossRef]
- Mao, Y.; Bakac, A. Photocatalytic Oxidation of Toluene to Benzaldehyde by Molecular Oxygen. J. Phys. Chem. 1996, 100, 4219–4223. [Google Scholar] [CrossRef]
- Borgaonkar, H.V.; Raverkar, S.R.; Chandalia, S.B. Liquid phase oxidation of toluene to benzaldehyde by air. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 455–458. [Google Scholar] [CrossRef]
- Eyal, G.; Ibrahim, A. Cerium-Catalyzed Selective Oxidation of Alkylbenzenes with Bromate Salts. Synth. Commun. 1995, 25, 3149–3154. [Google Scholar]
- Pierre, P.M. Process of Making Aromatic Aldehyde. US Patent 613460A, 1 November 1898. [Google Scholar]
- Bazlen, M. Process of Making Aromatic Aldehyde and Acid. US Patent 698355, 22 April 1902. [Google Scholar]
- Bazlen, M.; Labhardt, H. Process of Oxidizing Methyl Groups in Aromatic Hydrocarbons. US Patent 780404, 17 January 1905. [Google Scholar]














| Bond Distance (Å) | |||||||
|---|---|---|---|---|---|---|---|
| Complex | Ir–Cav | Ir–N(1) a | Ir–N(2) a | Ir–N(3) b | Ir–X c | ||
| 1 | 2.018(4) | 2.044(4) | 2.153(4) | 2.162(4) | 2.3458(11) | ||
| 2 | 2.023(3) | 2.174(3) | 2.046(3) | 2.187(3) | 2.3586(8) | ||
| 4 | 2.013(3) | 2.042(3) | 2.049(3) | 2.159(3) | 2.4395(8) | ||
| 5 | 2.036(3) | 2.058(2) | 2.035(2) | 2.149(2) | 2.4241(6) | ||
| 6 | 2.040(4) | 2.052(4) | 2.049(4) | 2.182(4) | 2.4476(11) | ||
| 8 | 2.041(5) | 2.054(4) | 2.051(4) | 2.159(3) | 2.7440(3) | ||
| 9 | 2.037(3) | 2.027(2) | 2.058(2) | 2.146(3) | 2.7336(2) | ||
| Bond Angles (°) | |||||||
| Complex | C(13)–Ir–C(26) | N(1)–Ir–N(2) | N(3)–Ir–X | ||||
| 1 | 94.79(18) | 98.40(14) | 90.74(10) | ||||
| 2 | 92.48(13) | 98.91(10) | 91.53(7) | ||||
| 4 | 87.75(12) | 175.67(11) | 91.12(7) | ||||
| 5 | 87.14(10) | 176.71(8) | 89.71(6) | ||||
| 6 | 92.37(17) | 172.75(14) | 89.17(10) | ||||
| 8 | 88.14(17) | 174.35(14) | 91.24(9) | ||||
| 9 | 85.50(12) | 173.60(10) | 92.59(7) | ||||
| Complex | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Para. | ||||||||||
| MW a | 695 | 709 | 719 | 768 | 781 | 791 | 859 | 873 | 884 | |
| WNRb | 11.4 | 13.1 | 14.5 | 10.3 | 11.9 | 13.2 | 9.2 | 10.7 | 11.8 | |
| TDNR c | 251 | 220 | 200 | 297 | 360 | 237 | 180 | 289 | 326 | |
| Entry | Conversion d (%) | Selectivity e (%) | TON f | TOF (h−1) g | Quantum Yield h |
|---|---|---|---|---|---|
| g-C3N4 a | 2.6 | 24 | 4.7 | 0.29 | - |
| mpg130 a | 2.6 | 99 | 23.1 | 1.44 | - |
| [UO2 2+] b | <1 | 30 | - | - | 0.01 |
| NaBr c | 10 | 40 | <1 | <0.25 | - |
| Complex 1 | 1.73 | 90 | 1620 | 270 | - |
| Complex 2 | 1.67 | 90 | 1580 | 262 | - |
| Complex 3 | 1.55 | 90 | 1460 | 244 | - |
| Complex 4 | 4.46 | 96 | 4180 | 698 | - |
| Complex 5 | 4.66 | 97 | 3320 | 553 | - |
| Complex 6 | 3.44 | 96 | 2810 | 468 | - |
| Complex 7 | 2.23 | 95 | 2090 | 349 | - |
| Complex 8 | 1.97 | 94 | 1850 | 308 | - |
| Complex 9 | 1.95 | 93 | 1830 | 304 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.H.-C.; Liu, P.-C.; Chen, T.-R.; Chen, J.-D. Cyclometalated Iridium(III) Complexes Containing Benzoxazole Derivatives and Different Ancillary Ligands for Catalytic Oxidation of Toluene. Inorganics 2018, 6, 118. https://doi.org/10.3390/inorganics6040118
Chen KH-C, Liu P-C, Chen T-R, Chen J-D. Cyclometalated Iridium(III) Complexes Containing Benzoxazole Derivatives and Different Ancillary Ligands for Catalytic Oxidation of Toluene. Inorganics. 2018; 6(4):118. https://doi.org/10.3390/inorganics6040118
Chicago/Turabian StyleChen, Kelvin H.-C., Pei-Chun Liu, Tsun-Ren Chen, and Jhy-Der Chen. 2018. "Cyclometalated Iridium(III) Complexes Containing Benzoxazole Derivatives and Different Ancillary Ligands for Catalytic Oxidation of Toluene" Inorganics 6, no. 4: 118. https://doi.org/10.3390/inorganics6040118
APA StyleChen, K. H. -C., Liu, P. -C., Chen, T. -R., & Chen, J. -D. (2018). Cyclometalated Iridium(III) Complexes Containing Benzoxazole Derivatives and Different Ancillary Ligands for Catalytic Oxidation of Toluene. Inorganics, 6(4), 118. https://doi.org/10.3390/inorganics6040118





