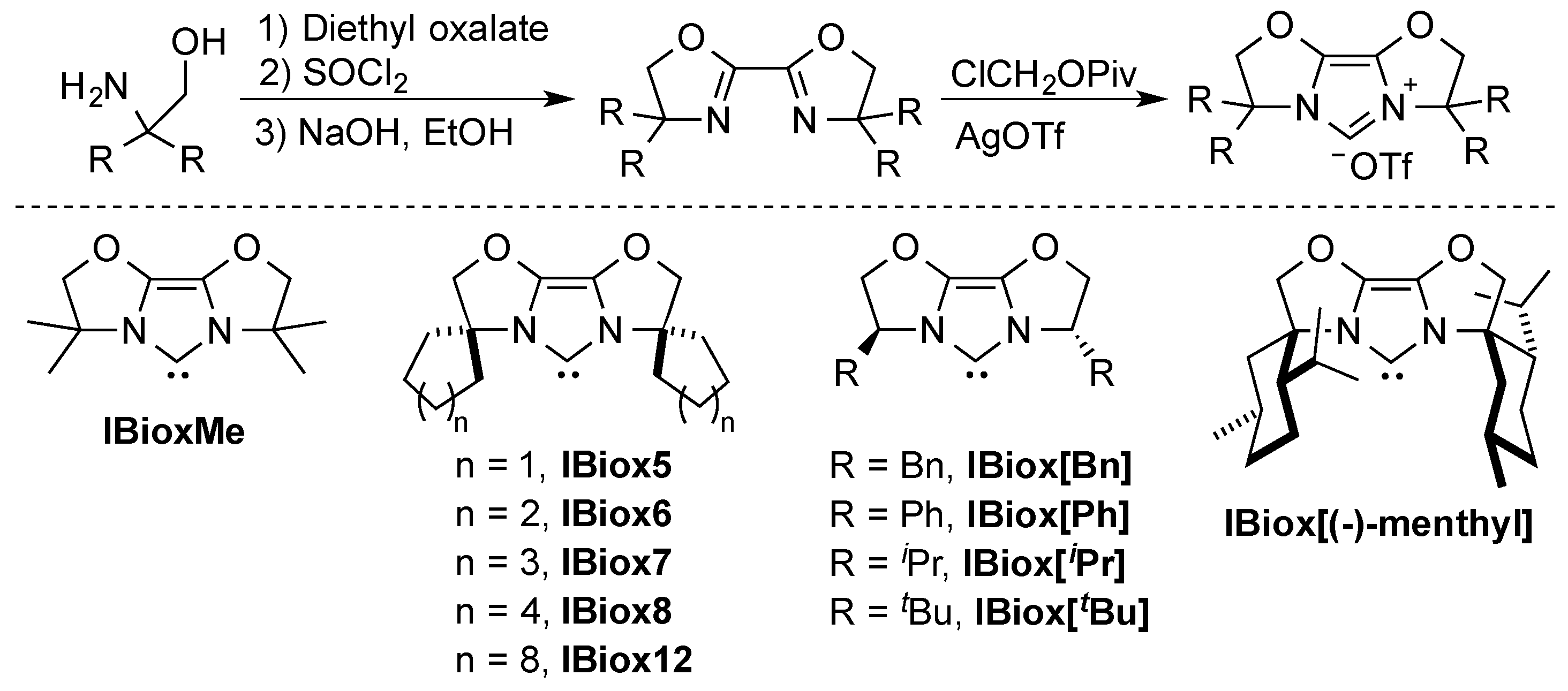Abstract
In recent years, several classes of new N-heterocyclic carbene (NHC) ligands were developed around the concept of “flexible steric bulk”. The steric hindrance of these ligands brings stability to the active species, while ligand flexibility still allows for the approach of the substrate. In this review, the synthesis of several types of new classes, such as IBiox, cyclic alkyl amino carbenes (CAAC), ITent, and IPr* are discussed, as well as how they move the state-of-the-art in palladium catalyzed cross-coupling forward.
1. Introduction
In recent years, interest on N-heterocyclic carbenes (NHCs) has been growing rapidly due to their excellent stability, diversity and a possible tunability of steric and electronic properties [1,2,3,4,5,6,7,8,9,10,11,12,13]. Fine-tuning the steric hindrance led to major breakthroughs and improvements in catalysis [14,15,16,17]. Bulkier ligands stabilize the active species and disfavor bimolecular decomposition and other routes of deactivation [18,19,20,21]. However, steric bulk disfavors the approach of the substrate, which might diminish catalytic activity. Therefore, a delicate balance between skeletal flexibility and steric bulk is required to enhance catalytic efficacy [18,19,20,21,22].
The focus of this review is on three different classes of bulky ligands: IBiox [23], cyclic alkyl amino carbenes (CAAC) [24] and N,N’-bis(aryl)imidazolylidenes (such as the ITent-, IPr*- and SICyoctNap series) [25,26,27,28,29]. Their synthesis is discussed, as well as their role in palladium-catalyzed cross-coupling reactions.
2. IBiox
Glorius was the first to report on the concept of “flexible steric bulk” with his work on bisoxazoline-derived N-heterocyclic carbene ligands (IBiox) [23,30]. The rigidity of these ligands comes from their tricyclic backbone. These ligands can be easily prepared, starting from their corresponding bisoxazolines [15,23,30] and tuning of steric bulk, flexibility, and chirality can be achieved by judicious substitutions [31,32] (Scheme 1).
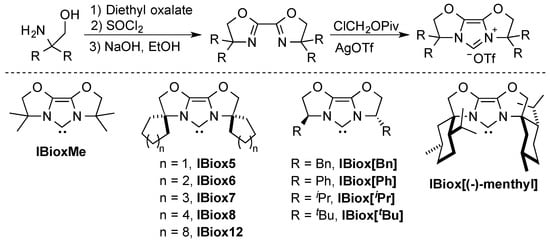
Scheme 1.
Synthesis of the IBiox ligand series [23,30,32,33,34].
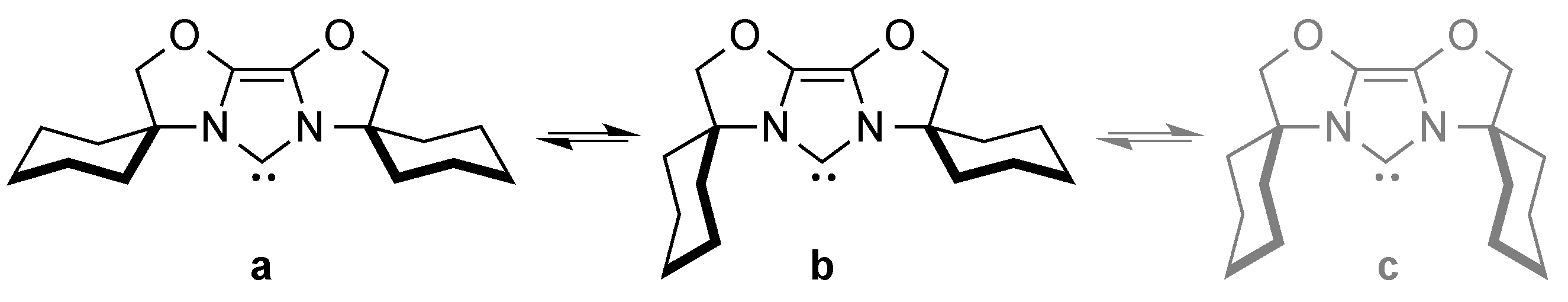
The flexibility of the steric hindrance of these ligands can be demonstrated by the equilibrium between various conformers of IBiox6 (Scheme 2) [30].
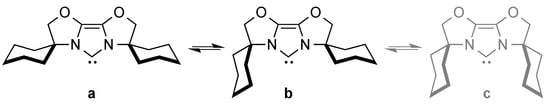
Scheme 2.
Equilibrium between conformations of IBiox6 (a–c) [30].
2.1. Suzuki–Miyaura Cross-Coupling
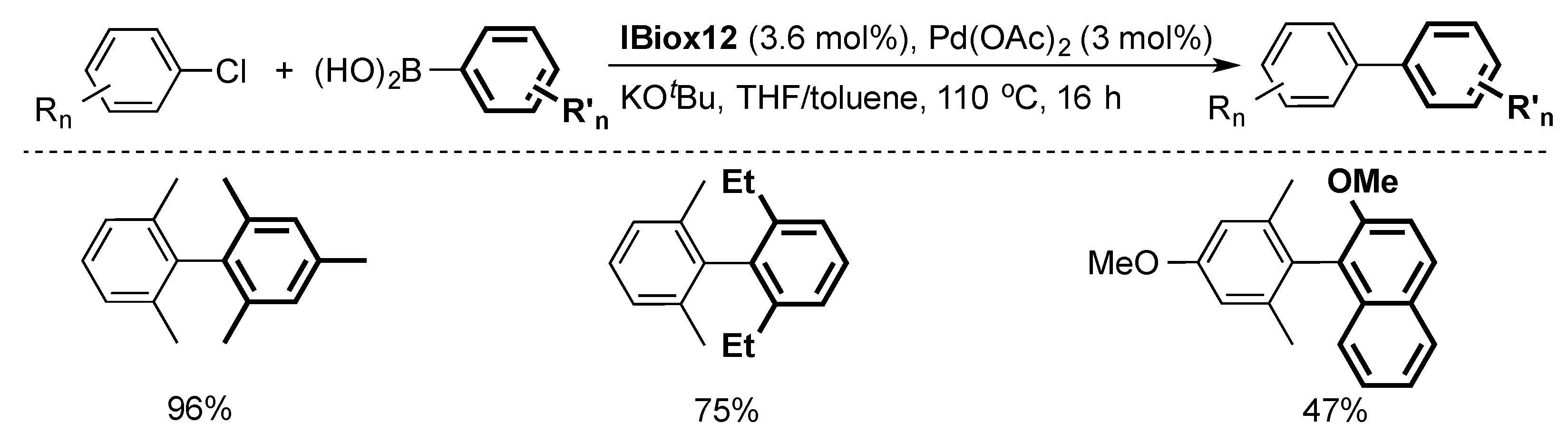
The steric flexibility of the IBiox series proved to be an important factor in enabling challenging cross-coupling reactions, such as the formation of ortho-substituted biaryls via a Suzuki–Miyaura coupling [15,23,30]. Screening of a series of IBiox ligands with cycloalkyl substituents showed that the formation of highly hindered tetra-ortho-substituted biaryls requires increased steric hindrance about the NHC ligand [23]. In that context, IBiox12 emerged as the desired catalyst for this reaction (Scheme 3) [23].
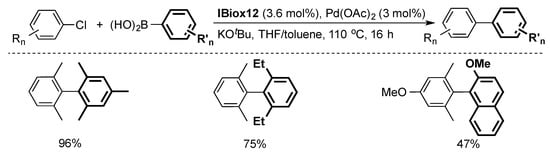
Scheme 3.
Selected examples of Suzuki–Miyaura cross-coupling using IBiox12 [23].
While this system is produced in situ, the well-defined IBiox12-based dimer [PdCl(µ-Cl)(IBiox12)]2 was subsequently isolated. The latter showed comparable results to its in situ counterpart in cross-coupling reactions [23].
2.2. Alkyl Sonogashira Cross-Coupling
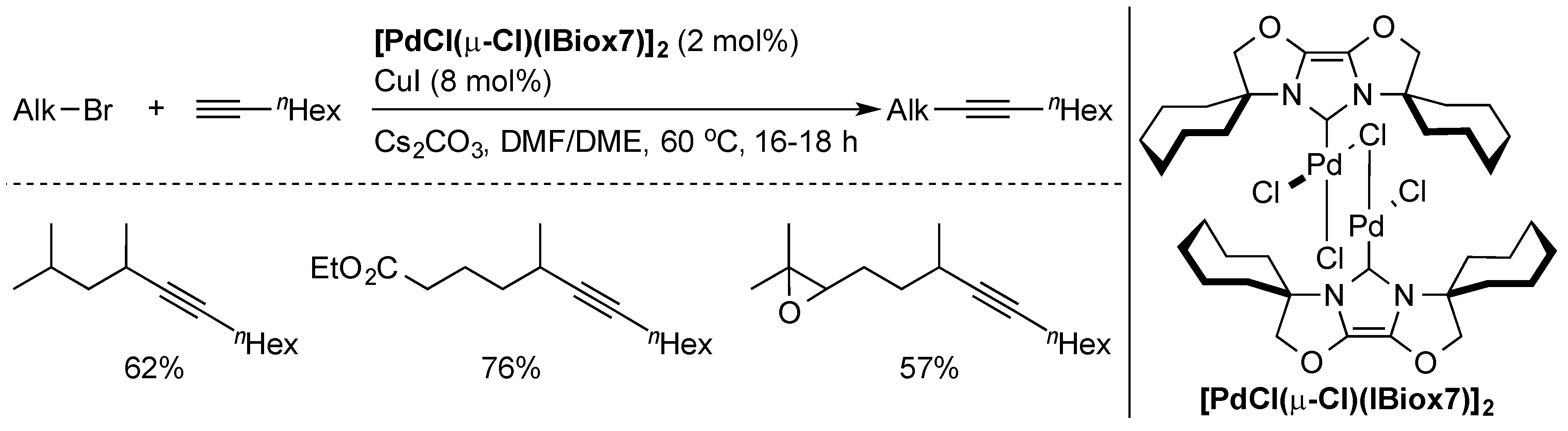
The Sonogashira cross-coupling [14,35,36] of alkynes with organic halides is an efficient method of obtaining functionalized alkynes. However, aromatic halides are typically used. Coupling of non-activated alkyl halides was always elusive due to problematic oxidative addition steps and side-reactions such as β-hydride elimination. The first successful example of this reaction [37] was the palladium-catalyzed coupling of primary alkyl halides with terminal alkynes by Fu and co-workers. Later, the first Sonogashira coupling of secondary bromides with 1-octyne was developed by Glorius and co-workers [38], using the well-defined [PdCl(µ-Cl)(IBiox7)]2 pre-catalyst (Scheme 4).
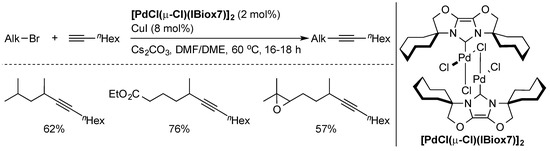
Scheme 4.
Selected examples of Sonogashira coupling using a well-defined Pd-IBiox complex [38].
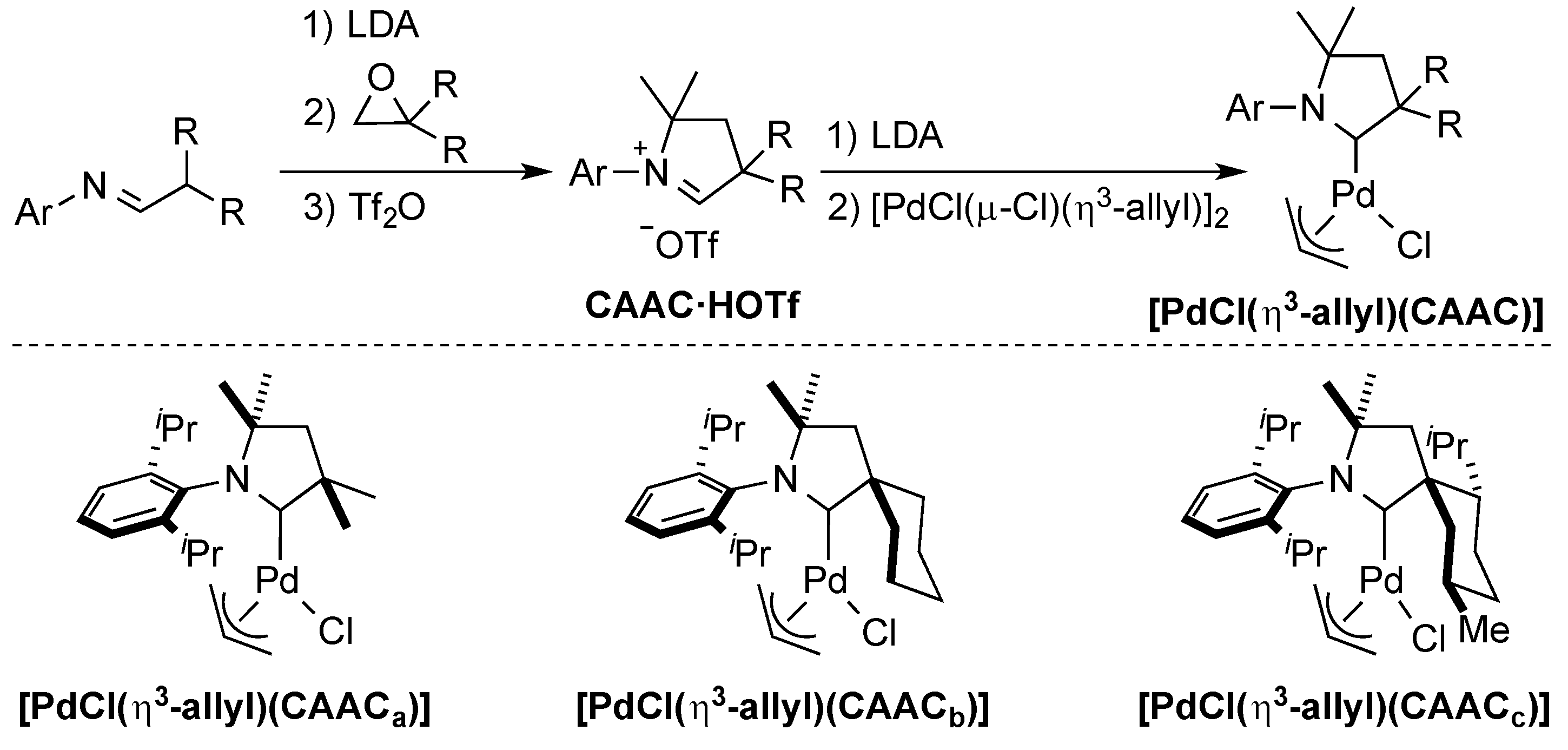
3. CAAC Series
Bertrand and co-workers recognized the concept of sterically flexible ligands in their development of the novel class of cyclic (alkyl)(amino)carbenes (CAACs) [24,39,40]. These electron-rich ligands are pyrrolidine-based and bear two quaternary carbons. They are prepared from the corresponding imines [24] and after deprotonation of the CAAC·HOTf, under harsh conditions, using LDA (lithium diisopropylamide), addition of palladium allyl chloride dimer leads to the formation of the well-defined [PdCl(η3-allyl)(CAAC)] complex (Scheme 5).
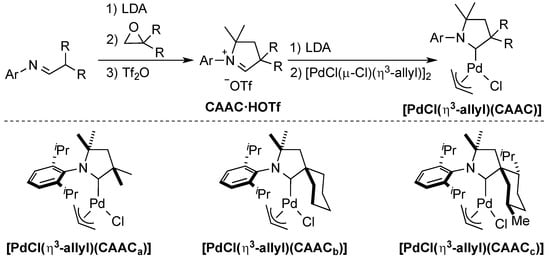
Scheme 5.
Synthesis of the CAAC ligand series [24].
The reactivity of these [PdCl(η3-allyl)(CAAC)] complexes was tested in the α-arylation of aryl chlorides with phenylethylketone [24]. It was found that the most sterically demanding ligand (CAACc) was the most effective when using an unsubstituted aryl chloride, whereas the flexibility of CAACb led to higher yields with more sterically hindered aryl chlorides.
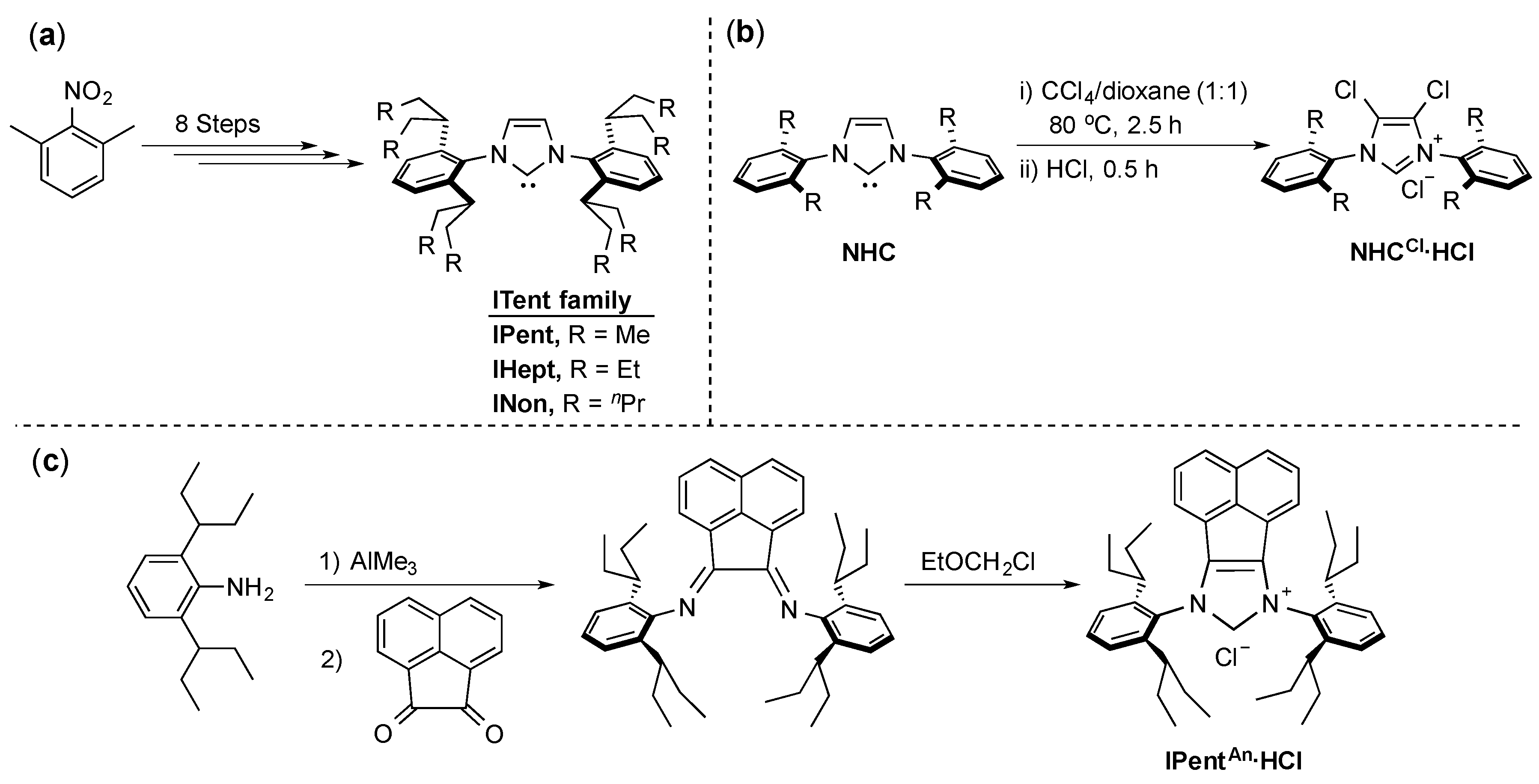
4. ITent
Recent studies showed that up to a certain point, there is a proportional relationship between the increase in the NHC steric bulk and the increase in catalytic activity [5,41]. This phenomenon led to the implementation of flexible bulk into the design of one of the most commonly used NHCs: IPr (N,N’-bis-[2,6-(di-iso-propyl)phenyl]imidazol-2-ylidene). This new series is known as ITent (after its tentacular structure) and the synthesis of these IPr-mimicking ligands was described by Nolan and co-workers (Scheme 6a) [42]. Analogues of these ligands with a chloro-substituted backbone were described by Organ and co-workers in their PEPPSI (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) series [43] (Scheme 6b), and more recently, Liu and co-workers described the synthesis of the acenaphthyl-substituted backbone analogue IPentAn (Scheme 6c) [44].
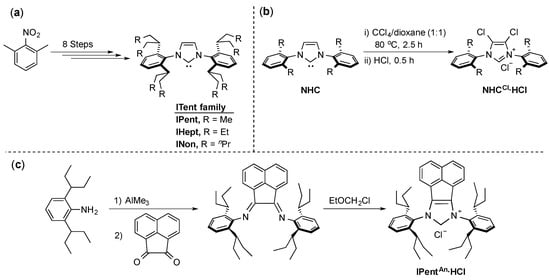
Scheme 6.
Synthetic access to ITent ligands (a–c) [42,43,44].
The Nolan group used this ITent series for the synthesis of their “custom-made” pre-catalysts [PdCl(η3-cin)(ITent)] (cin = cinnamyl) and [PdCl(acac)(ITent)] (acac = acetylacetonate) [42], while Organ used them to further optimize the PEPPSI series [16,27].
4.1. Suzuki–Miyaura Cross-Coupling
The synthesis of tetra-ortho-substituted biaryls under mild conditions remains a challenge in the Suzuki–Miyaura reaction. As shown above, the use of ligands with flexible bulk proved to be critical, and the first example using NHC ligands was described by Glorius in 2004 [23]. Organ was able to further optimize the reaction using the ITent series. More specifically, the well-defined PEPPSI-IPent pre-catalyst was used to achieve a wider scope than previous reports [27].
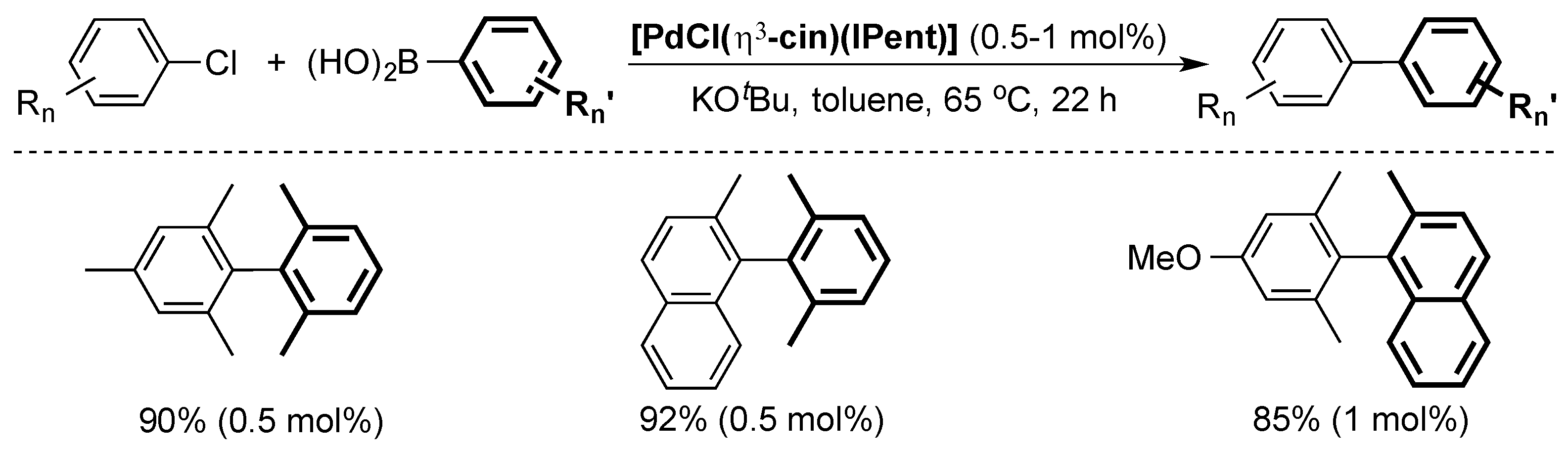
The Nolan group used the well-defined [PdCl(η3-cin)(ITent)] pre-catalyst series to further advance the state-of-the-art [42]. Of particular interest, [PdCl(η3-cin)(IPent)] was able to efficiently catalyze the cross-coupling of highly hindered aryl chlorides with boronic acids at low catalysts loading (Scheme 7).
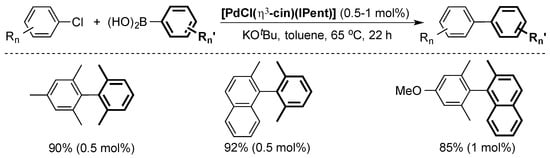
Scheme 7.
Selected examples of the Suzuki–Miyaura coupling using [PdCl(η3-cin)(IPent)] [42].
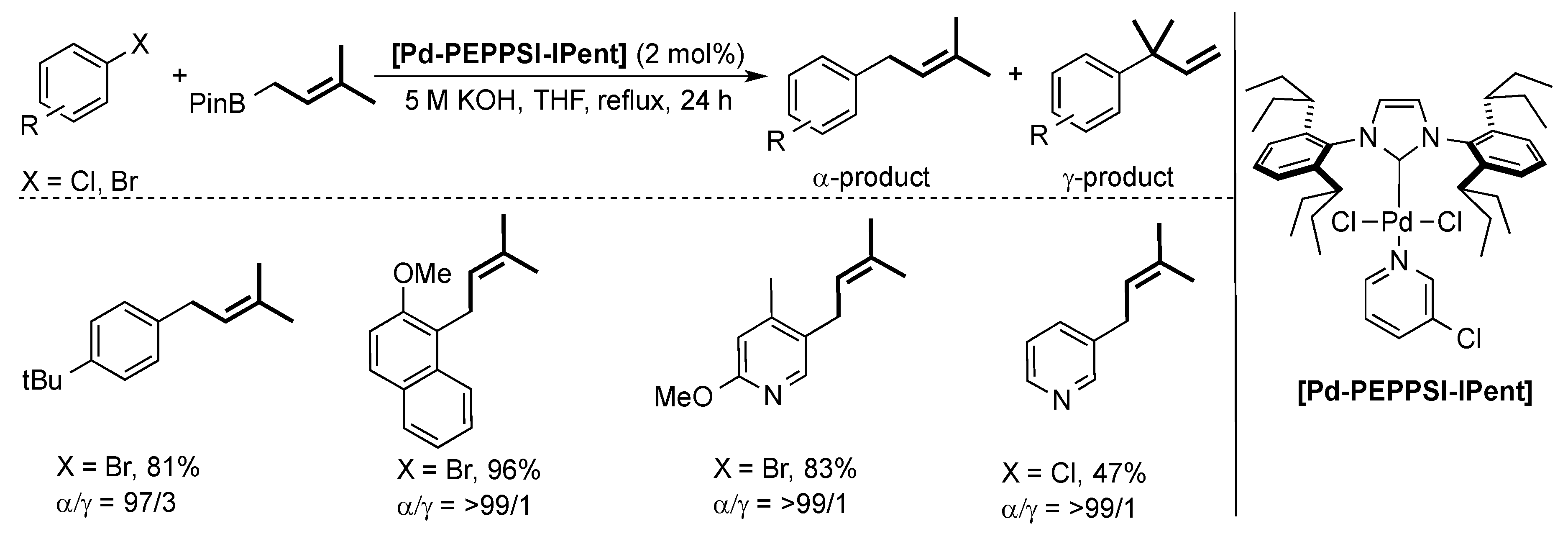
Organ and co-workers also used the [Pd-PEPPSI-IPent] pre-catalyst for the challenging coupling of allylboronic acid with different aryl halides [45]. In this context, (hetero)aryl halides were successfully coupled with high selectivity for the α-product (Scheme 8).
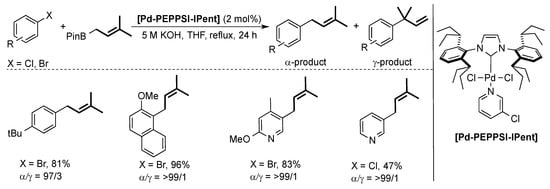
Scheme 8.
Examples of the Suzuki–Miyaura coupling using allylboronic esters and aryl halides [45].
Recently, Liu and co-workers showed that the [Pd-PEPPSI-IPentAn] precatalyst is efficient in the the Suzuki-Miyaura coupling of a wide range of hindered arylboronic acids and hindered aryl chlorides at 80 °C using a 1 mol % Pd loading [44].
4.2. Negishi Cross-Coupling
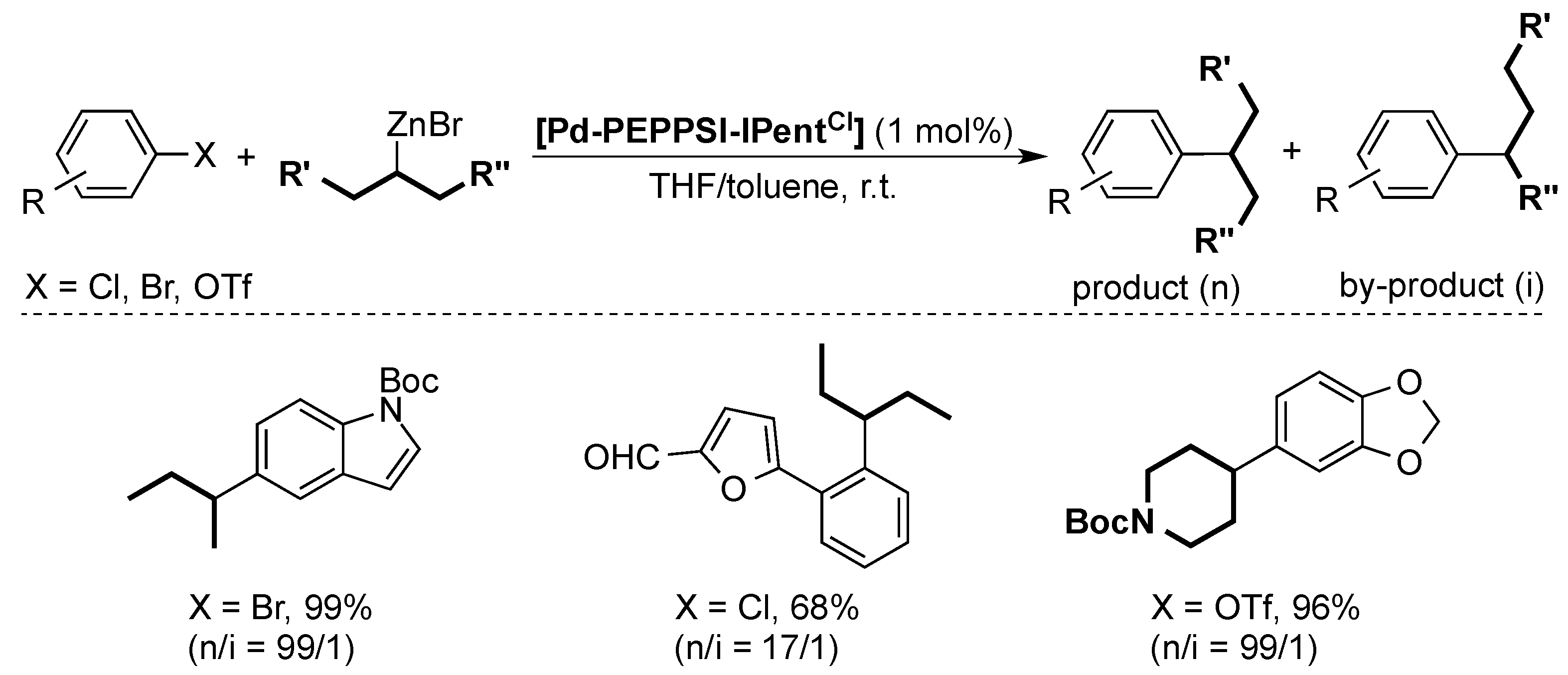
After the first Negishi coupling with the well-defined [Pd-PEPPSI-IPr] pre-catalyst [46], the Organ group showed that the [Pd-PEPPSI-IPent] pre-catalyst was even more active, especially in the synthesis of tetra-ortho-substituted biaryls [47,48]. The same palladium pre-catalyst also proved to be highly efficient in the coupling of secondary alkylzinc substrates to aryl halides [49]. The flexible steric bulk of the IPent ligand reduced the β-hydride elimination/migratory insertion considerably, limiting the formation of the isomeric by-product. Later, Organ and co-workers improved this selectivity even further by using [Pd-PEPPSI-IPentCl] (Scheme 9) [43].
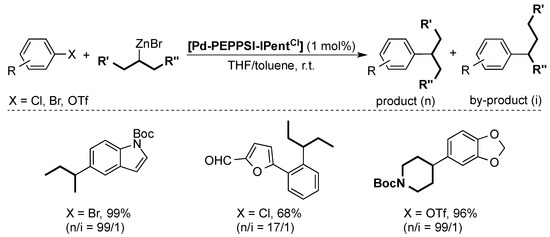
Scheme 9.
Selected examples for the Negishi cross-coupling of secondary alkylzinc substrates [43].
Recently, Organ and co-workers described a silica-supported pre-catalyst, [Pd-PEPPSI-IPent-SiO2], and evaluated its activity in challenging Negishi cross-coupling reactions under flow conditions [50]. The catalyst material was used in a packed-bed reactor at room temperature and small residence times (10 min or less) were enough to obtain high conversions.
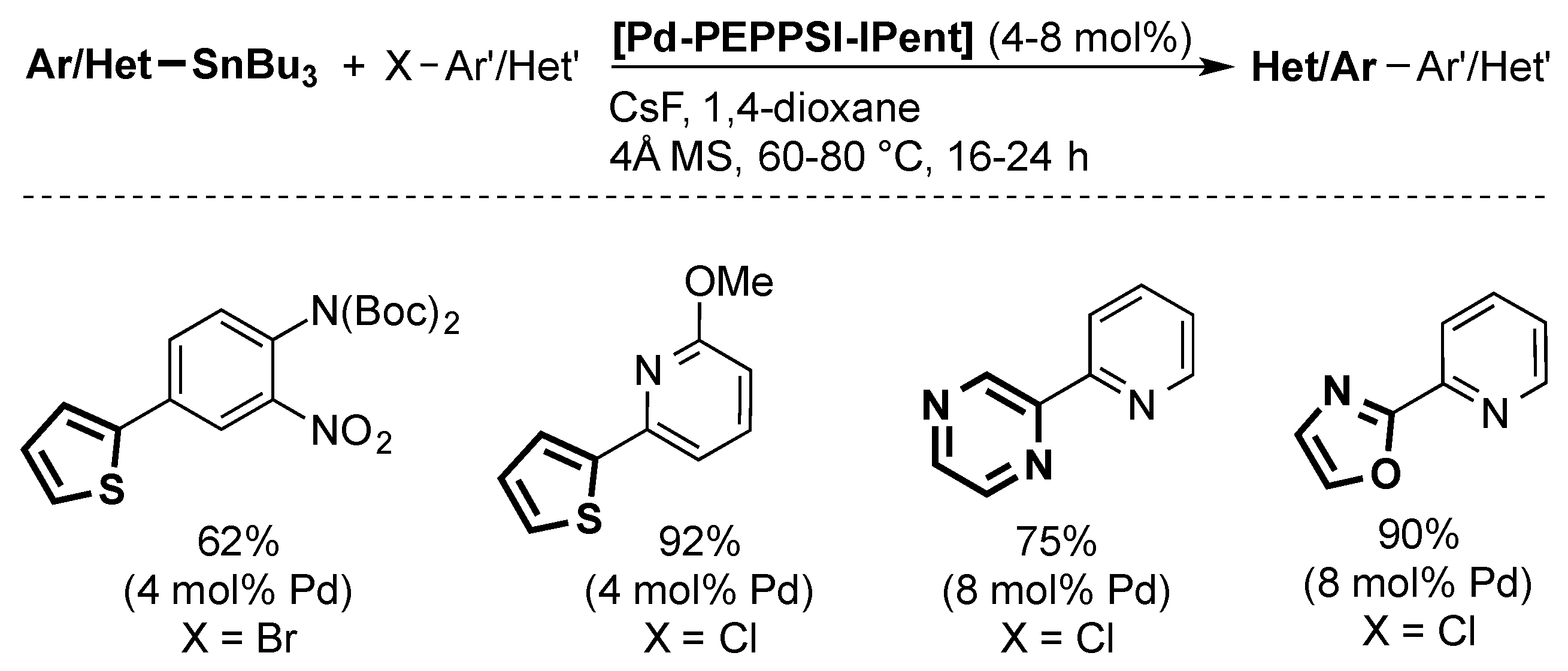
4.3. Stille Coupling
In 2010, Organ and co-workers reported a Stille coupling using [Pd-PEPPSI-IPent] (Scheme 10) [51]. While the method can be used for aryls and heteroaryl substrates, at relatively mild temperatures (60–80 °C), high catalyst loadings are needed in all cases (4–8 mol %).
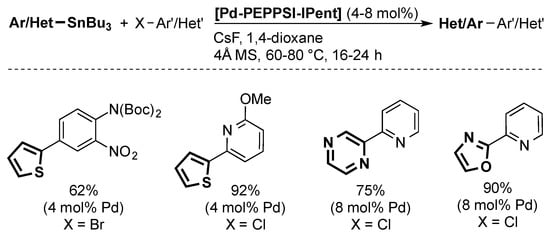
Scheme 10.
Examples of the Stille coupling catalyzed by [Pd-PEPPSI-IPent] [51].
4.4. Buchwald-Hartwig Cross-Coupling
After the first report of the use of palladium-NHC systems for the amination reaction by Nolan [52], the concept of ligands with “flexible bulk” advanced the state-of-the-art. Organ reported the use of [Pd-PEPPSI-IPent] [53] under milder conditions than the ones used with the [Pd-PEPPSI-IPr] analogue, allowing for a greater tolerance of functional groups. The IPent derivative was even capable of catalyzing some reactions that were previously unattainable using the IPr-based catalyst [54]. However, it must be noted that high catalyst loadings (4 mol %) were necessary to achieve good results. More recently, the same group successfully achieved the coupling of various amides with aryl and heteroaryl chlorides using [Pd(η3-cin)Cl(DiMeIHeptCl)] as catalyst, assisted by boron-derived Lewis acids [55].
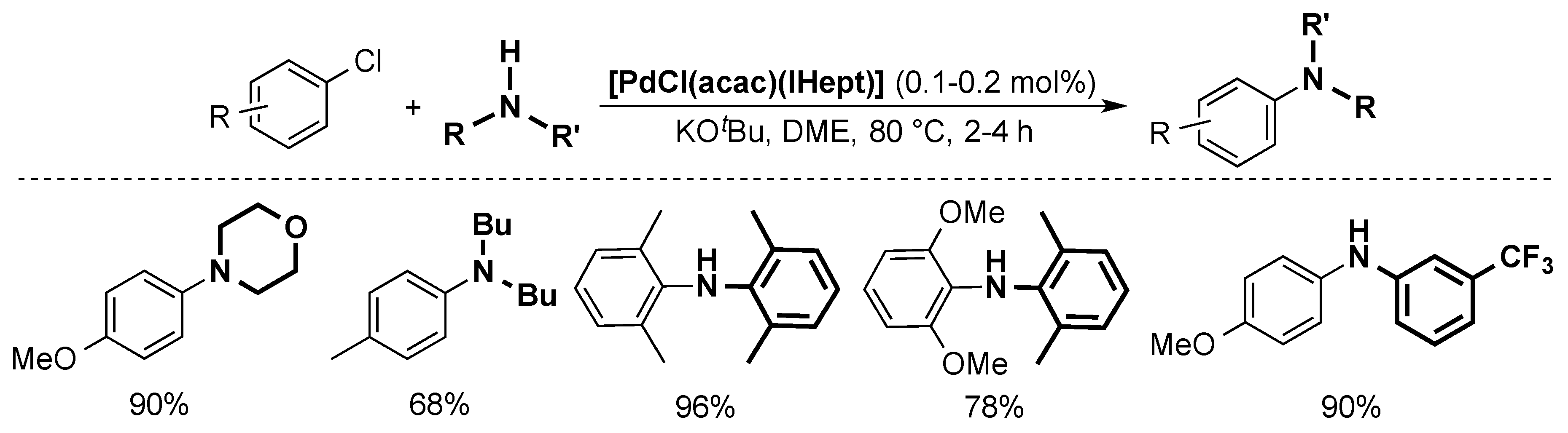
The Nolan group was able to reduce the catalyst loading by using [PdCl(acac)(ITent)] complexes (Scheme 11) [42]. More specifically, the use of the IHept ligand permitted to lower the catalyst loading to 0.1–0.2 mol %. This system allows for the use of otherwise difficult coupling partners, such as electron-poor anilines.
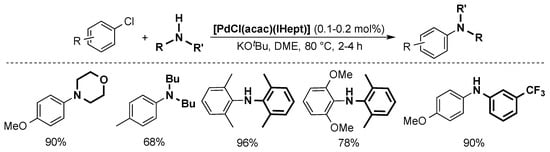
Scheme 11.
Examples of the Buchwald-Hartwig coupling catalyzed by [PdCl(acac)(IHept)] [42].
The Organ group investigated the coupling of 2-aminopyridine derivatives using [Pd-PEPPSI-IPentCl] [56]. Such catalysts were successful in the coupling of such aminopyridines with various aryl chlorides, but it must be mentioned that catalyst poisoning occurred due to metal coordination of the aminopyridine.
Recently, Liu and co-workers showed that [Pd-PEPPSI-IPentAn] was an efficient pre-catalyst in the amination of (hetero)aryl chlorides under aerobic conditions [57].
5. N-Naphthyl-Based NHCs
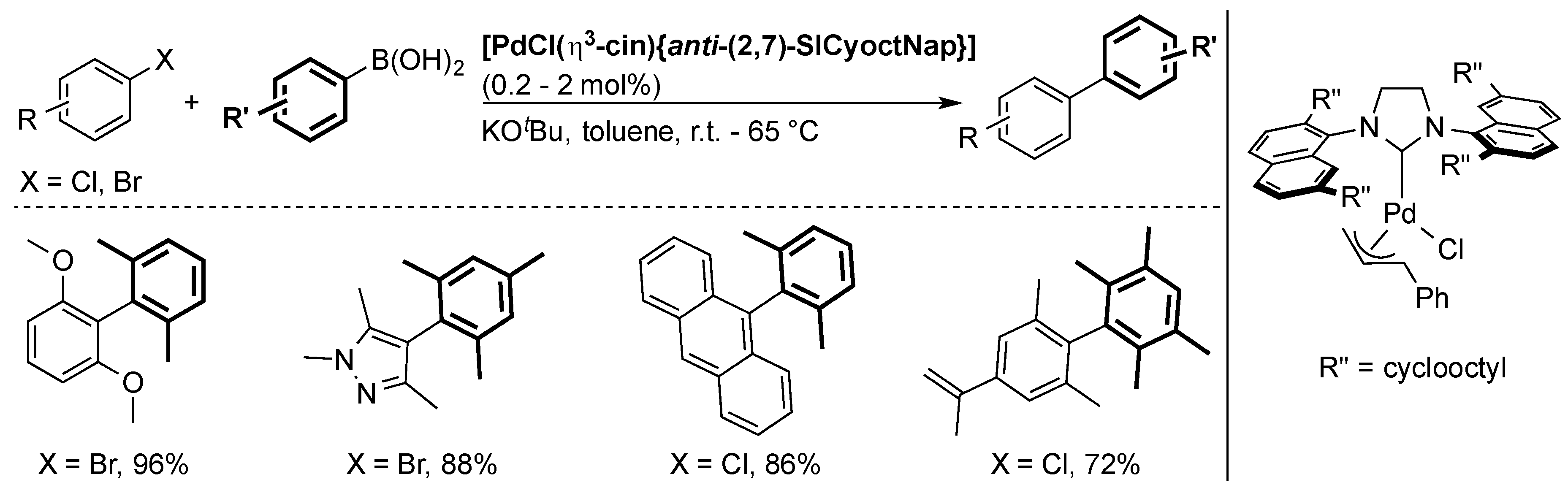
Another class of NHC ligands with naphthyl groups on the nitrogen atoms was synthesized by Dorta and co-workers and used to prepare well-defined palladium pre-catalysts [29,58,59,60]. [PdCl(η3-cin){anti-(2,7)-SICyoctNap}], one of the most important complexes of the series, was the first well-defined complex to promote the coupling of tetra-ortho-substituted biaryls via a Suzuki–Miyaura reaction at room temperature (Scheme 12) [29,60]. It showed that the success of this ligand lied with the NHC being twisted around the metal center with two bulky faces and two less hindered faces.
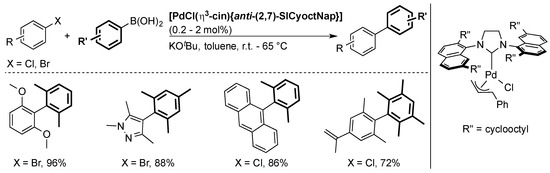
Scheme 12.
Selected examples using [PdCl(η3-cin){anti-(2,7)-SICyoctNap}] [29].
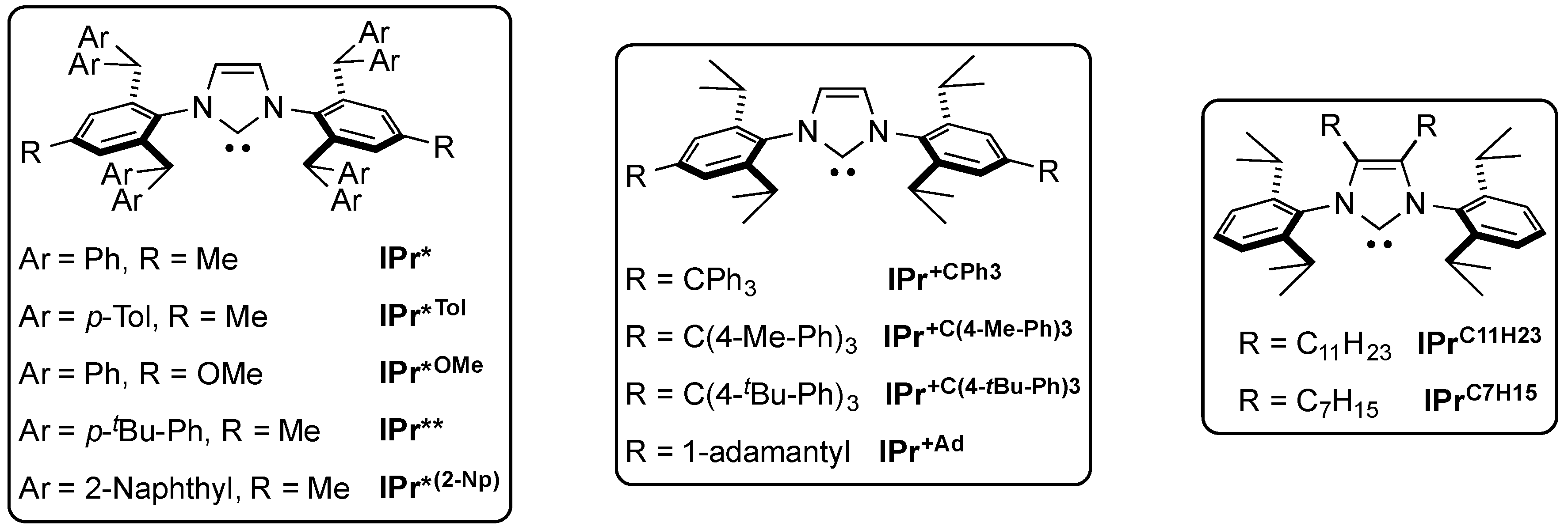
6. IPr* and Related NHCs
The IPr* ligand proved optimal for the balance between sterics and flexibility. This hindered ligand, which was first synthesized by Markó and co-workers [28], triggered interest and a number of congeners were developed [61,62,63,64]. Other manipulations of the IPr framework were investigated, such as the IPr+C series by Holland and co-workers [65] and the substitution of the NHC backbone with long alkyl chains by Glorius and co-workers [66] (Figure 1).
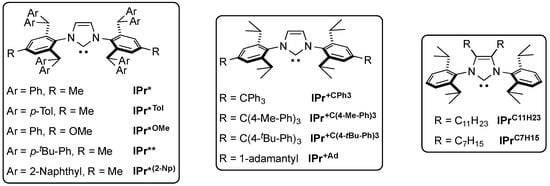
Figure 1.
Structure of the IPr*, IPr+C and IPrCxHy ligands [28,61,62,63,64].
6.1. Suzuki Miyaura Cross-Coupling
The [PdCl(η3-cin)(IPr*)] pre-catalyst proved to be efficient in the preparation of tetra-ortho-substituted biaryls via Suzuki–Miyaura coupling at room temperature using 1 mol % Pd loading [67].
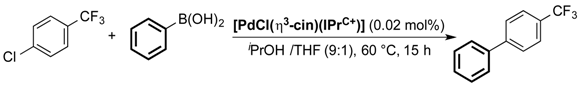
Holland and co-workers reported the [PdCl(η3-cin)(IPr+C)] series in Suzuki–Miyaura coupling of 4-chloro(trifluoromethyl)benzene and phenylboronic acid [65]. When compared with the IPr analogue, all complexes were found more active with a trend of increase in steric bulk directly proportional to increase of activity up to a certain tipping point; this is where flexibility is compromised because of a too large steric hindrance (Table 1).

Table 1.
Investigation of the [PdCl(η3-cin)(IPr+C)] series in Suzuki–Miyaura coupling [65].
Recently, Qian and co-workers reported a polymer analogue to the IPr ligand (Figure 2, left) for use as a recoverable catalyst [68]. This catalyst was tested in the cross-coupling involving activated aryl bromides and chlorides. Reusability of the catalyst was shown over 6 runs—however, no kinetic data was provided—thus the possibility of the system acting as a reservoir of active species cannot be ruled out.
An alternative approach to catalyst separation using membranes was undertaken by Ormerod and co-workers on IPr and related ligands. While early studies were carried out on IPr ligand [69], modification of the latter by appending long chains at the para-position of the phenyl ring (Figure 2, right) proved judicious for catalyst recovery through nanofiltration. This allowed the cross-coupling reaction in a semicontinuous mode in a membrane-assisted reactor [70].
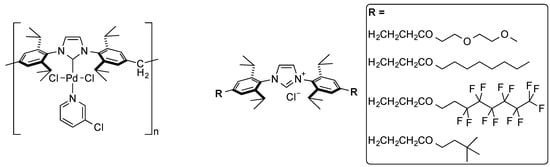
Figure 2.
Polymer-modified IPr-complex (left) [68] and para-tailed IPr ligands (right) for semicontinuous reactions [70].
Figure 2.
Polymer-modified IPr-complex (left) [68] and para-tailed IPr ligands (right) for semicontinuous reactions [70].
6.2. Buchwald Hartwig Cross-Coupling
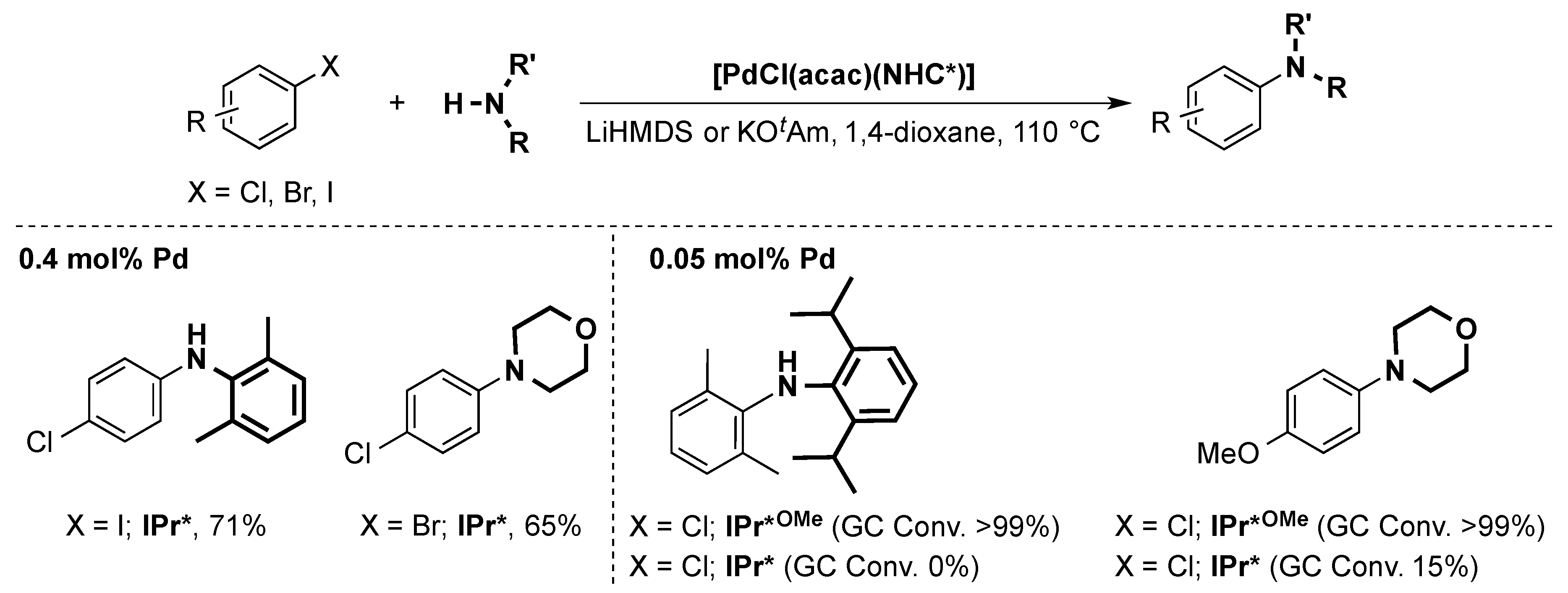
The Nolan group investigated the use of [PdCl(η3-cin)(IPr*)] and [Pd-PEPPSI-IPr*] in the Buchwald-Hartwig reaction [71,72,73] and found that they were the most active pre-catalysts for this reaction to date. Both complexes showed similar activity, which indicates that the active species is the same in both cases. The [PdCl(η3-cin)(IPr*)] pre-catalyst was also used in a solvent-free amination using 1 mol % of the complex in neat condition [74]. This allowed for the use of primary amines at room temperature, which was previously not possible.
The very easily prepared [PdCl(acac)(IPr*)] pre-catalyst was shown to be active in the same reaction [71] and showed chemoselective arylamination of various dihalides resulting in mono-aminated products. [PdCl(acac)(IPr*OMe)] was also tested and proved to be even more active, allowing for the coupling of challenging electron-poor anilines (Scheme 13) [62].
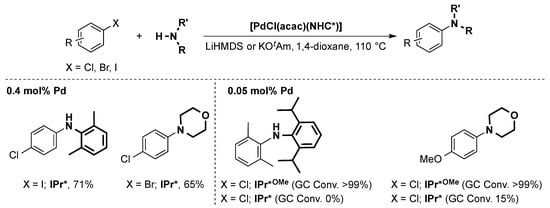
Scheme 13.
Examples of the Buchwald-Hartwig coupling using [PdCl(acac)(IPr*)] or [PdCl(acac)(IPr*OMe)] as pre-catalysts [62,71]. (KOtAm = potassium tert-amylate).
Glorius and co-workers used their series of long-carbon-chain-based palladium complexes in the Buchwald-Hartwig amination [66]. Of this series, [PdCl(η3-allyl)(IPrC11H23)] (see Figure 1) proved to be the most active (0.1 mol % Pd loading, 75 °C).
6.3. Other Palladium-Catalyzed Reactions
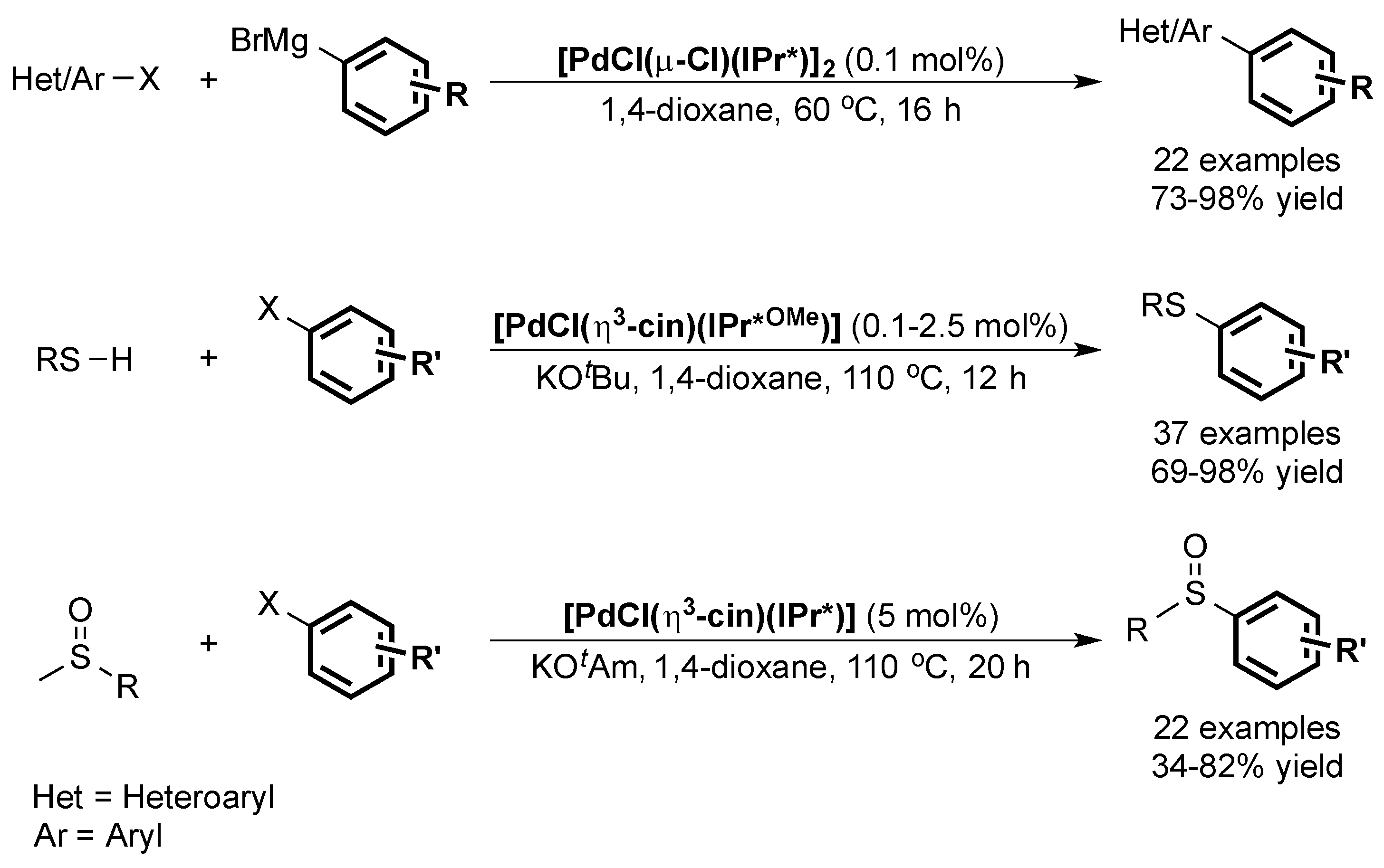
The IPr* ligand and its analogues were also used in the cross-coupling of Grignard reagents leading to the formation of tetra-ortho-substituted compounds [75] in high yields. Finally, the same ligand family was shown to lead to active catalysts in the formation of C–S bonds (Scheme 14) [76,77].
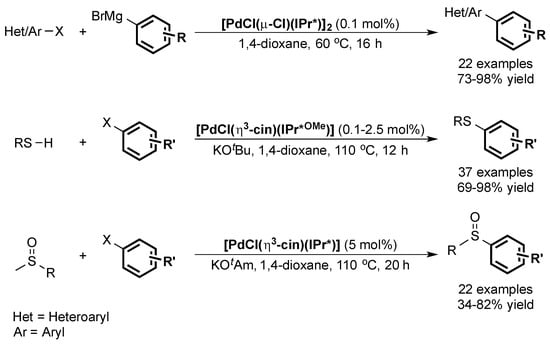
Scheme 14.
Other palladium-catalyzed cross-couplings using IPr* and IPr*OMe ligands [75,76,77].
7. Conclusions and Outlook
“Bulky yet flexible” ligands were recently investigated, greatly advancing the state-of-the-art, particularly in reactions involving sterically congested coupling partners. The correlation between flexible steric bulk and catalytic activity is not always straightforward, as is proven by the failure of some extremely large ligands.
Advancements in this field not only benefited palladium chemistry but were also successfully applied to other research areas and related metals such as nickel. Various reports on the design and applications of IPr- and IPr*-based (among other related derivatives) nickel catalysts by Nolan [78,79,80,81], Nakamura [82], Ackermann [83], Sun [84], Cramer [85], Newman [86], and Matsubara [87] pioneered the transfer of the “bulky yet flexible” concept from palladium to nickel-based coupling reactions. The combination of nickel with other “bulky yet flexible” ligands such as CAACs [88,89] and abnormal NHCs [90] also saw some success. Moreover, Montgomery [91], Louie [92] and Johnson [93] used this concept to further investigate and consequently advance the synthesis and catalytic application of styrene-based nickel complexes, thus building upon the work of Belderrain and Nicasio [94].
Future advances, in particular through mechanistic studies, will provide insights into the exact role of electronic and steric parameters of NHC ligands on catalyst activity, thus guiding further catalyst design efforts across the periodic table. Nonetheless, these “bulky yet flexible” NHCs have already pushed the limits of highly hindered cross-coupling reactions, and hold great promise for combined high activity and catalyst recycling for numerous other metal systems.
Funding
Part of this work was funded by a Research Foundation—Flanders (FWO) grant (180327/1S99819N).
Acknowledgments
The authors greatly acknowledge the Research Foundation—Flanders (FWO) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Cazin, C.S.J. (Ed.) N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Springer: London, UK, 2011; ISBN 978-90-481-2866-2. [Google Scholar]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.D. Diverse Chemical Applications of N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2009, 131, 15075–15077. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Cazin, C.S.J. (Eds.) Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis; Thieme: Stuttgart, Germany, 2016; Volume 1, ISBN 978-31-320-1291-2. [Google Scholar]
- Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in gold catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Gade, L.H.; Bellemin-Laponnaz, S. Chiral N-Heterocyclic Carbenes as Stereodirecting Ligands in Asymmetric Catalysis. In N-Heterocyclic Carbenes in Transition Metal Catalysis; Glorius, F., Ed.; Springer: Berlin, Germany, 2007; pp. 117–157. ISBN 978-3-540-36930-1. [Google Scholar]
- Lin, I.J.B.; Vasam, C.S. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar] [CrossRef]
- Kuhl, O. The chemistry of functionalised N-heterocyclic carbenes. Chem. Soc. Rev. 2007, 36, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, M.; Guest, D.; Navarro, O. (N-Heterocyclic Carbene)–Palladium Complexes in Catalysis. In N-Heterocycle Carbenes: Effective Tools for Organometallic Synthesis; Nolan, S.P., Ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 85–110. ISBN 978-35-273-3490-2. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabba, F.P.; Bertrand, G. Stable carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef]
- Chartoire, A.; Nolan, S.P. Advances in C–C and C–X Coupling Using Palladium–N-Heterocyclic Carbene (Pd–NHC) Complexes. In New Trends in Cross-Coupling: Theory and Applications; Colacot, T.J., Ed.; RSC: Cambridge, UK, 2014; pp. 139–227. ISBN 978-1-84973-896-5. [Google Scholar]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Würtz, S.; Glorius, F. Surveying Sterically Demanding N-Heterocyclic Carbene Ligands with Restricted Flexibility for Palladium-catalyzed Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1523–1533. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hou Hoi, K.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, F.; Manzini, S.; Nolan, S.P. The Use of the Sterically Demanding IPr* and Related Ligands in Catalysis. Chem. Commun. 2014, 50, 14926–14937. [Google Scholar] [CrossRef] [PubMed]
- Szilvási, T.; Veszprémi, T. Internal Catalytic Effect of Bulky NHC Ligands in Suzuki–Miyaura Cross-Coupling Reaction. ACS Catal. 2013, 3, 1984–1991. [Google Scholar] [CrossRef]
- Widenhoefer, R.A.; Buchwald, S.L. Halide and Amine Influence in the Equilibrium Formation of Palladium Tris(o-tolyl)phosphine Mono(amine) Complexes from Palladium Aryl Halide Dimers. Organometallics 1996, 15, 2755–2763. [Google Scholar] [CrossRef]
- Poater, A.; Cavallo, L. Deactivation of Ru-benzylidene Grubbs catalysts active in olefin metathesis. Theor. Chem. Acc. 2012, 131, 1155. [Google Scholar] [CrossRef]
- Poater, A.; Bahri-Laleh, N.; Cavallo, L. Rationalizing current strategies to protect N-heterocyclic carbene-based ruthenium catalysts active in olefin metathesis from C–H (de)activation. Chem. Commun. 2011, 47, 6674–6676. [Google Scholar] [CrossRef]
- Poater, A.; Falivene, L.; Urbina-Blanco, C.A.; Manzini, S.; Nolan, S.P.; Cavallo, L. How does the addition of steric hindrance to a typical N-heterocyclic carbene ligand affect catalytic activity in olefin metathesis? Dalton Trans. 2013, 42, 7433–7439. [Google Scholar] [CrossRef]
- Altenhoff, G.; Goddard, R.; Lehmann, C.W.; Glorius, F. Sterically Demanding, Bioxazoline-Derived N-Heterocyclic Carbene Ligands with Restricted Flexibility for Catalysis. J. Am. Chem. Soc. 2004, 126, 15195–15201. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem. Int. Ed. 2005, 44, 5705–5709. [Google Scholar] [CrossRef]
- Huang, J.; Nolan, S.P. Efficient Cross-Coupling of Aryl Chlorides with Aryl Grignard Reagents (Kumada Reaction) Mediated by a Palladium/Imidazolium Chloride System. J. Am. Chem. Soc. 1999, 121, 9889–9890. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Krafczyk, R.; Schmutzler, R.; Craig, H.A.; Goerlich, J.R.; Marshall, W.J.; Unverzagt, M. Imidazolylidenes, imidazolinylidenes and imidazolidines. Tetrahedron 1999, 55, 14523–14534. [Google Scholar] [CrossRef]
- Organ, M.G.; Çalimsiz, S.; Sayah, M.; Hoi, K.H.; Lough, A.J. Pd-PEPPSI-IPent: An Active, Sterically Demanding Cross-Coupling Catalyst and Its Application in the Synthesis of Tetra-Ortho-Substituted Biaryls. Angew. Chem. Int. Ed. 2009, 48, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Berthon-Gelloz, G.; Siegler, M.A.; Spek, A.L.; Tinant, B.; Reek, J.N.H.; Markó, I.E. IPr* an easily accessible highly hindered N-heterocyclic carbene. Dalton Trans. 2010, 39, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Drinkel, E.; Gaggia, F.; Capolicchio, S.; Linden, A.; Falivene, L.; Cavallo, L.; Dorta, R. Room-Temperature Synthesis of Tetra-ortho-Substituted Biaryls by NHC-Catalyzed Suzuki–Miyaura Couplings. Chem. Eur. J. 2011, 17, 12886–12890. [Google Scholar] [CrossRef] [PubMed]
- Altenhoff, G.; Goddard, R.; Lehmann, C.W.; Glorius, F. An N-Heterocyclic Carbene Ligand with Flexible Steric Bulk Allows Suzuki Cross-Coupling of Sterically Hindered Aryl Chlorides at Room Temperature. Angew. Chem. Int. Ed. 2003, 42, 3690–3693. [Google Scholar] [CrossRef] [PubMed]
- Bexrud, J.; Lautens, M. A Rhodium IBiox[(−)-menthyl] Complex as a Highly Selective Catalyst for the Asymmetric Hydroarylation of Azabicyles: An Alternative Route to Epibatidine. Org. Lett. 2010, 12, 3160–3163. [Google Scholar] [CrossRef] [PubMed]
- Würtz, S.; Lohre, C.; Fröhlich, R.; Bergander, K.; Glorius, F. IBiox[(−)-menthyl]: A Sterically Demanding Chiral NHC Ligand. J. Am. Chem. Soc. 2009, 131, 8344–8345. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Oxazolines as chiral building blocks for imidazolium salts and N-heterocyclic carbene ligands. Chem. Commun. 2002, 2704–2705. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Anastasia, L. Palladium-Catalyzed Alkynylation. Chem. Rev. 2003, 103, 1979–2018. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Fu, G.C. The First Applications of Carbene Ligands in Cross-Couplings of Alkyl Electrophiles: Sonogashira Reactions of Unactivated Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003, 125, 13642–13643. [Google Scholar] [CrossRef] [PubMed]
- Altenhoff, G.; Würtz, S.; Glorius, F. The first palladium-catalyzed Sonogashira coupling of unactivated secondary alkyl bromides. Tetrahedron Lett. 2006, 47, 2925–2928. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; DeHope, A.; Donnadieu, B.; Bertrand, G. A Rigid Cyclic (Alkyl)(amino)carbene Ligand Leads to Isolation of Low-Coordinate Transition-Metal Complexes. Angew. Chem. Int. Ed. 2005, 44, 7236–7239. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Tang, H.; Zeng, X.; Liu, L.; Melaimi, M.; Bertrand, G. Cyclic (Amino)(aryl)carbenes (CAArCs) as Strong σ-Donating and π-Accepting Ligands for Transition Metals. Angew. Chem. Int. Ed. 2015, 54, 14915–14919. [Google Scholar] [CrossRef] [PubMed]
- Organ, M.G.; Chass, G.A.; Fang, D.-C.; Hopkinson, A.C.; Valente, C. Pd-NHC (PEPPSI) Complexes: Synthetic Utility and Computational Studies into Their Reactivity. Synthesis 2008, 2776–2797. [Google Scholar] [CrossRef]
- Meiries, S.; Le Duc, G.; Chartoire, A.; Collado, A.; Speck, K.; Arachchige, K.S.A.; Slawin, A.M.Z.; Nolan, S.P. Large yet Flexible N-Heterocyclic Carbene Ligands for Palladium Catalysis. Chem. Eur. J. 2013, 19, 17358–17368. [Google Scholar] [CrossRef]
- Pompeo, M.; Froese, R.D.J.; Hadei, N.; Organ, M.G. Pd-PEPPSI-IPent(Cl): A highly effective catalyst for the selective cross-coupling of secondary organozinc reagents. Angew. Chem. Int. Ed. 2012, 51, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.D.; He, X.X.; Liu, F.S. Bulky yet flexible Pd-PEPPSI-IPentAn for the synthesis of sterically hindered biaryls in air. J. Org. Chem. 2017, 82, 10898–10911. [Google Scholar] [CrossRef]
- Farmer, J.L.; Hunter, H.N.; Organ, M.G. Regioselective Cross-Coupling of Allylboronic Acid Pinacol Ester Derivatives with Aryl Halides via Pd-PEPPSI-IPent. J. Am. Chem. Soc. 2012, 134, 17470–17473. [Google Scholar] [CrossRef]
- Organ, M.G.; Avola, S.; Dubovyk, I.; Hadei, N.; Kantchev, E.A.B.; O’Brien, C.J.; Valente, C. A User-Friendly, All-Purpose Pd–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for the Negishi Reaction: A Step Towards a Universal Cross-Coupling Catalyst. Chem. Eur. J. 2006, 12, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Calimsiz, S.; Sayah, M.; Mallik, D.; Organ, M.G. Pd-PEPPSI-IPent: Low-Temperature Negishi Cross-Coupling for the Preparation of Highly Functionalized, Tetra-ortho-Substituted Biaryls. Angew. Chem. Int. Ed. 2010, 49, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Belowich, M.E.; Hadei, N.; Organ, M.G. Pd-PEPPSI Complexes and the Negishi Reaction. Eur. J. Org. Chem. 2010, 2010, 4343–4354. [Google Scholar] [CrossRef]
- Calimsiz, S.; Organ, M.G. Negishi cross-coupling of secondary alkylzinc halides with aryl/heteroaryl halides using Pd–PEPPSI–IPent. Chem. Commun. 2011, 47, 5181–5183. [Google Scholar] [CrossRef] [PubMed]
- Price, G.A.; Hassan, A.; Chandrasoma, N.; Bogdan, A.R.; Djuric, S.W.; Organ, M.G. Pd-PEPPSI-IPent-SiO2: A supported catalyst for challenging Negishi coupling reactions in flow. Angew. Chem. Int. Ed. 2017, 56, 13347–13350. [Google Scholar] [CrossRef]
- Dowlut, M.; Mallik, D.; Organ, M.G. An Efficient Low-Temperature Stille–Migita Cross-Coupling Reaction for Heteroaromatic Compounds by Pd–PEPPSI–IPent. Chem. Eur. J. 2010, 16, 4279–4283. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Grasa, G.; Nolan, S.P. General and Efficient Catalytic Amination of Aryl Chlorides Using a Palladium/Bulky Nucleophilic Carbene System. Org. Lett. 1999, 1, 1307–1309. [Google Scholar] [CrossRef]
- Hoi, K.H.; Calimsiz, S.; Froese, R.D.J.; Hopkinson, A.C.; Organ, M.G. Amination with Pd–NHC Complexes: Rate and Computational Studies on the Effects of the Oxidative Addition Partner. Chem. Eur. J. 2011, 17, 3086–3090. [Google Scholar] [CrossRef]
- Hoi, K.H.; Calimsiz, S.; Froese, R.D.J.; Hopkinson, A.C.; Organ, M.G. Amination with Pd–NHC Complexes: Rate and Computational Studies Involving Substituted Aniline Substrates. Chem. Eur. J. 2012, 18, 145–151. [Google Scholar] [CrossRef]
- Sharif, S.; Day, J.; Hunter, H.N.; Lu, Y.; Mitchell, D.; Rodriguez, M.J.; Organ, M.G. Cross-coupling of primary amides to aryl and heteroaryl partners using (DiMeIHeptCl)Pd promoted by trialkylboranes or B(C6F5)3. J. Am. Chem. Soc. 2017, 139, 18436–18439. [Google Scholar] [CrossRef]
- Khadra, A.; Mayer, S.; Organ, M.G. Pd-PEPPSI-IPentCl: A useful catalyst for the coupling of 2-aminopyridine derivatives. Chem. Eur. J. 2017, 23, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-D.; Xu, C.; Lu, D.-D.; Shen, D.-S.; Li, T.; Liu, F.-S. Pd-PEPPSI-IPentAn promoted deactivated amination of aryl chlorides with amines under aerobic conditions. J. Org. Chem. 2018, 83, 9144–9155. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Mariz, R.; Gatti, M.; Costabile, C.; Poater, A.; Cavallo, L.; Linden, A.; Dorta, R. Identification and Characterization of a New Family of Catalytically Highly Active Imidazolin-2-ylidenes. J. Am. Chem. Soc. 2008, 130, 6848–6858. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Luan, X.; Mariz, R.; Blumentritt, S.; Linden, A.; Dorta, R. A New Class of Stable, Saturated N-Heterocyclic Carbenes with N-Naphthyl Substituents: Synthesis, Dynamic Behavior, and Catalytic Potential. Eur. J. Inorg. Chem. 2009, 2009, 1861–1870. [Google Scholar] [CrossRef]
- Gatti, M.; Wu, L.; Drinkel, E.; Gaggia, F.; Blumentritt, S.; Linden, A.; Dorta, R. The effect of substituents on the syn-anti conformer ratio in naphthyl-based imidazolinium salts and their corresponding N-heterocyclic carbenes. ARKIVOC 2011, 6, 176–198. [Google Scholar] [CrossRef]
- Martin, A.R.; Chartoire, A.; Slawin, A.M.Z.; Nolan, S.P. Extending the utility of [Pd(NHC)(cinnamyl)Cl] precatalysts: Direct arylation of heterocycles. Beilstein J. Org. Chem. 2012, 8, 1637–1643. [Google Scholar] [CrossRef]
- Meiries, S.; Speck, K.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*OMe)(acac)Cl]: Tuning the N-Heterocyclic Carbene in Catalytic C–N Bond Formation. Organometallics 2013, 32, 330–339. [Google Scholar] [CrossRef]
- Weber, S.G.; Loos, C.; Rominger, F.; Straub, B.F. Synthesis of an extremely sterically shielding N-heterocyclic carbene ligand. ARKIVOC 2012, 3, 226–242. [Google Scholar] [CrossRef]
- Dierick, S.; Dewez, D.F.; Markó, I.E. IPr*(2-Np)—An Exceedingly Bulky N-Heterocyclic Carbene. Organometallics 2014, 33, 677–683. [Google Scholar] [CrossRef]
- Dible, B.R.; Cowley, R.E.; Holland, P.L. Remote Substitution on N-Heterocyclic Carbenes Heightens the Catalytic Reactivity of Their Palladium Complexes. Organometallics 2011, 30, 5123–5132. [Google Scholar] [CrossRef]
- Rühling, A.; Rakers, L.; Glorius, F. Long Alkyl Chain NHC Palladium Complexes for the Amination and Hydrodehalogenation of Aryl Chlorides in Lipophilic Media. ChemCatChem 2017, 9, 547–550. [Google Scholar] [CrossRef]
- Chartoire, A.; Lesieur, M.; Falivene, L.; Slawin, A.M.Z.; Cavallo, L.; Cazin, C.S.J.; Nolan, S.P. [Pd(IPr*)(cinnamyl)Cl]: An Efficient Pre-catalyst for the Preparation of Tetra-ortho-substituted Biaryls by Suzuki–Miyaura Cross-Coupling. Chem. Eur. J. 2012, 18, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, W.; Deng, C.; Wang, Z.; Zhang, Y.; Qian, H. Preparation of poly(bulky N-heterocyclic carbene) as the self-supporting catalyst toward Suzuki–Miyaura Reaction. Macromol. Chem. Phys. 2018, 219, 1800156. [Google Scholar] [CrossRef]
- Ormerod, D.; Lefevre, N.; Dorbec, M.; Eyskens, I.; Vloemans, P.; Duyssens, K.; Diez de la Torre, V.; Kaval, N.; Merkul, E.; Sergeyev, S.; et al. Potential of homogeneous Pd catalyst separation by ceramic membranes. Application to downstream and continuous flow processes. Org. Process Res. Dev. 2016, 20, 911–920. [Google Scholar] [CrossRef]
- Ormerod, D.; Dorbec, M.; Merkul, E.; Kaval, N.; Lefèvre, N.; Hostyn, S.; Eykens, L.; Lievens, J.; Sergeyev, S.; Maes, B.U.W. Synthesis of Pd complexes containing tailed NHC ligands and their use in a semicontinuous membrane-assisted Suzuki cross-coupling process. Org. Process Res. Dev. 2018, 22, 1509–1517. [Google Scholar] [CrossRef]
- Meiries, S.; Chartoire, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(acac)Cl]: An Easily Synthesized, Bulky Precatalyst for C–N Bond Formation. Organometallics 2012, 31, 3402–3409. [Google Scholar] [CrossRef]
- Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(3-Cl-pyridinyl)Cl2]: A Novel and Efficient PEPPSI Precatalyst. Organometallics 2012, 31, 6947–6951. [Google Scholar] [CrossRef]
- Chartoire, A.; Frogneux, X.; Nolan, S.P. An Efficient Palladium-NHC (NHC = N-Heterocyclic Carbene) and Aryl Amination Pre-Catalyst: [Pd(IPr*)(cinnamyl)Cl]. Adv. Synth. Catal. 2012, 354, 1897–1901. [Google Scholar] [CrossRef]
- Chartoire, A.; Boreux, A.; Martin, A.R.; Nolan, S.P. Solvent-free aryl amination catalysed by [Pd(NHC)] complexes. RSC Adv. 2013, 3, 3840–3843. [Google Scholar] [CrossRef]
- Lesieur, M.; Slawin, A.M.Z.; Cazin, C.S.J. [Pd(μ-Cl)Cl(IPr*)]2: A highly hindered pre-catalyst for the synthesis of tetra-ortho-substituted biaryls via Grignard reagent cross-coupling. Org. Biomol. Chem. 2014, 12, 5586–5589. [Google Scholar] [CrossRef]
- Bastug, G.; Nolan, S.P. Carbon–Sulfur Bond Formation Catalyzed by [Pd(IPr*OMe)(cin)Cl] (cin = cinnamyl). J. Org. Chem. 2013, 78, 9303–9308. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, F.; Chartoire, A.; Nolan, S.P. Direct S-Arylation of Unactivated Arylsulfoxides Using [Pd(IPr*)(cin)Cl]. ACS Catal. 2013, 3, 2190–2193. [Google Scholar] [CrossRef]
- Martin, A.R.; Makida, Y.; Meiries, S.; Slawin, A.M.Z.; Nolan, S.P. Enhanced Activity of [Ni(NHC)CpCl] Complexes in Arylamination Catalysis. Organometallics 2013, 32, 6265–6270. [Google Scholar] [CrossRef]
- Martin, A.R.; Nelson, D.J.; Meiries, S.; Slawin, A.M.Z.; Nolan, S.P. Efficient C-N and C-S Bond Formation Using the Highly Active [Ni(allyl)Cl(IPr*OMe)] Precatalyst. Eur. J. Org. Chem. 2014, 15, 3127–3131. [Google Scholar] [CrossRef]
- Fernández-Salas, J.A.; Marelli, E.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. General and Mild Ni0-Catalyzed α-arylation of ketones Using Aryl Chlorides. Chem. Eur. J. 2015, 21, 3906–3909. [Google Scholar] [CrossRef]
- Fernández-Salas, J.A.; Marelli, E.; Nolan, S.P. Synthesis of (diarylmethyl)amines using Ni-catalyzed arylation of C(sp3)-H bonds. Chem. Sci. 2015, 6, 4973–4977. [Google Scholar] [CrossRef]
- Matsubara, K.; Fukahori, Y.; Inatomi, T.; Tazaki, S.; Yamada, Y.; Koga, Y.; Kanegawa, S.; Nakamura, T. Monomeric Three-Coordinate N-Heterocyclic Carbene Nickel(I) Complexes: Synthesis, Structures, and catalytic Applications in Cross-Coupling Reactions. Organometallics 2016, 35, 3281–3287. [Google Scholar] [CrossRef]
- Nakanowatari, S.; Müller, T.; Oliveira, J.C.A.; Ackermann, L. Bifurcated Nickel-Catalyzed Functionalizations: Heteroarene C–H Activation with Allenes. Angew. Chem. Int. Ed. 2017, 56, 15891–15895. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, G.; Xu, J.; Sun, H.; Shen, Q. Nickel-Catalyzed Reductive Cross-Coupling of Benzyl Chlorides with Aryl Chlorides/Fluorides: A One-Pot Synthesis of Diarylmethanes. Org. Lett. 2016, 18, 2860–2863. [Google Scholar] [CrossRef]
- Diesel, J.; Finogenova, A.M.; Cramer, N. Nickel-Catalyzed Enantioselective Pyridone C–H Functionalizations Enabled by a Bulky N-Heterocyclic Carbene Ligand. J. Am. Chem. Soc. 2018, 140, 4489–4493. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Newman, S.G. Methyl Esters as Cross-Coupling Electrophiles: Direct Synthesis of Amide bonds. ACS Catal. 2019, 9, 4426–4433. [Google Scholar] [CrossRef]
- Inatomi, T.; Fukahori, Y.; Yamada, Y.; Ishikawa, R.; Kanegawa, S.; Koga, Y.; Matsubara, K. Ni(I)-Ni(III) cycle in Buchwald-Hartwig amination of aryl bomide mediated by NHC-ligated Ni(I) complexes. Catal. Sci. Technol. 2019, 9, 1784–1793. [Google Scholar] [CrossRef]
- Pelties, S.; Wolf, R. Iron(II), Cobalt(II), and Nickel(II) Complexes of a Cyclic (Alkyl)(amino)carbene. Z. Anorg. Allg. Chem. 2013, 639, 2581–2585. [Google Scholar] [CrossRef]
- Henrion, M.; Cardoso, B.P.; César, V.; Chetcutti, M.J.; Ritleng, V. Nickel(II) Complexes of Highly σ-Donating Cyclic (Alkyl)(Amino)- and Malonate-Carbenes: Syntheses and Catalytic Studies. Organometallics 2017, 36, 1113–1121. [Google Scholar] [CrossRef]
- Vijaykumar, G.; Jose, A.; Vardhanapu, P.K.; Mandal, S.K. Abnormal-NHC-Supported Nickel Catalysts for Hydroheteroarylation of Vinylarenes. Organometallics 2017, 36, 4753–4758. [Google Scholar] [CrossRef]
- Nett, A.J.; Cañellas, S.; Higuchi, Y.; Robo, M.T.; Kochkodan, J.M.; Haynes, M.T., II; Kampf, J.W.; Montgomery, J. Stable, Well-Defined Nickel(0) Catalysts for Catalytic C–C and C–N Bond Formation. ACS Catal. 2018, 8, 6606–6611. [Google Scholar] [CrossRef]
- Felten, S.; Marshall, S.F.; Groom, A.J.; Vanderlinden, R.T.; Stolley, R.M.; Louie, J. Synthesis and Characterization of [(NHC)Ni(styrene)2] Complexes: Isolation of Monocarbene Nickel Complexes and Benchmarking of %Vbur in (NHC)Ni-π Systems. Organometallics 2018, 37, 3687–3697. [Google Scholar] [CrossRef]
- Elsby, M.R.; Liu, J.; Zhu, S.; Hu, L.; Huang, G.; Johnson, S.A. Influence of N-Heterocyclic Carbene dteric Bulk on Selectivity in Nickel Catalyzed C–H Bond Silylation, Germylation, and Stannylation. Organometallics 2019, 38, 436–450. [Google Scholar] [CrossRef]
- Iglesia, M.J.; Blandez, J.F.; Fructos, M.R.; Prieto, A.; Álvarez, E.; Belderrain, T.R.; Nicasio, M.C. Synthesis, Structural Characterization, and Catalytic Activity of IPrNi(styrene)2 in the Amination of Aryl Tosylates. Organometallics 2012, 31, 6312–6316. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).