Characterization and Photophysical Properties of a Luminescent Aluminum Hydride Complex Supported by a β-Diketiminate Ligand
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
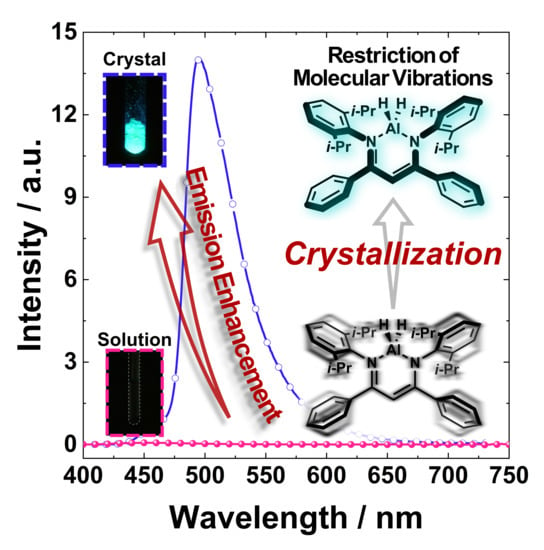
2.2. Photophysical Properties
2.3. Theoretical Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of LAlH
3.3. NMR Spectroscopy
3.4. High Resolution Mass Spectrometry
3.5. Single Crystal X-Ray Analysis
3.6. Photophysical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finholt, A.E.; Bond, A.C.; Schlesinger, H.I. Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of their Applications in Organic and Inorganic Chemistry. J. Am. Chem. Soc. 1947, 69, 1199–1203. [Google Scholar] [CrossRef]
- Li, W.; Ma, X.; Walawalkar, M.G.; Yang, Z.; Roesky, H.W. Soluble aluminum hydrides function as catalysts in deprotonation, insertion, and activation reactions. Coord. Chem. Rev. 2017, 350, 14–29. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Wang, S. Luminescence and electroluminescence of Al(III), B(III), Be(II) and Zn(II) complexes with nitrogen donors. Coord. Chem. Rev. 2001, 215, 79–98. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S. Luminescence and reactivity of 7-azaindole derivatives and complexes. Chem. Rev. 2010, 39, 3142–3156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, M.; Ma, X.; Nijesh, K.; De, S.; Parameswaran, P.; Roesky, H.W. An Aluminum Dihydride Working as a Catalyst in Hydroboration and Dehydrocoupling. J. Am. Chem. Soc. 2016, 138, 2548–2551. [Google Scholar] [CrossRef]
- Piers, W. Non-cyclopentadienyl ancillaries in organogroup 3 metal chemistry: A fine balance in ligand design. Coord. Chem. Rev. 2002, 233–234, 131–155. [Google Scholar] [CrossRef]
- Bourget-Merle, L.; Lappert, M.F.; Severn, J.R. The Chemistry of β-Diketiminatometal Complexes. Chem. Rev. 2002, 102, 3031–3066. [Google Scholar] [CrossRef]
- Roesky, H.W.; Singh, S.; Jancik, V.; Chandrasekhar, V. A Paradigm Change in Assembling OH Functionalities on Metal Centers. Acc. Chem. Res. 2004, 37, 969–981. [Google Scholar] [CrossRef]
- Wu, J.; Yu, T.-L.; Chen, C.-T.; Lin, C.-C. Recent developments in main group metal complexes catalyzed/initiated polymerization of lactides and related cyclic esters. Coord. Chem. Rev. 2006, 250, 602–626. [Google Scholar] [CrossRef]
- Hill, M.S.; Hitchcock, P.B.; Pongtavornpinyo, R. A Linear Homocatenated Compound Containing Six Indium Centers. Science 2006, 311, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Mindiola, D.J. Nacnac … Are You Still There? The Evolution of β-Diketiminate Complexes of Nickel. Angew. Chem. Int. Ed. 2009, 48, 6198–6200. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C. The chemistry of univalent metal β-diketiminates. Coord. Chem. Rev. 2012, 256, 722–758. [Google Scholar] [CrossRef]
- Bakthavachalam, K.; Reddy, N.D. Synthesis of Aluminum Complexes of Triaza Framework Ligands and Their Catalytic Activity toward Polymerization of ε-Caprolactone. Organometallics 2013, 32, 3174–3184. [Google Scholar] [CrossRef]
- Chen, C.; Bellows, S.M.; Holland, P.L. Tuning steric and electronic effects in transition-metal β-diketiminate complexes. Dalton Trans. 2015, 44, 16654–16670. [Google Scholar] [CrossRef]
- Camp, C.; Arnold, J. On the non-innocence of “Nacnacs”: Ligand-based reactivity in β-diketiminate supported coordination compounds. Dalton Trans. 2016, 45, 14462–14498. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Roesky, H.W.; Schmidt, H.G.; Noltemeyer, M.; Hao, H.; Cimpoesu, F. Synthesis and structure of a monomeric aluminum(I) compound [{HC(CMeNAr)2}Al] (AR = 2,6-iPr2C6H3): A stable aluminum analogue of a carbene. Angew. Chem. Int. Ed. 2000, 39, 4274–4276. [Google Scholar] [CrossRef]
- Roesky, H.W.; Kumar, S.S. Chemistry of aluminium(I). Chem. Commun. 2005, 32, 4027–4038. [Google Scholar] [CrossRef]
- Nagendran, S.; Roesky, H.W. The Chemistry of Aluminum(I), Silicon(II), and Germanium(II). Organometallics 2008, 27, 457–492. [Google Scholar] [CrossRef]
- Chu, T.; Nikonov, G.I. Oxidative Addition and Reductive Elimination at Main-Group Element Centers. Chem. Rev. 2018, 118, 3608–3680. [Google Scholar] [CrossRef]
- Bakewell, C.; White, A.J.P.; Crimmin, M.R. Reactions of Fluoroalkenes with an Aluminium(I) Complex. Angew. Chem. Int. Ed. 2018, 57, 6638–6642. [Google Scholar] [CrossRef]
- Macedo, F.P.; Gwengo, C.; Lindeman, S.V.; Smith, M.D.; Gardinier, J.R. β-Diketonate, β-Ketoiminate, and β-Diiminate Complexes of Difluoroboron. Eur. J. Inorg. Chem. 2008, 2008, 3200–3211. [Google Scholar] [CrossRef]
- Yoshii, R.; Hirose, A.; Tanaka, K.; Chujo, Y. Boron Diiminate with Aggregation-Induced Emission and Crystallization-Induced Emission-Enhancement Characteristics. Chem. Eur. J. 2014, 20, 8320–8324. [Google Scholar] [CrossRef]
- Yoshii, R.; Hirose, A.; Tanaka, K.; Chujo, Y. Functionalization of Boron Diiminates with Unique Optical Properties: Multicolor Tuning of Crystallization-Induced Emission and Introduction into the Main Chain of Conjugated Polymers. J. Am. Chem. Soc. 2014, 136, 18131–18139. [Google Scholar] [CrossRef]
- Ito, S.; Hirose, A.; Yamaguchi, M.; Tanaka, K.; Chujo, Y. Size-discrimination of volatile organic compounds utilizing gallium diiminate by luminescent chromism of crystallization-induced emission via encapsulation-triggered crystal–crystal transition. J. Mater. Chem. C 2016, 4, 5564–5571. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ito, S.; Hirose, A.; Tanaka, K.; Chujo, Y. Modulation of sensitivity to mechanical stimulus in mechanofluorochromic properties by altering substituent positions in solid-state emissive diiodo boron diiminates. J. Mater. Chem. C 2016, 4, 5314–5319. [Google Scholar] [CrossRef]
- Ito, S.; Hirose, A.; Yamaguchi, M.; Tanaka, K.; Chujo, Y. Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element. Polymers 2017, 9, 68. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Luminescent color tuning with polymer films composed of boron diiminate conjugated copolymers by changing the connection points to comonomers. Polym. Chem. 2018, 9, 1942–1946. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishidoshiro, M.; Irie, Y.; Imoto, H.; Naka, K.; Tanaka, K.; Inagi, S.; Tomita, I. Arsole-Containing π-Conjugated Polymer by the Post-Element-Transformation Technique. Angew. Chem. Int. Ed. 2016, 55, 15040–15043. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, J.; Payne, S.J.; Kooi, S.E.; Demas, J.N.; Fraser, C.L. Multi-Emissive Difluoroboron Dibenzoylmethane Polylactide Exhibiting Intense Fluorescence and Oxygen-Sensitive Room-Temperature Phosphorescence. J. Am. Chem. Soc. 2007, 129, 8942–8943. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, J.; Sabat, M.; Fraser, C.L. Polymorphism and Reversible Mechanochromic Luminescence for Solid-State Difluoroboron Avobenzone. J. Am. Chem. Soc. 2010, 132, 2160–2162. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M. Reversible Mechanochromic Luminescence of [(C6F5Au)2(μ-1,4-Diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044–10045. [Google Scholar] [CrossRef]
- Zhao, D.; Li, G.; Wu, D.; Qin, X.; Neuhaus, P.; Cheng, Y.; Yang, S.; Lu, Z.; Pu, X.; Long, C.; et al. Regiospecific N-Heteroarylation of Amidines for Full-Color-Tunable Boron Difluoride Dyes with Mechanochromic Luminescence. Angew. Chem. Int. Ed. 2013, 52, 13676–13680. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Zou, B.; Ye, K.; Zhang, H.; Wang, Y. Luminescent Chromism of Boron Diketonate Crystals: Distinct Responses to Different Stresses. Adv. Mater. 2015, 27, 2918–2922. [Google Scholar] [CrossRef]
- Hirai, Y.; Nakanishi, T.; Kitagawa, Y.; Fushimi, K.; Seki, T.; Ito, H.; Hasegawa, Y. Triboluminescence of Lanthanide Coordination Polymers with Face-to-Face Arranged Substituents. Angew. Chem. Int. Ed. 2017, 56, 7171–7175. [Google Scholar] [CrossRef]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent progress in the development of advanced element-block materials. Polym. J. 2018, 50, 109–126. [Google Scholar] [CrossRef]
- Gon, M.; Tanaka, K.; Chujo, Y. Creative Synthesis of Organic–Inorganic Molecular Hybrid Materials. Bull. Chem. Soc. Jpn. 2017, 90, 463–474. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Wang, C.; Roesky, H.W. Studies of the Ligand Effect on the Synthesis of Dialuminoxanes by Various β-Diketiminato Ligands. Inorg. Chem. 2012, 51, 2204–2211. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Crimmin, M.R.; Hill, M.S.; Kociok-Köhn, G. Beryllium derivatives of a phenyl-substituted β-diketiminate: A well-defined ring opening reaction of tetrahydrofuran. Dalton Trans. 2013, 42, 9720–9726. [Google Scholar] [CrossRef]
- Jana, B.; Uhl, W. New aluminum and gallium complexes of β-diketiminato and β-ketiminato ligands. Inorganica Chim. Acta 2017, 455, 61–69. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, S.B.; Park, S.Y.; Yi, Y.S.; Ahn, C.W. Structure of Amorphous Aluminum Oxide. Phys. Rev. Lett. 2009, 103, 095501. [Google Scholar] [CrossRef]
- Salem, L. Molecular Orbital Theory of Conjugated Systems; W. A. Benjamin, Inc.: New York, NY, USA, 1966; ISBN 0805384014. [Google Scholar]
- Twamley, B.; Hardman, N.J.; Power, P.P. A primary monomeric alane: [N,N’-bis(2,6-diisopropylphenyl)pentane-2,4-diiminato-N,N’]dihydridoaluminium. Acta Crystallogr. Sect. E Struct. Rep. Online 2001, 57, m227–m228. [Google Scholar] [CrossRef]
- González-Gallardo, S.; Jancik, V.; Cea-Olivares, R.; Toscano, R.A.; Moya-Cabrera, M. Preparation of Molecular Alumoxane Hydrides, Hydroxides, and Hydrogensulfides. Angew. Chem. Int. Ed. 2007, 46, 2895–2898. [Google Scholar] [CrossRef]
- Stender, M.; Eichler, B.E.; Hardman, N.J.; Power, P.P.; Prust, J.; Noltemeyer, M.; Roesky, H.W. Synthesis and Characterization of HC{C(Me)N(C6H3-2,6-i-Pr2)}2MX2(M = Al, X = Cl, I; M = Ga, In, X = Me, Cl, I): Sterically Encumbered β-Diketiminate Group 13 Metal Derivatives. Inorg. Chem. 2001, 40, 2794–2799. [Google Scholar] [CrossRef]
- Strickler, S.J.; Berg, R.A. Relationship between Absorption Intensity and Fluorescence Lifetime of Molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef]
- Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Huang, K.; Rhys, A. Theory of light absorption and non-radiative transitions in F-centres. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1950, 204, 406–423. [Google Scholar]
- Kubo, R. Thermal Ionization of Trapped Electrons. Phys. Rev. 1952, 86, 929–937. [Google Scholar] [CrossRef]
- Yu, G.; Yin, S.; Liu, Y.; Chen, J.; Xu, X.; Sun, X.; Ma, D.; Zhan, X.; Peng, Q.; Shuai, Z.; et al. Structures, Electronic States, Photoluminescence, and Carrier Transport Properties of 1,1-Disubstituted 2,3,4,5-Tetraphenylsiloles. J. Am. Chem. Soc. 2005, 127, 6335–6346. [Google Scholar] [CrossRef]
- Yin, S.; Peng, Q.; Shuai, Z.; Fang, W.; Wang, Y.-H.; Luo, Y. Aggregation-enhanced luminescence and vibronic coupling of silole molecules from first principles. Phys. Rev. B 2006, 73, 205409. [Google Scholar] [CrossRef]
- Peng, Q.; Yi, Y.; Shuai, Z.; Shao, J. Toward Quantitative Prediction of Molecular Fluorescence Quantum Efficiency: Role of Duschinsky Rotation. J. Am. Chem. Soc. 2007, 129, 9333–9339. [Google Scholar] [CrossRef]
- Peng, Q.; Niu, Y.; Wu, Q.; Gao, X.; Shuai, Z. Theoretical Understanding of AIE Phenomena Through Computational Chemistry. In Aggregation-Induced Emission: Fundamentals; John Wiley and Sons Ltd.: Chichester, UK, 2013; pp. 357–398. [Google Scholar]
- Jin, J.-L.; Geng, Y.; Su, Z.-M. Recent Theoretical Advances in Understanding the Mechanism of Aggregation-Induced Emission for Small Organic Molecules. In Aggregation-Induced Emission: Fundamentals; John Wiley and Sons Ltd.: Chichester, UK, 2013; pp. 399–418. [Google Scholar]
- Higashi, T. ABSCOR. Program for Absorption Correction; Rigaku Corporation: Tokyo, Japan, 1995. [Google Scholar]
- Sheldrick, G.M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Wakita, K.; Yadokari, X.G. Program for Crystal Structure Analysis. 2000. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]






| Distances | |||
| Atom1 | Atom2 | Length/Å | |
| Al1 | N1 | 1.898(1) | |
| Al1 | N2 | 1.899(1) | |
| N1 | C1 | 1.339(2) | |
| N2 | C3 | 1.334(2) | |
| C1 | C2 | 1.405(2) | |
| C2 | C3 | 1.410(2) | |
| Angles | |||
| Atom1 | Atom2 | Atom3 | Angle/deg |
| N1 | Al1 | N2 | 96.84(5) |
| Al1 | N1 | C1 | 121.98(8) |
| Al1 | N2 | C3 | 121.01(8) |
| N1 | C1 | C2 | 122.3(1) |
| N2 | C3 | C2 | 122.5(1) |
| C1 | C2 | C3 | 127.9(1) |
| Dihedral Angles | |||
| Plane1 a | Plane2 b | Dihedral Angle/deg | |
| C3N2 | AlN2 | 23.95 | |
| C3N2 | C4–9 | 40.29 | |
| C3N2 | C10–15 | 45.99 | |
| C3N2 | C16–21 | 81.48 | |
| C3N2 | C22–27 | 69.84 | |
| λabs/nm a | εmax/104 M−1 cm−1 b | λem/nm c | ΦFLd | ΦPhosd | ||
|---|---|---|---|---|---|---|
| solution e | r.t. | 394 | 1.4 | 457 | <0.01 | <0.01 |
| 80 K | — f | — f | 430, 449 | 0.80 | 0.13 | |
| crystal | r.t. | — f | — f | 501 | 0.34 | <0.01 |
| 80 K | — f | — f | 456, 479 | 0.83 | <0.01 |
| τ1/ns; f1 (%) a | τ2/ns; f2 (%) a | χ2b | <τ>/ns c | kFL/108 s−1 d | knrS/108 s−1 e | ||
|---|---|---|---|---|---|---|---|
| solution | 80 K | 1.43 f; 7.72 f | 3.27 f; 87.32 f | 1.20 | 3.0 | 2.7 | <0.68 |
| crystal | r.t. | 1.39; 31.07 | 2.75; 68.93 | 1.01 | 2.3 | 1.5 | 2.8 |
| 80 K | 2.07; 21.07 | 3.27; 78.93 | 0.94 | 3.0 | 2.8 | 0.56 |
| Geometry | Transition | Composition | Coefficient a | f b | λ/nm c |
|---|---|---|---|---|---|
| S0 geom | S0–S1 | HOMO–LUMO | 0.68764 | 0.4205 | 347.05 |
| S1 geom | S0–S1 | HOMO–LUMO | 0.69035 | 0.3807 | 404.73 |
| Mode Index, i | ωi/cm−1 | Si | λi/cm−1 a |
|---|---|---|---|
| 1 | 15.2511 | 10.2582 | 156.44883 |
| 3 | 33.1647 | 6.53073 | 216.5897 |
| 4 | 39.4657 | 1.14514 | 45.19375 |
| 6 | 51.8166 | 0.76687 | 39.73634 |
| 8 | 56.779 | 0.98173 | 55.74187 |
| 13 | 80.6648 | 0.86301 | 69.61493 |
| 124 | 1065.3059 | 0.5273 | 561.74006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Tanaka, K.; Chujo, Y. Characterization and Photophysical Properties of a Luminescent Aluminum Hydride Complex Supported by a β-Diketiminate Ligand. Inorganics 2019, 7, 100. https://doi.org/10.3390/inorganics7080100
Ito S, Tanaka K, Chujo Y. Characterization and Photophysical Properties of a Luminescent Aluminum Hydride Complex Supported by a β-Diketiminate Ligand. Inorganics. 2019; 7(8):100. https://doi.org/10.3390/inorganics7080100
Chicago/Turabian StyleIto, Shunichiro, Kazuo Tanaka, and Yoshiki Chujo. 2019. "Characterization and Photophysical Properties of a Luminescent Aluminum Hydride Complex Supported by a β-Diketiminate Ligand" Inorganics 7, no. 8: 100. https://doi.org/10.3390/inorganics7080100
APA StyleIto, S., Tanaka, K., & Chujo, Y. (2019). Characterization and Photophysical Properties of a Luminescent Aluminum Hydride Complex Supported by a β-Diketiminate Ligand. Inorganics, 7(8), 100. https://doi.org/10.3390/inorganics7080100