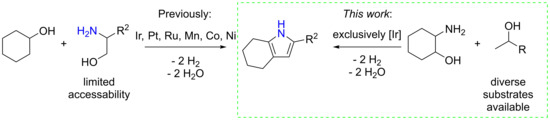
Iridium Catalyzed Synthesis of Tetrahydro-1H-Indoles by Dehydrogenative Condensation
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. General Procedure for the Synthesis of Tetrahydro-1H-indoles
5.2. General Procedure for the Dehydrogenation Reaction
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vispute, T.P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G.W. Renewable Chemical Commodity Feedstocks from Integrated Catalytic Processing of Pyrolysis Oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.O.; Perez, E.; Horvath, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Applications of Acceptorless Dehydrogenation and Related Transformations in Chemical Synthesis. Science 2013, 341, 1229712. [Google Scholar] [CrossRef] [PubMed]
- Chelucci, G. Metal-catalyzed dehydrogenative synthesis of pyrroles and indoles from alcohols. Coord. Chem. Rev. 2017, 331, 37–53. [Google Scholar] [CrossRef]
- Lamberth, C.; Dinges, J. (Eds.) Bioactive Heterocyclic Compound Classes: Pharmaceuticals; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2012; ISBN 978-3-527-33395-0. [Google Scholar]
- Lamberth, C.; Dinges, J. (Eds.) Bioactive Heterocyclic Compound Classes: Agrochemicals; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2012; ISBN 978-3-527-33396-7. [Google Scholar]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; ISBN 1405133007. [Google Scholar]
- Van Order, R.B.; Lindwall, H.G. Indole. Chem. Rev. 1942, 30, 69–96. [Google Scholar] [CrossRef]
- Robinson, B. The Fischer Indole Synthesis. Chem. Rev. 1963, 63, 373–401. [Google Scholar] [CrossRef]
- Taber, D.F.; Tirunahari, P.K. Indole synthesis: A review and proposed classification. Tetrahedron 2011, 67, 7195–7210. [Google Scholar] [CrossRef] [PubMed]
- Srimani, D.; Ben-David, Y.; Milstein, D. Direct Synthesis of Pyrroles by Dehydrogenative Coupling of β-Aminoalcohols with Secondary Alcohols Catalyzed by Ruthenium Pincer Complexes. Angew. Chem. Int. Ed. 2013, 52, 4012–4015. [Google Scholar] [CrossRef]
- Siddiki, S.M.A.H.; Touchy, A.S.; Chaudhari, C.; Kon, K.; Toyao, T.; Shimizu, K. Synthesis of 2,5-disubstituted pyrroles via dehydrogenative condensation of secondary alcohols and 1,2-amino alcohols by supported platinum catalysts. Org. Chem. Front. 2016, 3, 846–851. [Google Scholar] [CrossRef]
- Forberg, D.; Obenauf, J.; Friedrich, M.; Hühne, S.M.; Mader, W.; Motz, G.; Kempe, R. The synthesis of pyrroles via acceptorless dehydrogenative condensation of secondary alcohols and 1,2-amino alcohols mediated by a robust and reusable catalyst based on nanometer-sized iridium particles. Catal. Sci. Technol. 2014, 4, 4188–4192. [Google Scholar] [CrossRef]
- Kallmeier, F.; Dudziec, B.; Irrgang, T.; Kempe, R. Manganese-Catalyzed Sustainable Synthesis of Pyrroles from Alcohols and Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 7261–7265. [Google Scholar] [CrossRef] [PubMed]
- Michlik, S.; Kempe, R. A sustainable catalytic pyrrole synthesis. Nat. Chem. 2013, 5, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Neumann, H.; Beller, M. Selective Ruthenium-Catalyzed Three-Component Synthesis of Pyrroles. Angew. Chem. Int. Ed. 2013, 52, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Daw, P.; Chakraborty, S.; Garg, J.A.; Ben-David, Y.; Milstein, D. Direct Synthesis of Pyrroles by Dehydrogenative Coupling of Diols and Amines Catalyzed by Cobalt Pincer Complexes. Angew. Chem. Int. Ed. 2016, 55, 14373–14377. [Google Scholar] [CrossRef] [PubMed]
- Midya, S.P.; Landge, V.G.; Sahoo, M.K.; Rana, J.; Balaraman, E. Cobalt-catalyzed acceptorless dehydrogenative coupling of aminoalcohols with alcohols: Direct access to pyrrole, pyridine and pyrazine derivatives. Chem. Commun. 2017, 54, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Vellakkaran, M.; Banerjee, D. A nitrogen-ligated nickel-catalyst enables selective intermolecular cyclisation of β- And γ-amino alcohols with ketones: Access to five and six-membered N-heterocycles. Green Chem. 2018, 20, 2250–2256. [Google Scholar] [CrossRef]
- Iida, K.; Miura, T.; Ando, J.; Saito, S. The Dual Role of Ruthenium and Alkali Base Catalysts in Enabling a Conceptually New Shortcut to N-Unsubstituted Pyrroles through Unmasked α-Amino Aldehydes. Org. Lett. 2013, 15, 1436–1439. [Google Scholar] [CrossRef]
- Michlik, S.; Kempe, R. Regioselectively functionalized pyridines from sustainable resources. Angew. Chem. Int. Ed. 2013, 52, 6326–6329. [Google Scholar] [CrossRef]
- Hille, T.; Irrgang, T.; Kempe, R. The synthesis of benzimidazoles and quinoxalines from aromatic diamines and alcohols by iridium-catalyzed acceptorless dehydrogenative alkylation. Chem. Eur. J. 2014, 20, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Hille, T.; Irrgang, T.; Kempe, R. Synthesis of meta-Functionalized Pyridines by Selective Dehydrogenative Heterocondensation of β- and γ-Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Forberg, D.; Schwob, T.; Kempe, R. Catalytic condensation for the formation of polycyclic heteroaromatic compounds. Nat. Commun. 2018, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-catalyst high-weight% hydrogen storage in an N-heterocycle synthesized from lignin hydrogenolysis products and ammonia. Nat. Commun. 2016, 7, 13201. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forberg, D.; Kallmeier, F.; Kempe, R. Iridium Catalyzed Synthesis of Tetrahydro-1H-Indoles by Dehydrogenative Condensation. Inorganics 2019, 7, 97. https://doi.org/10.3390/inorganics7080097
Forberg D, Kallmeier F, Kempe R. Iridium Catalyzed Synthesis of Tetrahydro-1H-Indoles by Dehydrogenative Condensation. Inorganics. 2019; 7(8):97. https://doi.org/10.3390/inorganics7080097
Chicago/Turabian StyleForberg, Daniel, Fabian Kallmeier, and Rhett Kempe. 2019. "Iridium Catalyzed Synthesis of Tetrahydro-1H-Indoles by Dehydrogenative Condensation" Inorganics 7, no. 8: 97. https://doi.org/10.3390/inorganics7080097
APA StyleForberg, D., Kallmeier, F., & Kempe, R. (2019). Iridium Catalyzed Synthesis of Tetrahydro-1H-Indoles by Dehydrogenative Condensation. Inorganics, 7(8), 97. https://doi.org/10.3390/inorganics7080097