Preparation and Characterization of Large Area Li-NASICON Electrolyte Thick Films
Abstract
:1. Introduction
2. Results
2.1. Structural and Microstructral Characterization
2.2. Confocal Raman Characterization
2.3. NMR Characterization
2.3.1. 31P (I = 1/2) MAS-NMR Spectroscopy
2.3.2. 27Al (I = 5/2) MAS-NMR Spectroscopy
2.3.3. 7Li (I = 3/2) MAS-NMR Spectroscopy
2.4. Mechano-Elastic Characterization
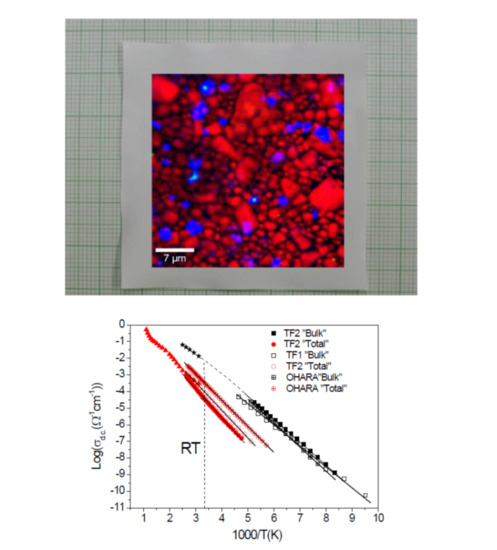
2.5. Electrical Characterization
3. Discussion
4. Materials and Methods
4.1. LATP Powder Preparation
4.2. Slurry and Thick Film Preparation
4.3. Samples Characterization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Brent, C.M.; Tarascon, J.M. Design and preparation of materials for advanced electrochemical storage. Acc. Chem. Res. 2013, 46, 1226–1238. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Lee, S.H.; Cho, K.H.; Shin, D.W.; Sun, Y.K. Li2O–B2O3–P2O5 solid electrolyte for thin film batteries. J. Power Sources 2006, 163, 223–228. [Google Scholar] [CrossRef]
- Money, B.K.; Hariharan, K. Relation between structural and conductivity relaxation in PEO and PEO based electrolytes. Solid State Ionics 2008, 179, 1273–1277. [Google Scholar] [CrossRef]
- Takada, K.; Inada, T.; Kajiyama, A.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Solid-state lithium battery with graphite anode. Solid State Ionics 2003, 158, 269–274. [Google Scholar] [CrossRef]
- Kotobuki, M.; Isshiki, Y.; Munakata, H.; Kanamura, K. All-solid-state lithium battery with a three-dimensionally ordered Li1.5Al0.5Ti1.5(PO4)3 electrode. Electrochim. Acta 2010, 55, 68–92. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D Integrated all-solid-state rechargeable batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Sun, Y. Lithium ion conducting membranes for lithium-air batteries. Nanomater. Energy 2013, 2, 801–816. [Google Scholar] [CrossRef]
- Usiskin, R.E.; Maier, J. Guidelines for optimizing the architecture of battery insertion electrodes based on the concept of wiring lengths. Phys. Chem. Chem. Phys. 2018, 20, 16449–16462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraki, S.; Oki, H.; Takagi, Y.; Suzuki, T.; Kumatani, A.; Shimizu, R.; Haruta, M.; Ohsawa, T.; Sato, Y.; Ikuhara, Y.; et al. Fabrication of all-solid-state battery using epitaxial LiCoO2 thin films. J. Power Sources 2014, 267, 881–887. [Google Scholar] [CrossRef]
- Haruta, M.; Shiraki, S.; Suzuki, T.; Kumatani, A.; Ohsawa, T.; Takagi, Y.; Shimizu, R.; Hitosugi, T. Negligible “Negative Space-Charge effects” at oxide-electrolyte/electrode interfaces of thin-film Batteries. Nano Lett. 2015, 15, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Choi, Z.; Kramer, D.; Mönig, R. Correlation of stress and structural evolution in Li4Ti5O12-based electrodes for lithium ion batteries. J. Power Sources 2013, 240, 245–251. [Google Scholar] [CrossRef]
- Arbi, K.; Mandal, S.; Rojo, J.M.; Sanz, J. Dependence of ionic conductivity of composition of fast ionic conductors Li1+xTi2−xAlx(PO4)3, 0 ≤ x ≤ 0.7. A parallel NMR and electric impedance study. Chem. Mater. 2002, 14, 1091–1097. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G.Y. The electrical properties of ceramic electrolytes for Li1+xMxTi2−x(PO4)3 + yLi2O, M = Ge, Sn, Hf and Zr systems. J. Electrochem. Soc. 1993, 140, 1827–1833. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N.; Aono, H. Fast Li+ conducting ceramic electrolytes. Adv. Mater. 1996, 8, 127–135. [Google Scholar] [CrossRef]
- Fu, J. Superionic conductivity of glass-ceramics in the system Li2O–Al2O3–TiO2–P2O5. Solid State Ionics 1997, 96, 195–200. [Google Scholar] [CrossRef]
- Arbi, K.; Rojo, J.M.; Sanz, J. Lithium mobility in titanium based Nasicon Li1+xTi2−xAlx(PO4)3 and LiTi2−x Zrx(PO4)3 materials followed by NMR and impedance spectroscopy. J. Eur. Ceram. Soc. 2007, 27, 4215–4218. [Google Scholar] [CrossRef]
- Jurków, D.; Roguszczak, H.; Golonka, L. Cold chemical lamination of ceramic green tapes. J. Eur. Ceram. Soc. 2009, 29, 703–709. [Google Scholar] [CrossRef]
- Park, H.G.; Moon, H.; Park, S.C.; Lee, J.J.; Yoon, D.; Hyun, S.H.; Kim, D.H. Performance improvement of anode-supported electrolytes for planar solid oxide fuel cells via a tape-casting/lamination/co-firing technique. J. Power Sources 2010, 195, 2463–2469. [Google Scholar] [CrossRef]
- Jurków, D.; Golonka, L. Low-pressure thermo compressive lamination. J. Eur. Ceram. Soc. 2012, 32, 2431–2441. [Google Scholar] [CrossRef]
- Jimenez, R.; del Campo, A.; Calzada ML Sanz, J.; Kobylianska, S.D.; Solopan, S.O.; Belous, A.G. Lithium La0.57Li0.33TiO3 perovskite and Li1.3Al0.3Ti1.7(PO4)3 Li-NASICON supported thick films electrolytes prepared by tape casting method. J. Electrochem. Soc. 2016, 163, A1653–A1659. [Google Scholar] [CrossRef]
- Jimenez, R.; del Campo, A.; Calzada, M.L.; Sanz, J.; Kobylianska, S.D.; Liniova, B.O.; Belous, A.G.; Ragulya, A.V. Improved conductivity in tape casted Li-NASICON supported thick films: Effect of temperature treatments and lamination. J. Eur. Ceram. Soc. 2018, 38, 1679–1687. [Google Scholar] [CrossRef]
- Fu, Y.; Ming, H.; Zhao, S.; Guo, J.; Chen, M.; Zhou, Q.; Zheng, J. A new insight into the LiTiOPO4 as anode material for Li ion bateries. Electrochim. Acta 2015, 185, 211–217. [Google Scholar] [CrossRef]
- Arbi, K.; Bucheli, W.; Jiménez, R.; Sanz, J. High lithium ion conducting solid electrolytes based on NASICON Li1+xAlxM2−x(PO4)3 materials (M = Ti, Ge and 0 ≤ x ≤ 0.5). J. Eur. Ceram. Soc. 2015, 35, 1477–1484. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Fan, Z. A new approach for the Young’s Modulus-Porosity Correlation of Ceramic materials. Ceram. Int. 1997, 12, 239–245. [Google Scholar] [CrossRef]
- Davidge, R.W. Cracking at grain Boundaries in polycystalline brittle materials. Acta Metall. 1981, 29, 1695–1702. [Google Scholar] [CrossRef]
- Case, E.D.; Smyth, J.R.; Hunter, O. Grain—Size dependence of microcrack initiation in brittle materials. J. Mater. Sci. 1980, 15, 149–153. [Google Scholar] [CrossRef]
- Bucharsky, E.C.; Schell, K.G.; Hintennach, A.; Hoffmann, M.J. Preparation and characterization of sol-gel derived high Lithium ion conductive NZP—Type ceramics Li1−xAlxTi2−x(PO4)3. Solid State Ionics 2015, 274, 77–82. [Google Scholar] [CrossRef]
- Nakajima, K.; Katoh, T.; Inda, Y.; Hoffman, B. Lithium Ion Conductive Glass Ceramics: Properties and Application in Lithium Metal Batteries. In Proceedings of the Symposium on Energy Storage Beyond Lithium Ion, Oak Ridge, TN, USA, 7–8 October 2010. [Google Scholar]
- Jackman, S.D.; Cutler, R.A. Effect of microcracking on ionic conductivity in LATP. J. Power Sources 2012, 218, 65–72. [Google Scholar] [CrossRef]
- Hupfer, T.; Bucharsky, E.C.; Schell, K.G.; Hoffmann, M.J. Influence of the secondary phase LiTiOPO4 on the properties of Li1−xAlxTi2−x(PO4)3 (x = 0; 0.3). Solid State Ionics 2017, 302, 49–53. [Google Scholar] [CrossRef]
- Arbi, K.; Hoelzel, M.; Kuhn, A.; Garcia-Alvarado, F.; Sanz, J. Structural Factors That Enhance Lithium Mobility in Fast-Ion Li1+xTi2−xAlx(PO4) (0 ≤ x ≤ 0.4) Conductors Investigated by Neutron Diffraction in the Temperature Range 100–500 K. Inorg. Chem. 2013, 52, 9290–9296. [Google Scholar] [CrossRef] [PubMed]
- Rettenwander, D.; Welzl, A.; Pristat, S.; Tietz, F.; Taibl, S.; Redhammer, G.J.; Fleig, J. A microcontact impedance study on NASICON-type Li1+xAlxTi2−x(PO4)3 (0 ≤ x ≤ 0.5) single crystals. J. Mater. Chem. A 2016, 4, 1506–1513. [Google Scholar] [CrossRef]
- Robertson, A.; Fletcher, J.G.; Skakle, J.M.S.; West, A.R. Synthesis of LiTiPO5 and LiTiAsO5 with the α-Fe2PO5 Structure. J. Solid State Chem. 1994, 109, 53–59. [Google Scholar] [CrossRef]
- Taher, Y.B.; Oueslati, A.; Khirouni, K.; Gargouri, M. Impedance Spectroscopy and conduction mechanism of LiAlP2O7 material. Mater. Res. Bull. 2016, 78, 148–157. [Google Scholar] [CrossRef]
- Kežionis, A.; Kazlauskas, S.; Petrulionis, D.; Orliukas, A.F. Broadband Method for the Determination of Small Sample’s Electrical and Dielectric Properties at High Temperatures. IEEE Trans. Microw. Theory Tech. 2014, 62, 2456–2461. [Google Scholar] [CrossRef]
- Kežionis, A.; Butvilas, P.; Šalkus, T.; Kazlauskas, S.; Petrulionis, D.; Žukauskas, T.; Kazakevičius, E.; Orliukas, A.F. Four-electrode impedance spectrometer for investigation of solid ion conductors. Rev. Sci. Instrum. 2013, 84, 013902. [Google Scholar] [CrossRef]
- Kežionis, A.; Kazakevičius, E.; Šalkus, T.; Orliukas, A.F. Broadband high frequency impedance spectrometer with working temperatures up to 1200 K. Solid State Ionics 2011, 188, 110–113. [Google Scholar] [CrossRef]








| Sample | Y’ (GPa) | tanδ |
|---|---|---|
| OHGC | 48 ± 9.6 | 0.04 |
| LATPT1 | 13 ± 2.6 | 0.3 |
| LATPT2 | 7.5 ± 1.5 | 0.4 |
| Sample | σbulk195 K (Ω−1·cm−1) | σtotal.300 K (Ω−1·cm−1) | Ea bulk (eV) | Ea total (eV) |
|---|---|---|---|---|
| OHGC | 2.0 × 10−5 | 3.4 × 10−4 | 0.29 | 0.31 |
| LATPT1 | 1.0 × 10−5 | 9.5 × 10−5 | 0.24 | 0.33 |
| LATPT2 | 3.4 × 10−5 | 3.8 × 10−5 | 0.27 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, R.; Sobrados, I.; Martínez-Chaparro, S.; del Campo, A.A.; Calzada, M.L.; Sanz, J.; Tsai, S.Y.; Lin, M.R.; Fung, K.Z.; Kazakevicius, E.; et al. Preparation and Characterization of Large Area Li-NASICON Electrolyte Thick Films. Inorganics 2019, 7, 107. https://doi.org/10.3390/inorganics7090107
Jiménez R, Sobrados I, Martínez-Chaparro S, del Campo AA, Calzada ML, Sanz J, Tsai SY, Lin MR, Fung KZ, Kazakevicius E, et al. Preparation and Characterization of Large Area Li-NASICON Electrolyte Thick Films. Inorganics. 2019; 7(9):107. https://doi.org/10.3390/inorganics7090107
Chicago/Turabian StyleJiménez, Ricardo, Isabel Sobrados, Sandra Martínez-Chaparro, Angel Adolfo del Campo, M. Lourdes Calzada, Jesús Sanz, Shu Yi Tsai, Ming Rui Lin, Kuan Zong Fung, Edvardas Kazakevicius, and et al. 2019. "Preparation and Characterization of Large Area Li-NASICON Electrolyte Thick Films" Inorganics 7, no. 9: 107. https://doi.org/10.3390/inorganics7090107
APA StyleJiménez, R., Sobrados, I., Martínez-Chaparro, S., del Campo, A. A., Calzada, M. L., Sanz, J., Tsai, S. Y., Lin, M. R., Fung, K. Z., Kazakevicius, E., & Kežionis, A. (2019). Preparation and Characterization of Large Area Li-NASICON Electrolyte Thick Films. Inorganics, 7(9), 107. https://doi.org/10.3390/inorganics7090107