Recent Progress on Supramolecular Luminescent Assemblies Based on Aurophilic Interactions in Solution
Abstract
:1. Introduction
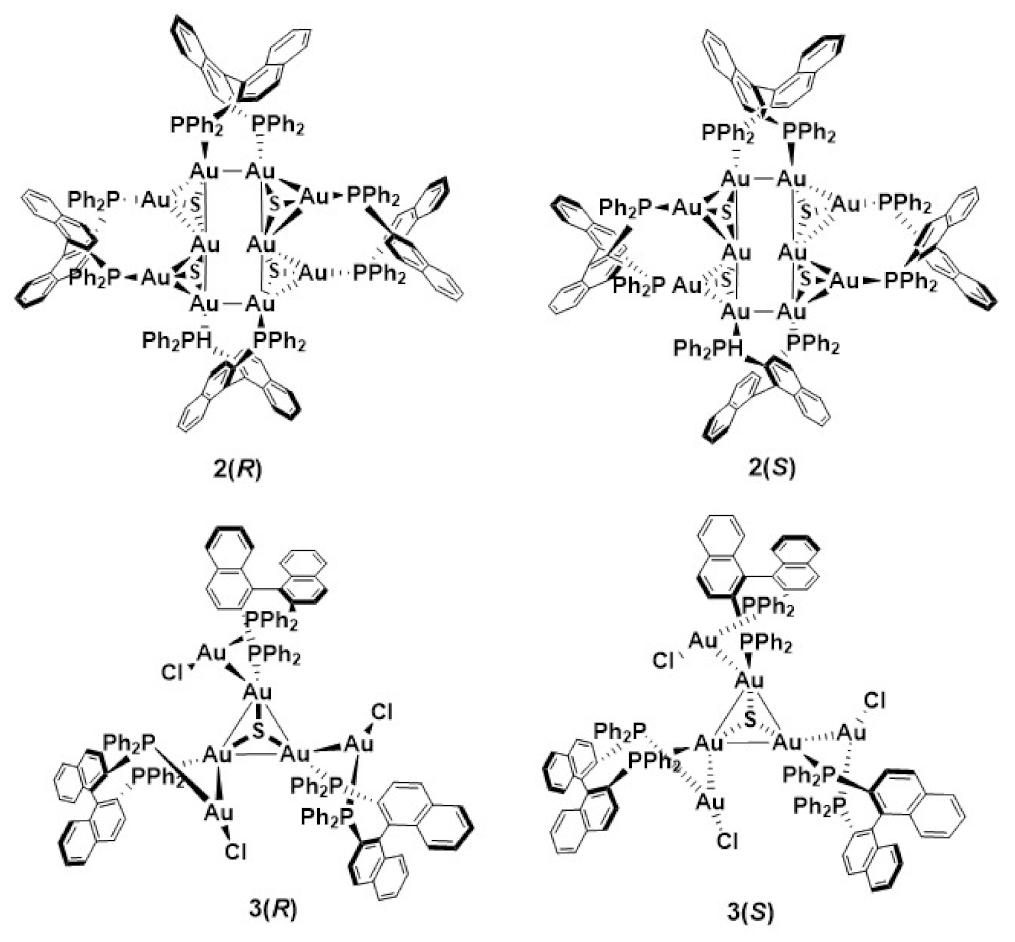
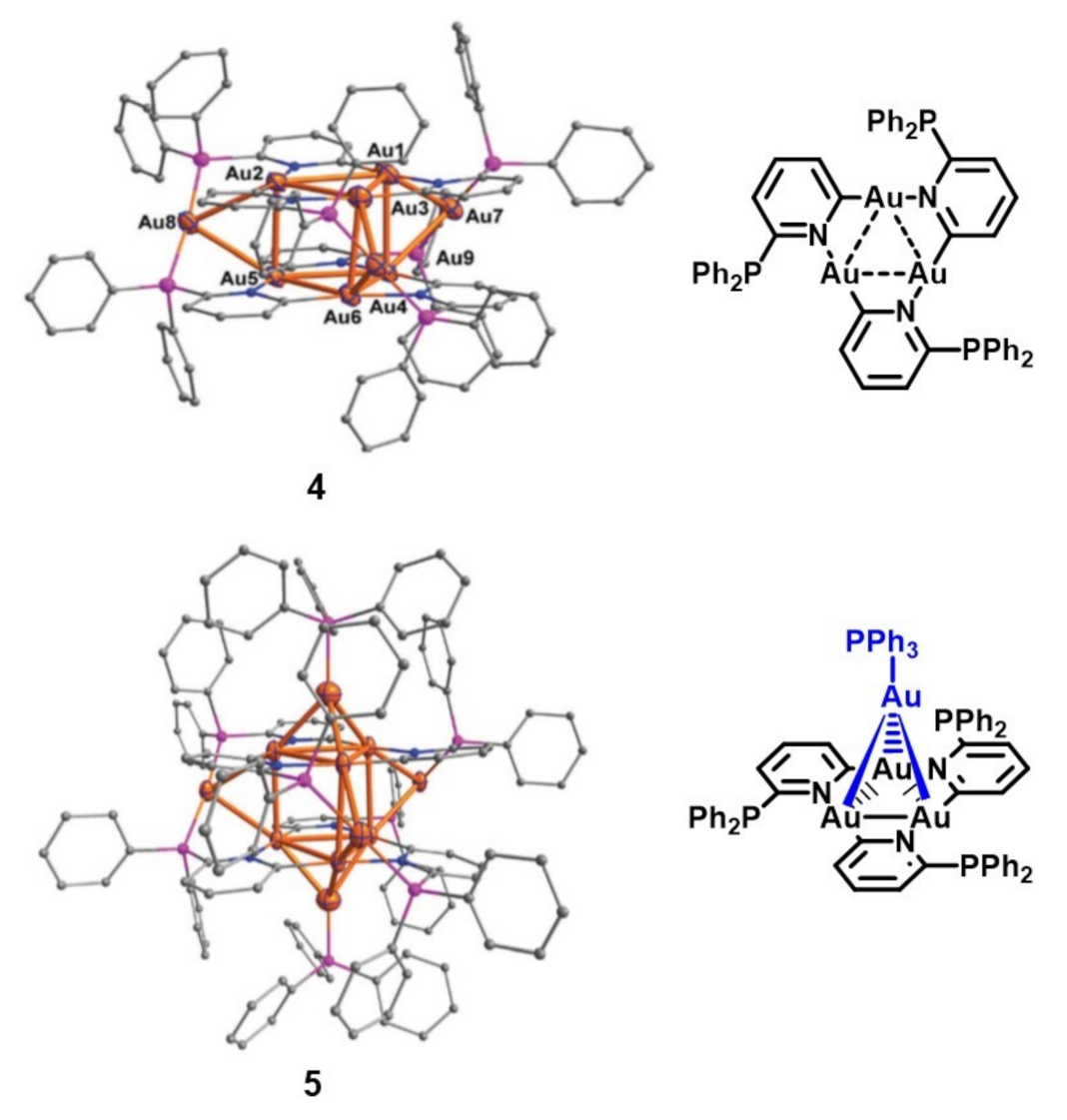
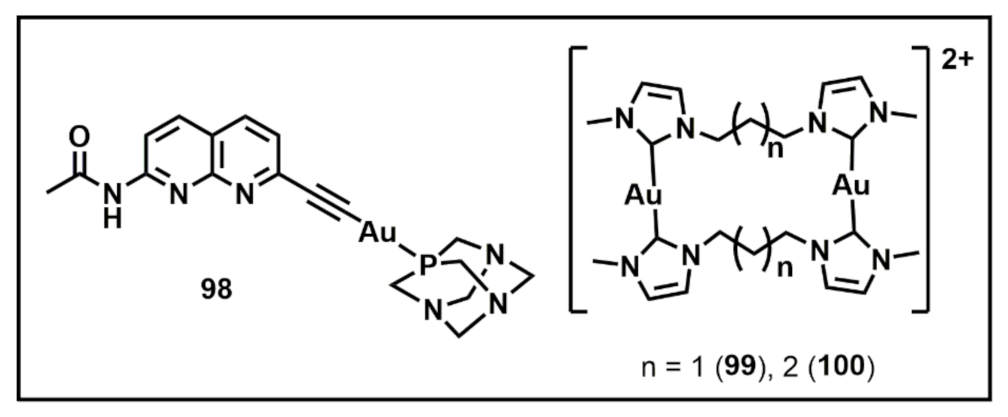
2. Gold(I) Clusters as Discrete Luminescent Units
3. Self-assembly of Gold(I) Complexes
3.1. Complexes that Display AIE Properties
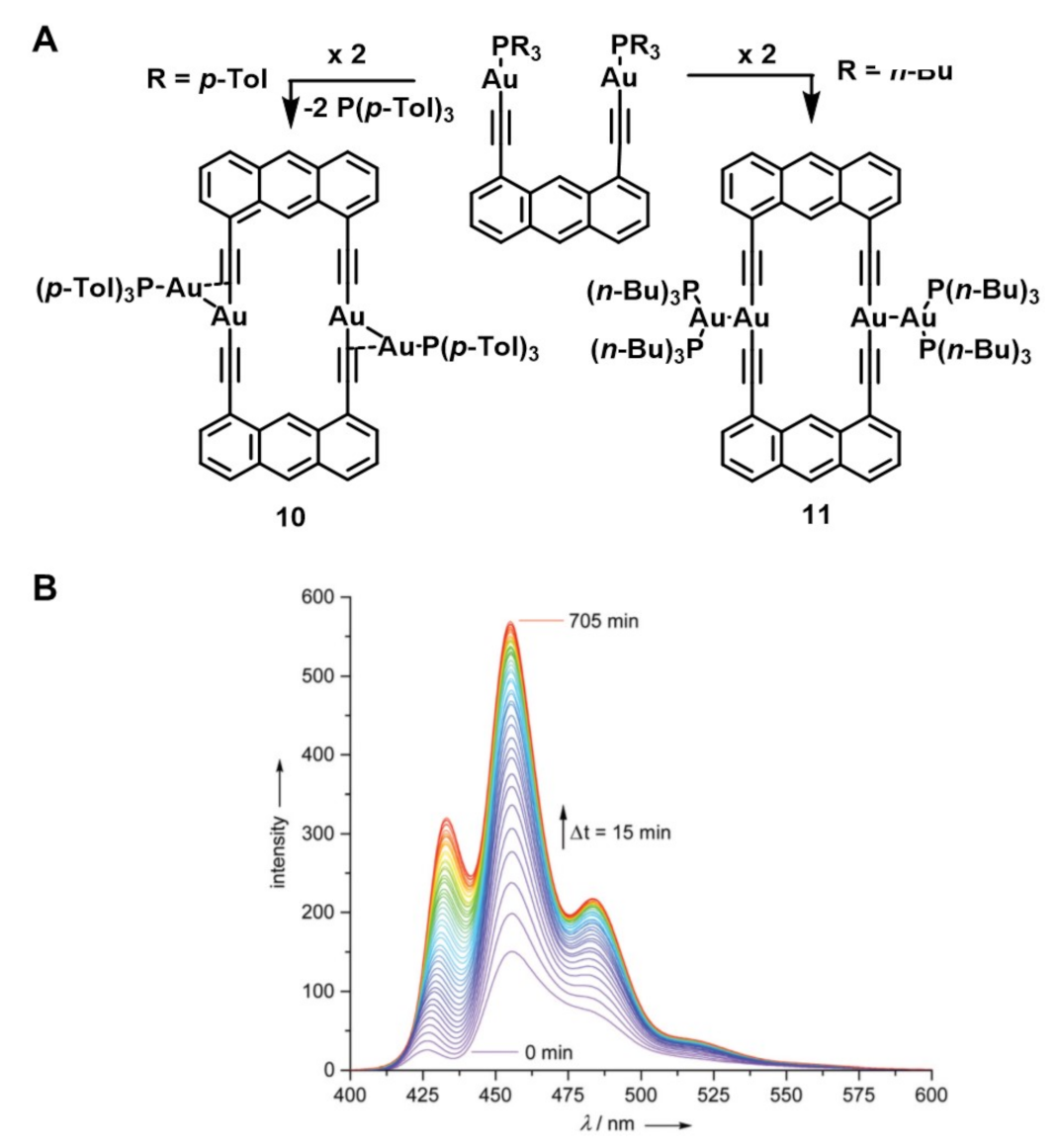
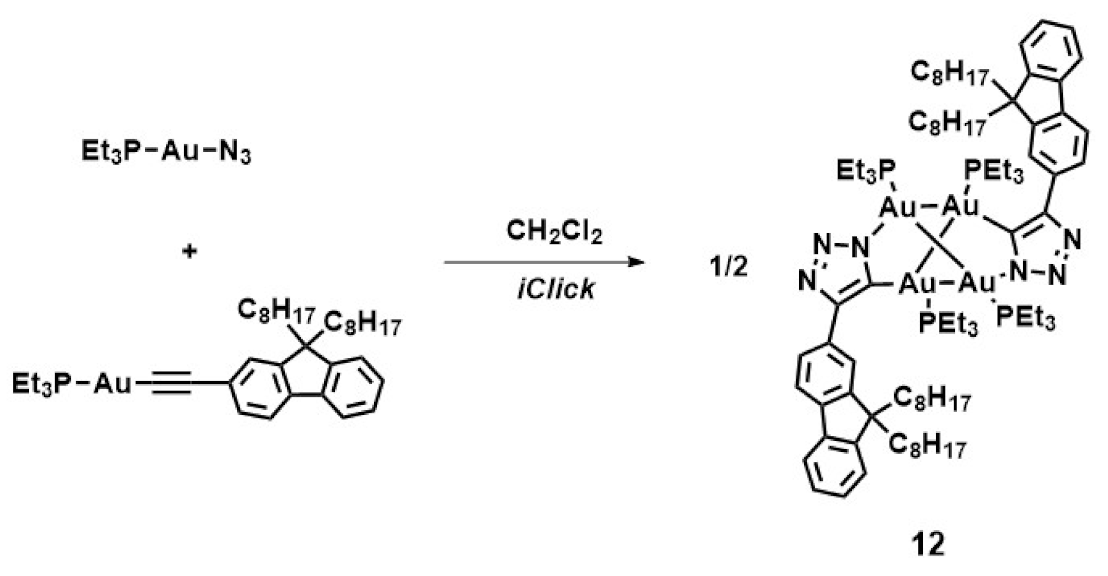
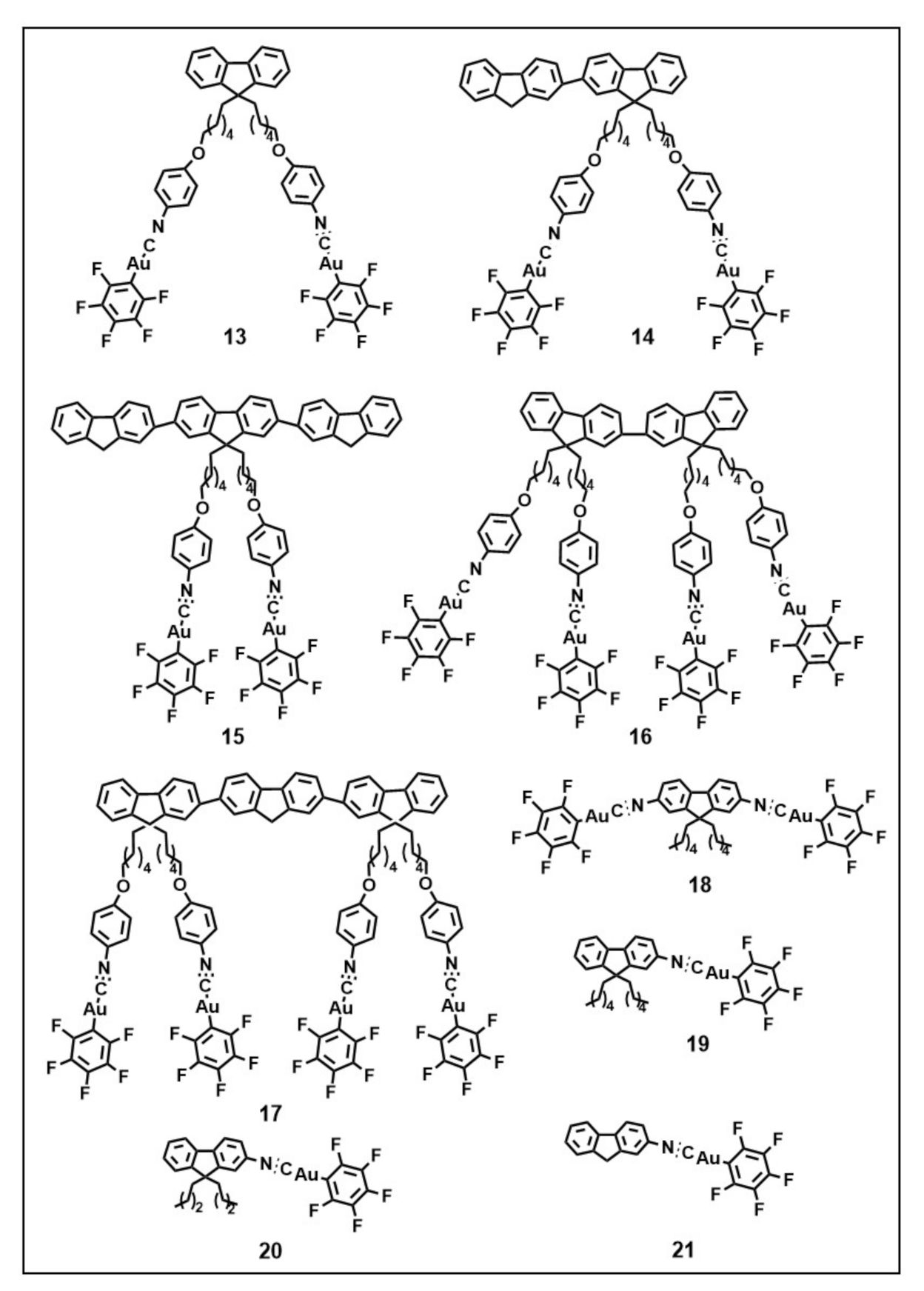
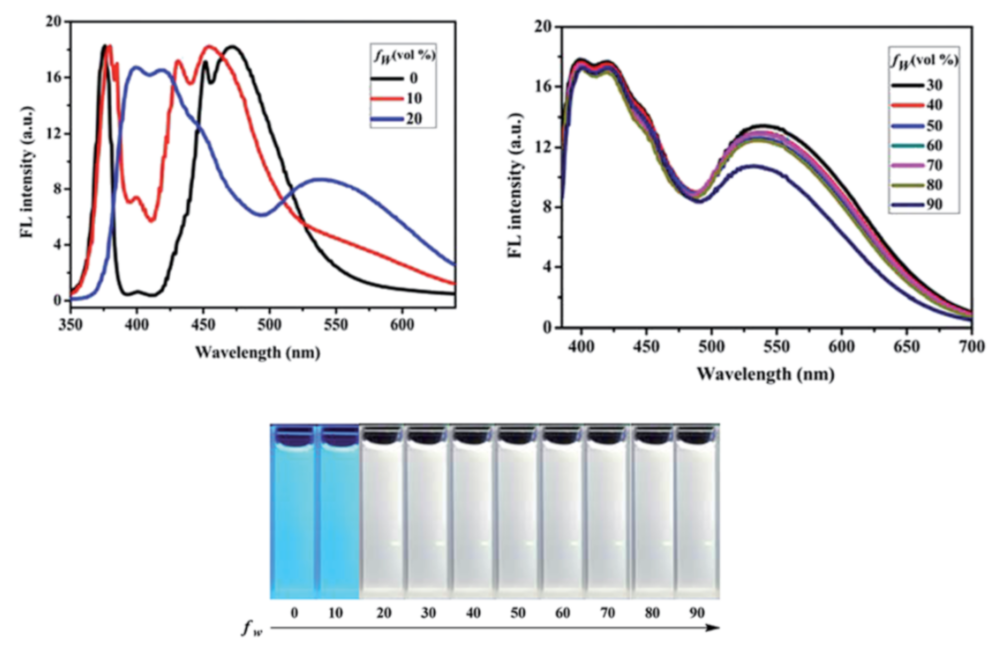
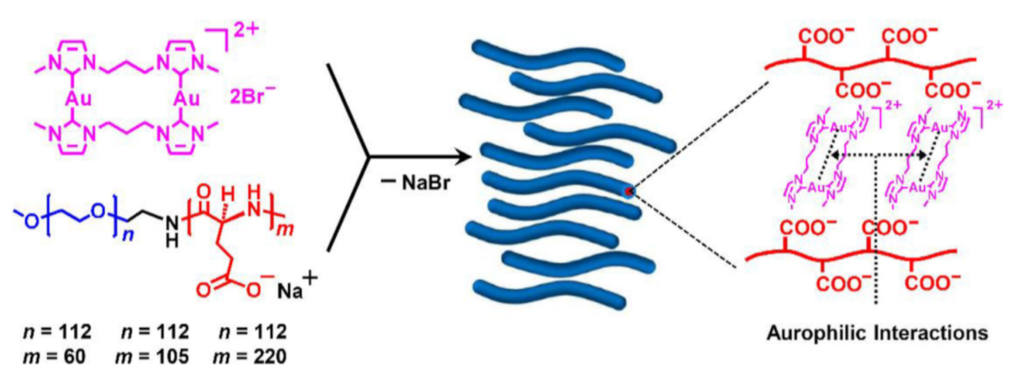
3.2. Gold(I) Complexes that Self-assemble in Well-defined Superstructures
4. Modulation of Luminescent Supramolecular Assemblies Based on the Presence of Cations and Anions
4.1. Detection of Metal Ions Based on the “On”-“Off” Switching of Au(I)···Au(I) Intramolecular Interactions
4.2. Assembly/disassembly Processes Based on the Interaction of Gold(I) Complexes with Other Species
4.3. Interaction with Luminophores
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Aurophilic interactions as a subject of current research: An up-date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef]
- Herrera, R.P.; Gimeno, M.C. Main Avenues in Gold Coordination Chemistry. Chem. Rev. 2021. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong closed-shell interactions in inorganic chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef]
- Mirzadeh, N.; Privér, S.H.; Blake, A.J.; Schmidbaur, H.; Bhargava, S.K. Innovative Molecular Design Strategies in Materials Science following the Aurophilicity Concept. Chem. Rev. 2020, 120, 7551–7591. [Google Scholar] [CrossRef]
- Pujadas, M.; Rodríguez, L. Luminescent phosphine gold(I) alkynyl complexes. Highlights from 2010 to 2018. Coord. Chem. Rev. 2020, 408, 213179. [Google Scholar] [CrossRef]
- Pinto, A.; Svahn, N.; Lima, J.C.; Rodríguez, L. Aggregation induced emission of gold(i) complexes in water or water mixtures. Dalt. Trans. 2017, 46, 11125–11139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam, V.W.W.; Au, V.K.M.; Leung, S.Y.L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Rodríguez, L. Applications of gold(i) alkynyl systems: A growing field to explore. Chem. Soc. Rev. 2011, 40, 5442–5456. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Connick, W.B.; Lachicotte, R.J.; Gysling, H.J.; Eisenberg, R. Linear Chain Au(I) Dimer Compounds as Environmental Sensors: A Luminescent Switch for the Detection of Volatile Organic Compounds. J. Am. Chem. Soc. 1998, 120, 1329–1330. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, M.; Chang, X.; Gao, Q.; Chen, Y.; Lu, W. Correlating thermochromic and mechanochromic phosphorescence with polymorphs of a complex gold(i) double salt with infinite aurophilicity. Chem. Commun. 2018, 54, 12844–12847. [Google Scholar] [CrossRef] [PubMed]
- López-De-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Quintana, J.; Rodríguez-Castillo, M. Stimuli-Responsive Solvatochromic Au(I)-Ag(I) Clusters: Reactivity and Photophysical Properties Induced by the Nature of the Solvent. Inorg. Chem. 2019, 58, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Rodríguez, L. Supramolecular gold metallogelators: The key role of metallophilic interactions. Inorganics 2015, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, K.; Zang, S.Q. Progress in Atomically Precise Coinage Metal Clusters with Aggregation-Induced Emission and Circularly Polarized Luminescence. Adv. Opt. Mater. 2020, 8, 1–26. [Google Scholar] [CrossRef]
- Gimeno, M.C.; Laguna, A. Chalcogenide centred gold complexes. Chem. Soc. Rev. 2008, 37, 1952–1966. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Jiang, X.F.; Li, H.; Yu, S.Y. Self-Assembly of a Au16 ring via metal-metal bonding interactions. Chem. An Asian J. 2015, 10, 1146–1149. [Google Scholar] [CrossRef]
- Yao, L.Y.; Lee, T.K.M.; Yam, V.W.W. Thermodynamic-Driven Self-Assembly: Heterochiral Self-Sorting and Structural Reconfiguration in Gold(I)-Sulfido Cluster System. J. Am. Chem. Soc. 2016, 138, 7260–7263. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Zhang, J.Y.; Guan, Z.J.; Wang, Q.M. Intensely luminescent gold(i) phosphinopyridyl clusters: Visualization of unsupported aurophilic interactions in solution. Chem. Commun. 2017, 53, 10902–10905. [Google Scholar] [CrossRef]
- Chu, A.; Hau, F.K.W.; Yao, L.Y.; Yam, V.W.W. Synthesis, structural characterization, and photophysical studies of hexanuclear gold(I) chalcogenido complexes. J. Chin. Chem. Soc. 2019, 66, 1100–1104. [Google Scholar] [CrossRef]
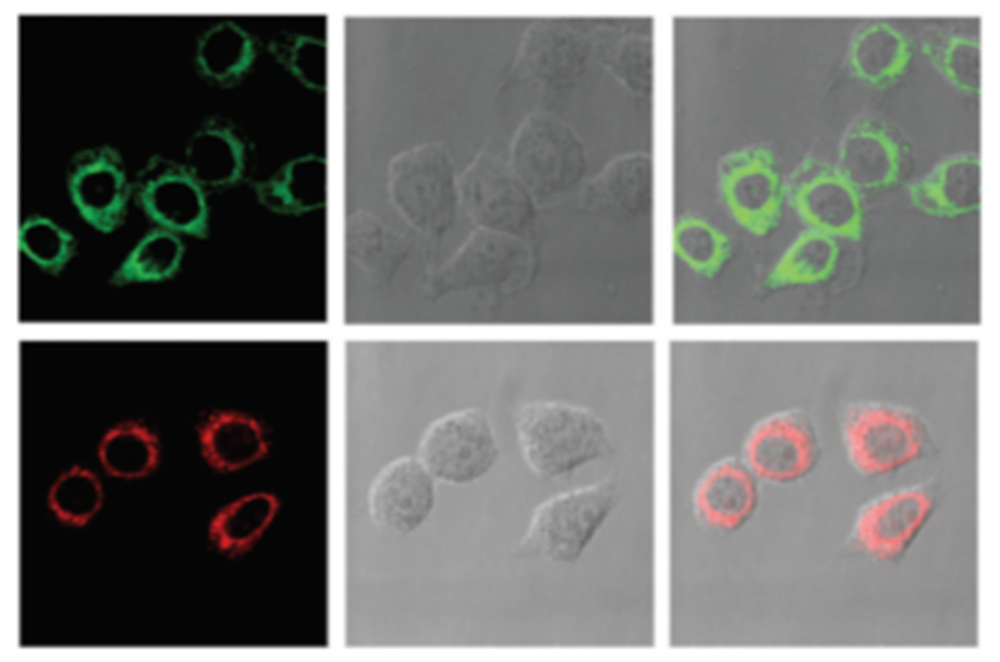
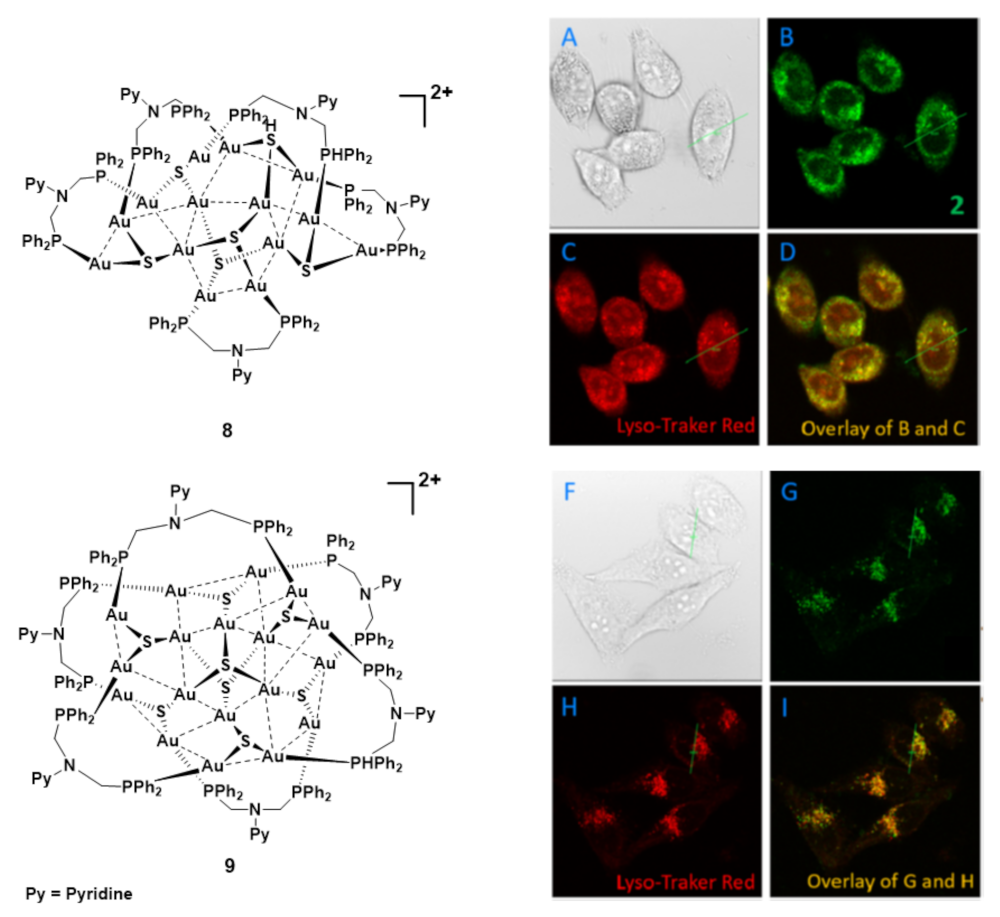
- Liu, C.Y.; Wei, X.R.; Chen, Y.; Wang, H.F.; Ge, J.F.; Xu, Y.J.; Ren, Z.G.; Braunstein, P.; Lang, J.P. Tetradecanuclear and Octadecanuclear Gold(I) Sulfido Clusters: Synthesis, Structures, and Luminescent Selective Tracking of Lysosomes in Living Cells. Inorg. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Niermeier, P.; Wickemeyer, L.; Neumann, B.; Stammler, H.G.; Goett-Zink, L.; Kottke, T.; Mitzel, N.W. Aurophilicity in action: Stepwise formation of dinuclear Au(i) macrocycles with rigid 1,8-dialkynylanthracenes. Dalt. Trans. 2019, 48, 4109–4113. [Google Scholar] [CrossRef]
- Beto, C.C.; Zeman, C.J.; Yang, Y.; Bullock, J.D.; Holt, E.D.; Kane, A.Q.; Makal, T.A.; Yang, X.; Ghiviriga, I.; Schanze, K.S.; et al. An Application Exploiting Aurophilic Bonding and iClick to Produce White Light Emitting Materials. Inorg. Chem. 2020, 59, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Climent, C.; Alemany, P.; Laskar, I.R. “Aggregation-induced emission” of transition metal compounds: Design, mechanistic insights, and applications. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100317. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Chen, Z.; Han, X.; Zhang, J.; Wu, D.; Yu, G.A.; Yin, J.; Liu, S.H. Fluorene-based novel gold(i) complexes with aggregation-induced emission (AIE) or aggregate fluorescence change characteristics: From green to white emission. RSC Adv. 2015, 5, 15341–15349. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, D.; Han, X.; Liang, J.; Yin, J.; Yu, G.A.; Liu, S.H. A novel fluorene-based gold(i) complex with aggregate fluorescence change: A single-component white light-emitting luminophor. Chem. Commun. 2014, 50, 11033–11035. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Song, M.; Yin, J.; Yu, G.A.; Liu, S.H. A novel fluorene-based aggregation-induced emission (AIE)-active gold(i) complex with crystallization-induced emission enhancement (CIEE) and reversible mechanochromism characteristics. Chem. Commun. 2015, 51, 326–329. [Google Scholar] [CrossRef]
- Chen, Z.; Nie, Y.; Liu, S.H. Fluorene-based mononuclear gold(i) complexes: The effect of alkyl chain, aggregation-induced emission (AIE) and mechanochromism characteristics. RSC Adv. 2016, 6, 73933–73938. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Hu, Y.; Wu, D.; Yin, J.; Yu, G.A.; Liu, S.H. Carbazole-based gold(i) complexes with alkyl chains of different lengths: Tunable solid-state fluorescence, aggregation-induced emission (AIE), and reversible mechanochromism characteristics. RSC Adv. 2015, 5, 93757–93764. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Hu, F.; Yu, G.A.; Yin, J.; Liu, S.H. Novel carbazole-based aggregation-induced emission-active gold(I) complexes with various mechanofluorochromic behaviors. Dye. Pigment. 2016, 125, 169–178. [Google Scholar] [CrossRef]
- Gavara, R.; Lima, J.C.; Rodríguez, L. Effect of solvent polarity on the spectroscopic properties of an alkynyl gold(i) gelator. the particular case of water. Photochem. Photobiol. Sci. 2016, 15, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Liu, G.; Pu, S.; Liu, S.H. Carbazole-based aggregation-induced emission (AIE)-active gold(I) complex: Persistent room-temperature phosphorescence, reversible mechanochromism and vapochromism characteristics. Dye. Pigment. 2017, 143, 409–415. [Google Scholar] [CrossRef]
- Han, X.; Lü, X.; Chen, Z.; Yu, G.; Yin, J.; Liu, S. A Fluorescent Probe for Hg2+ Based on Gold(I) Complex with An Aggregation-Induced Emission Feature. Chin. J. Chem. 2015, 33, 1064–1068. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Yang, L.; Liang, J.; Yin, J.; Yu, G.A.; Liu, S.H. Novel diisocyano-based dinuclear gold(I) complexes with aggregation-induced emission and mechanochromism characteristics. Dye. Pigment. 2015, 121, 170–177. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, P.S.; Li, Z.; Yin, J.; Yu, G.A.; Liu, S.H. Triisocyano-based trinuclear gold(I) complexes with aggregation-induced emission (AIE) and mechanochromic luminescence characteristics. Inorg. Chim. Acta 2015, 432, 192–197. [Google Scholar] [CrossRef]
- Li, W.B.; Luo, W.J.; Li, K.X.; Yuan, W.Z.; Zhang, Y.M. Aggregation-induced phosphorescence and mechanochromic luminescence of a tetraphenylethene-based gold(I) isocyanide complex. Chin. Chem. Lett. 2017, 28, 1300–1305. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, J.; Dong, Y.B.; Zhang, Y.; Yin, J.; Liu, S.H. Different structures modulated mechanochromism and aggregation-induced emission in a series of Gold(I) complexes. Dye. Pigment. 2018, 156, 74–81. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Ghiviriga, I.; Abboud, K.A.; Veige, A.S. Organogold oligomers: Exploiting iClick and aurophilic cluster formation to prepare solution stable Au4 repeating units. Dalt. Trans. 2015, 44, 11437–11443. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.; Chen, J.; Sun, Z.; Yao, T.; Jiang, Y.; Wei, S. Luminescence of Au(I)-thiolate complex affected by solvent. Radiat. Phys. Chem. 2017, 137, 68–71. [Google Scholar] [CrossRef]
- Leung, F.C.M.; Yam, V.W.W. Cation- and Solvent-Induced Supramolecular Aggregation Studies of Crown Ether-Containing Dinuclear Alkynylgold(I) Isocyanide Complexes. Eur. J. Inorg. Chem. 2017, 2017, 5271–5278. [Google Scholar] [CrossRef] [Green Version]
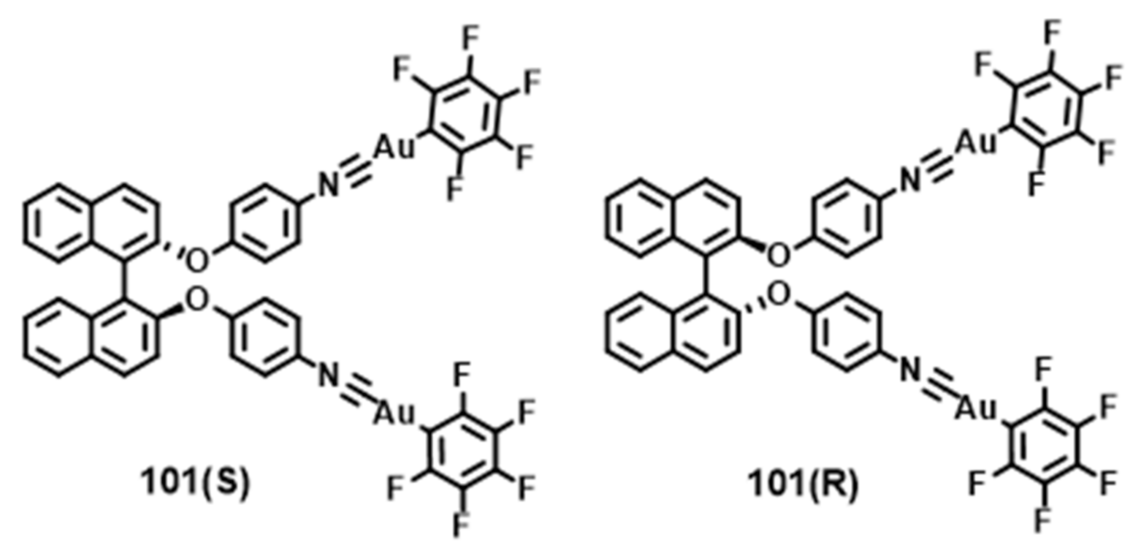
- Han, Z.; Zhao, X.; Peng, P.; Li, S.; Zhang, C.; Cao, M.; Li, K.; Wang, Z.Y.; Zang, S.Q. Intercluster aurophilicity-driven aggregation lighting circularly polarized luminescence of chiral gold clusters. Nano Res. 2020, 13, 3248–3252. [Google Scholar] [CrossRef]
- Gavara, R.; Llorca, J.; Lima, J.C.; Laura, R. A luminescent hydrogel based on a new au(i) complex. Chem. Commun. 2013, 49, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Aguiló, E.; Gavara, R.; Lima, J.C.; Llorca, J.; Rodríguez, L. From Au(i) organometallic hydrogels to well-defined Au(0) nanoparticles. J. Mater. Chem. C 2013, 1, 5538–5547. [Google Scholar] [CrossRef]
- Gavara, R.; Aguiló, E.; Guerra, C.F.; Rodríguez, L.; Lima, J.C. Thermodynamic aspects of aurophilic hydrogelators. Inorg. Chem. 2015, 54, 5195–5203. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.J.; Rome, B.; Aguiló, E.; Arcau, J.; Puttreddy, R.; Rissanen, K.; Lima, J.C.; Rodríguez, L. A coumarin based gold(i)-alkynyl complex: A new class of supramolecular hydrogelators. Org. Biomol. Chem. 2015, 13, 2026–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemper, B.; Hristova, Y.R.; Tacke, S.; Stegemann, L.; Van Bezouwen, L.S.; Stuart, M.C.A.; Klingauf, J.; Strassert, C.A.; Besenius, P. Facile synthesis of a peptidic Au(i)-metalloamphiphile and its self-assembly into luminescent micelles in water. Chem. Commun. 2015, 51, 5253–5256. [Google Scholar] [CrossRef] [Green Version]
- Aguiló, E.; Gavara, R.; Baucells, C.; Guitart, M.; Lima, J.C.; Llorca, J.; Rodríguez, L. Tuning supramolecular aurophilic structures: The effect of counterion, positive charge and solvent. Dalt. Trans. 2016, 45, 7328–7339. [Google Scholar] [CrossRef] [Green Version]
- Gavara, R.; Aguiló, E.; Schur, J.; Llorca, J.; Ott, I.; Rodríguez, L. Study of the effect of the chromophore and nuclearity on the aggregation and potential biological activity of gold(I) alkynyl complexes. Inorg. Chim. Acta 2016, 446, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Aguiló, E.; Moro, A.J.; Gavara, R.; Alfonso, I.; Pérez, Y.; Zaccaria, F.; Guerra, C.F.; Malfois, M.; Baucells, C.; Ferrer, M.; et al. Reversible Self-Assembly of Water-Soluble Gold(I) Complexes. Inorg. Chem. 2018, 57, 1017–1028. [Google Scholar] [CrossRef]
- Chu, A.; Hau, F.K.W.; Yam, V.W.W. AuI⋅⋅⋅AuI Interaction Assisted Host–Guest Interactions and Stimuli-Responsive Self-Assembly in Tetranuclear Alkynylgold(I) Calix[4]arene-Based Isocyanide Complexes. Chem. Eur. J. 2017, 23, 11076–11084. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Wang, C.; Gao, Z.; Gao, Z.; Wang, F. Cooperative self-assembly and gelation of organogold(i) complexes: Via hydrogen bonding and aurophilic Au⋯Au interactions. Chem. Commun. 2017, 53, 11552–11555. [Google Scholar] [CrossRef]
- Zhou, N.; Hailes, R.; Zhang, Y.; Chen, Z.; Manners, I.; He, X. Controlling the supramolecular polymerization of dinuclear isocyanide gold(i) arylethynylene complexes through tuning the central π-conjugated moiety. Polym. Chem. 2020, 11, 2700–2707. [Google Scholar] [CrossRef]
- Blasco, D.; López-De-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pascual, D.; Rodríguez-Castillo, M. Cooperative Au(I)···Au(I) Interactions and Hydrogen Bonding as Origin of a Luminescent Adeninate Hydrogel Formed by Ultrathin Molecular Nanowires. Inorg. Chem. 2018, 57, 3805–3817. [Google Scholar] [CrossRef]
- Chang, H.Y.; Tseng, Y.T.; Yuan, Z.; Chou, H.L.; Chen, C.H.; Hwang, B.J.; Tsai, M.C.; Chang, H.T.; Huang, C.C. The effect of ligand-ligand interactions on the formation of photoluminescent gold nanoclusters embedded in Au(i)-thiolate supramolecules. Phys. Chem. Chem. Phys. 2017, 19, 12085–12093. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Chang, H.Y.; Harroun, S.G.; Wu, C.W.; Wei, S.C.; Yuan, Z.; Chou, H.L.; Chen, C.H.; Huang, C.C.; Chang, H.T. Self-Assembled Chiral Gold Supramolecules with Efficient Laser Absorption for Enantiospecific Recognition of Carnitine. Anal. Chem. 2018, 90, 7283–7291. [Google Scholar] [CrossRef]
- Wu, Z.; Du, Y.; Liu, J.; Yao, Q.; Chen, T.; Cao, Y.; Zhang, H.; Xie, J. Aurophilic Interactions in the Self-Assembly of Gold Nanoclusters into Nanoribbons with Enhanced Luminescence. Angew. Chem. Int. Ed. 2019, 58, 8139–8144. [Google Scholar] [CrossRef]
- Yam, V.W.W.; Li, C.K.; Chan, C.L. Proof of potassium ions by luminescence signaling based on weak gold–Gold interactions in dinuclear gold(I) complexes. Angew. Chem. Int. Ed. 1998, 37, 2857–2859. [Google Scholar] [CrossRef]
- Leung, F.C.M.; Yam, V.W.W. Photophysical and ion-binding studies of a tetranuclear alkynylgold(I) isonitrile complex. J. Photochem. Photobiol. A Chem. 2018, 355, 212–219. [Google Scholar] [CrossRef]
- Guo, Y.; Tong, X.; Ji, L.; Wang, Z.; Wang, H.; Hu, J.; Pei, R. Visual detection of Ca2+ based on aggregation-induced emission of Au(i)-Cys complexes with superb selectivity. Chem. Commun. 2015, 51, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Maeba, J.; Nozaki, K.; Iwamura, M. Considerable enhancement of emission yields of [Au(CN)2]− oligomers in aqueous solutions by coexisting cations. Inorg. Chem. 2016, 55, 7739–7746. [Google Scholar] [CrossRef]
- Toohara, S.; Tanaka, Y.; Sakurai, S.; Ikeda, T.; Tanaka, K.; Gon, M.; Chujo, Y.; Kuroiwa, K. Self-assembly of [Au(CN)2]− complexes with tomato (Solanum lycopersicum) steroidal alkaloid glycosides to form sheet or tubular structures. Chem. Lett. 2018, 47, 1010–1013. [Google Scholar] [CrossRef]
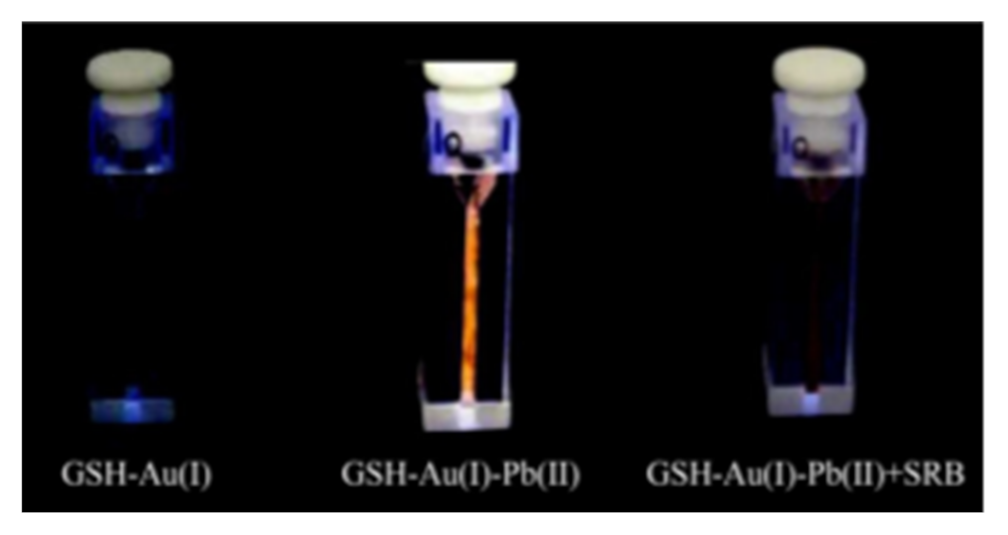
- Zheng, L.; Ye, X.; Qi, P.; Zhang, D.; Sun, Y. Fluorometric detection of sulfate-reducing bacteria via the aggregation-induced emission of glutathione-gold(I) complexes. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Spigolon, G.; Gavara, R.; Zonta, C.; Licini, G.; Rodríguez, L. Tripodal gold(i) polypyridyl complexes and their Cu+and Zn2+heterometallic derivatives. Effects on luminescence. Dalt. Trans. 2020, 49, 14613–14625. [Google Scholar] [CrossRef]
- Giestas, L.; Gavara, R.; Aguiló, E.; Svahn, N.; Lima, J.C.; Rodríguez, L. Modulation of supramolecular gold(I) aggregates by anion’s interaction. Supramol. Chem. 2018, 30, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Moro, A.J.; Avó, J.; Malfois, M.; Zaccaria, F.; Fonseca Guerra, C.; Caparrós, F.J.; Rodríguez, L.; Lima, J.C. Aggregation induced emission of a new naphthyridine-ethynyl-gold(i) complex as a potential tool for sensing guanosine nucleotides in aqueous media. Dalt. Trans. 2020, 49, 171–178. [Google Scholar] [CrossRef]
- Guo, P.; He, Q.; Wang, C.; Hou, Z.; Yu, B.; Bu, W. Intensely phosphorescent block copolymer micelles containing gold(I) complexes. Soft Matter 2018, 14, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Jin, R.; Wang, M.; He, Q.; Cai, C.; Zhao, Q.; Bu, W. Chiral gold(I)-containing polymeric composites: Chiroptical sensing and circularly polarized luminescence. J. Organomet. Chem. 2021, 931, 121616. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wu, W.; Peng, J.; Zhang, H.; Song, F.; He, B.; Wang, X.; Sung, H.H.Y.; Chen, M.; et al. Real-Time Monitoring of Hierarchical Self-Assembly and Induction of Circularly Polarized Luminescence from Achiral Luminogens. ACS Nano 2019, 13, 3618–3628. [Google Scholar] [CrossRef] [PubMed]



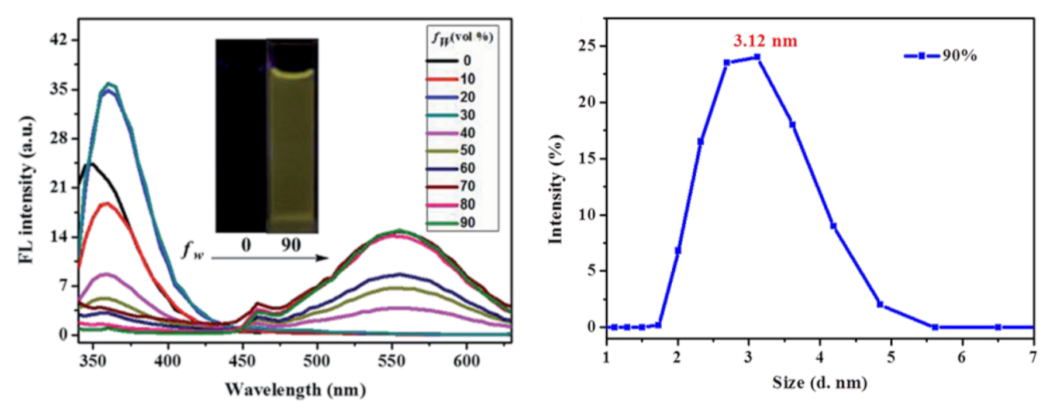
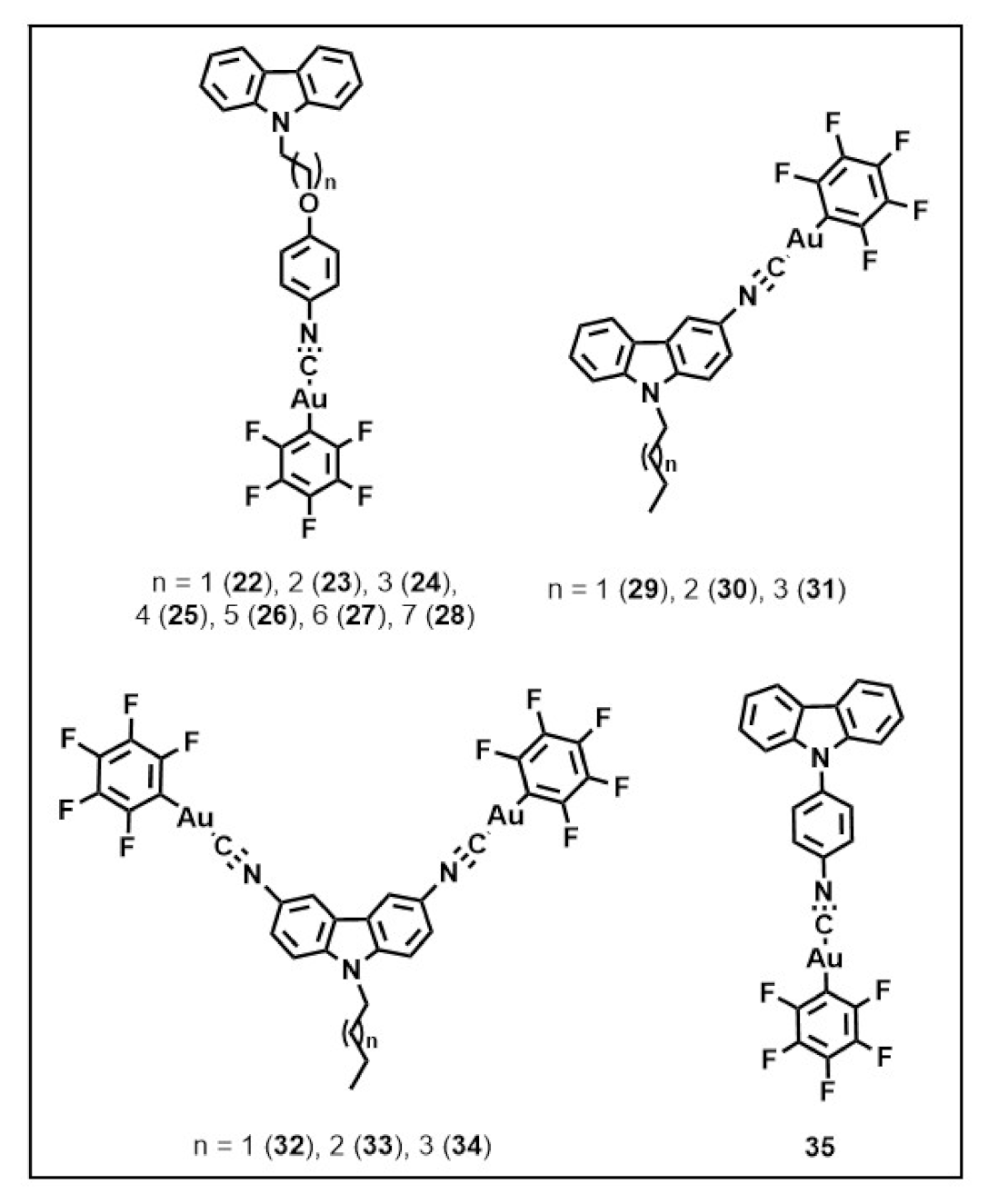

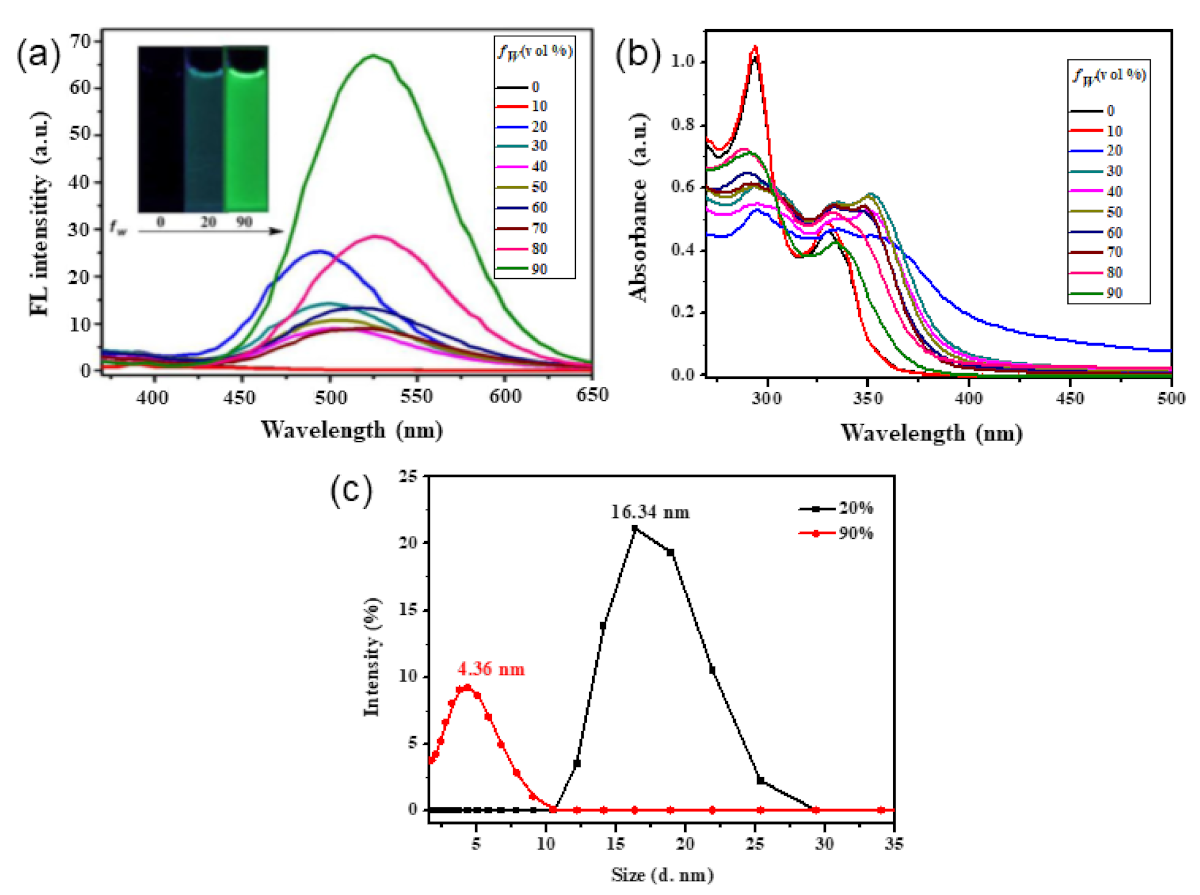

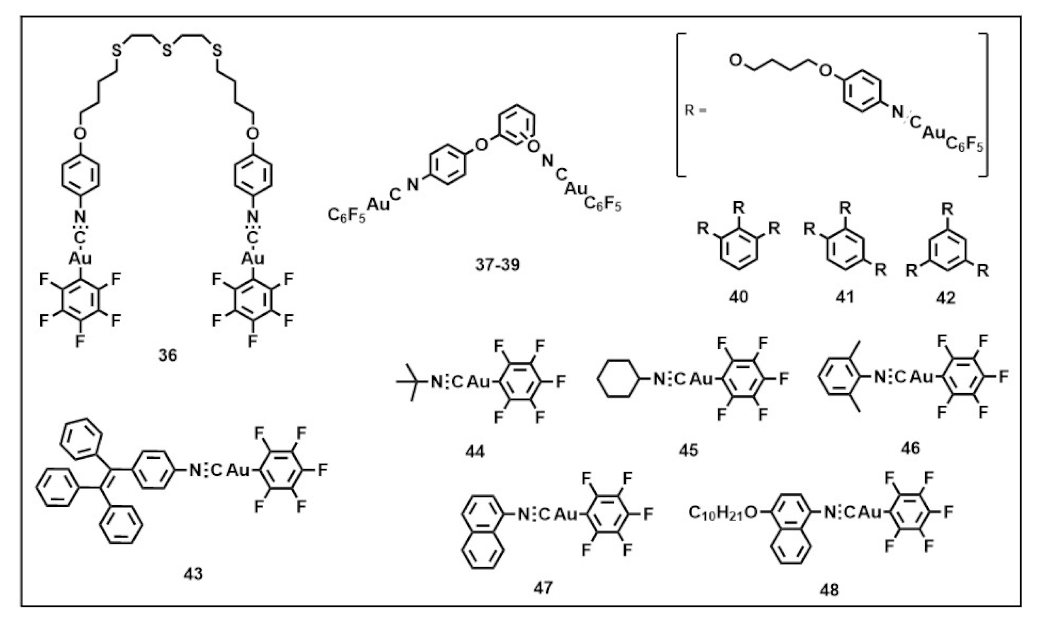
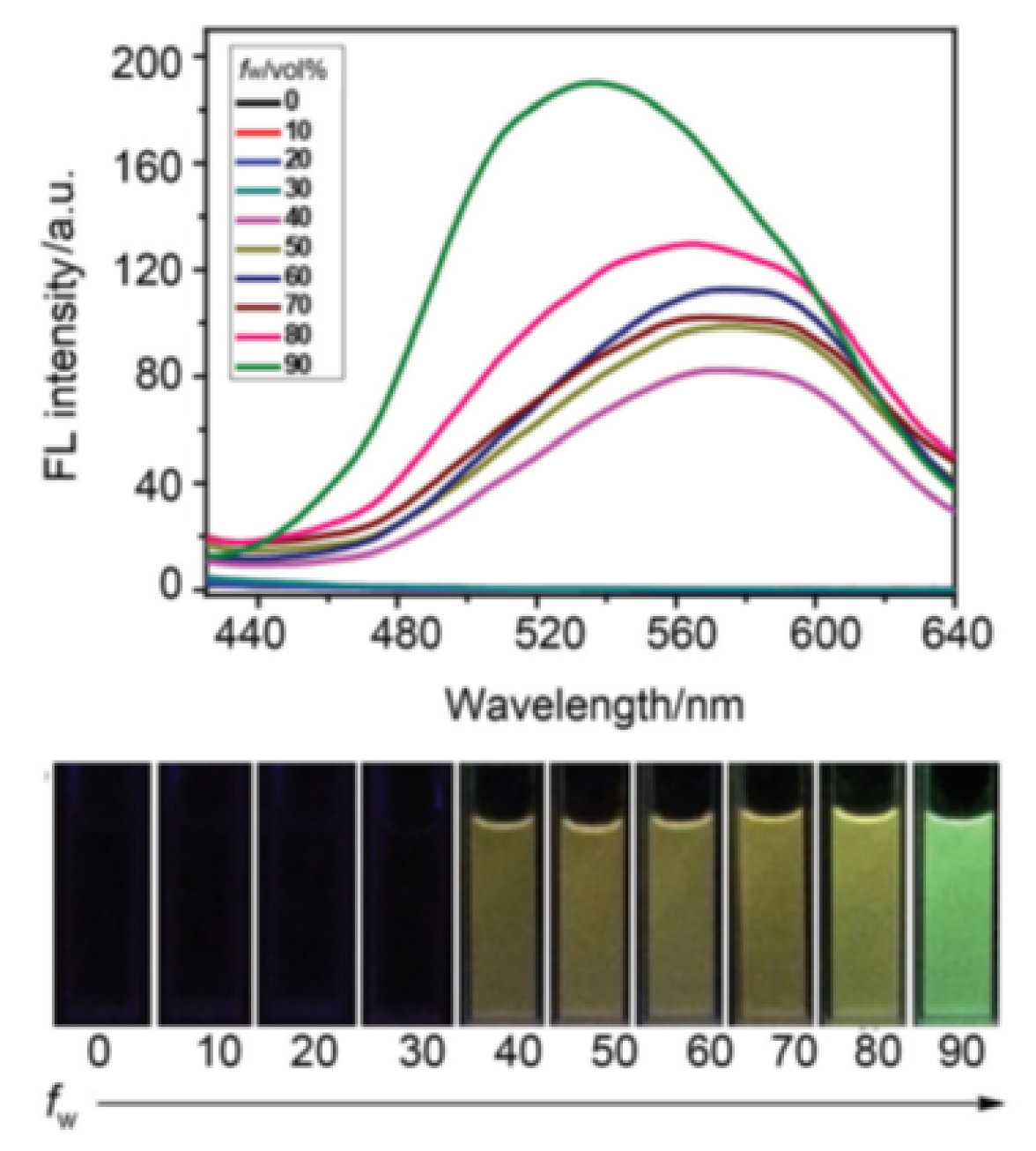
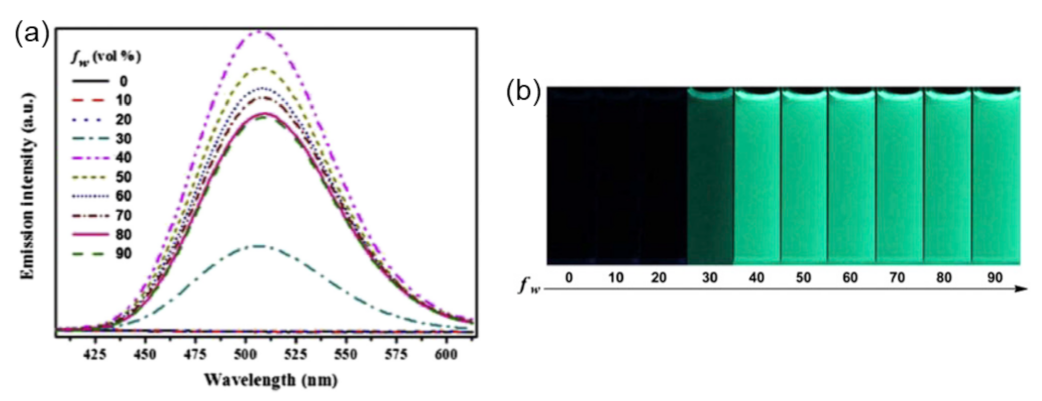
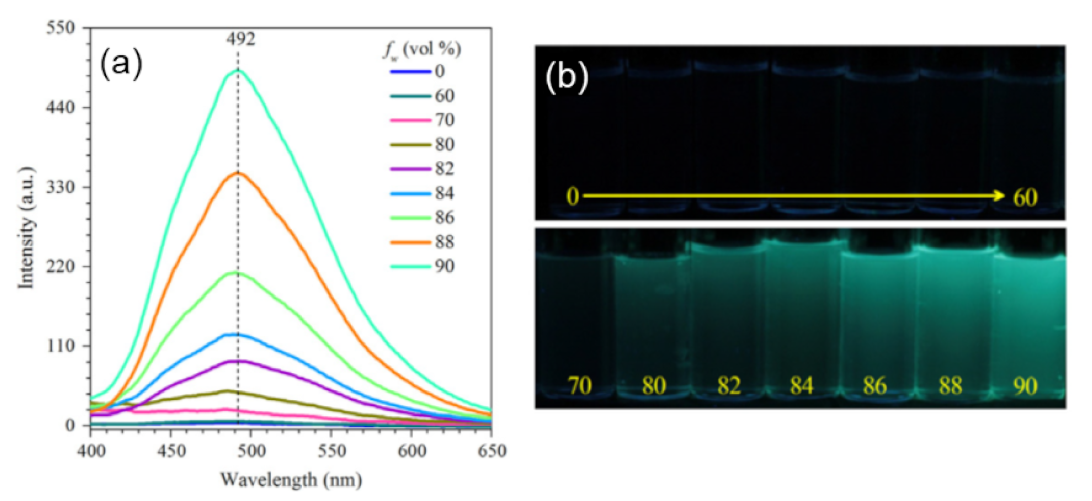
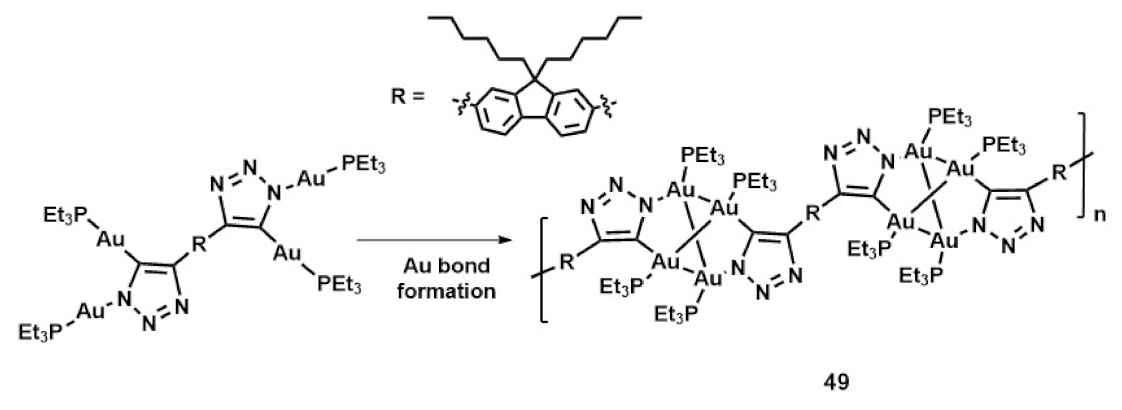
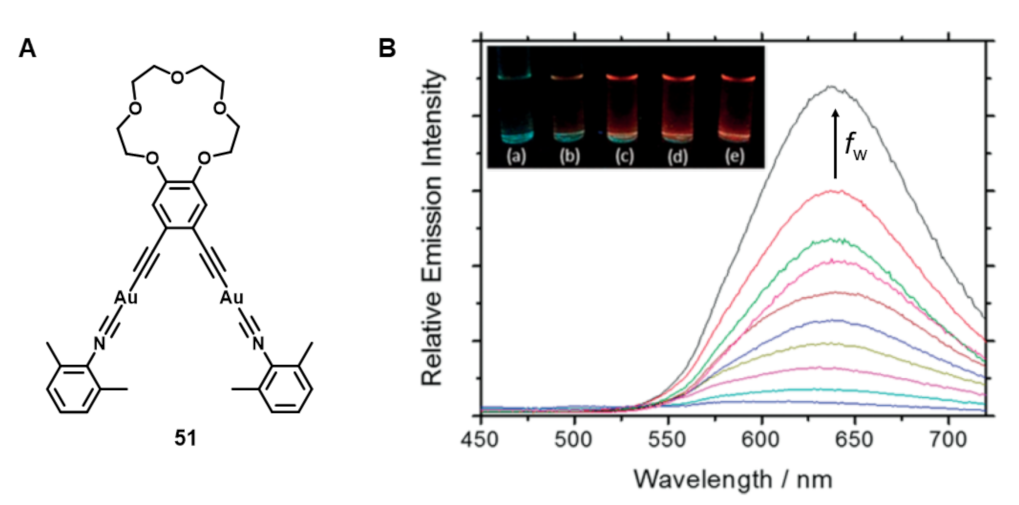
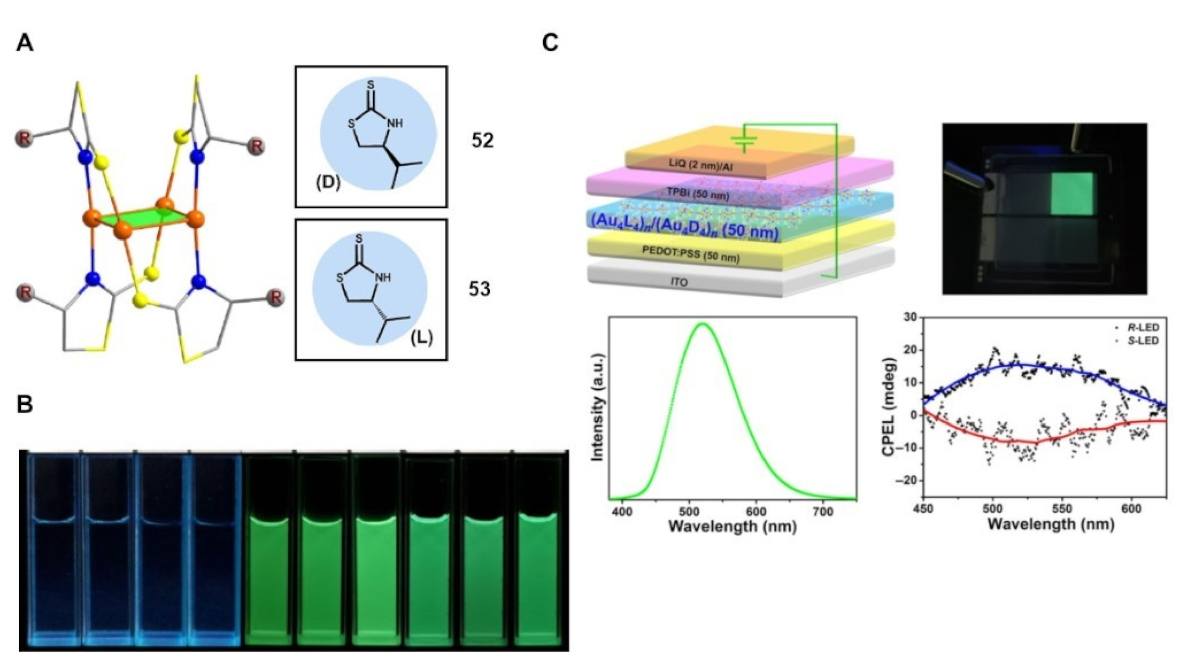
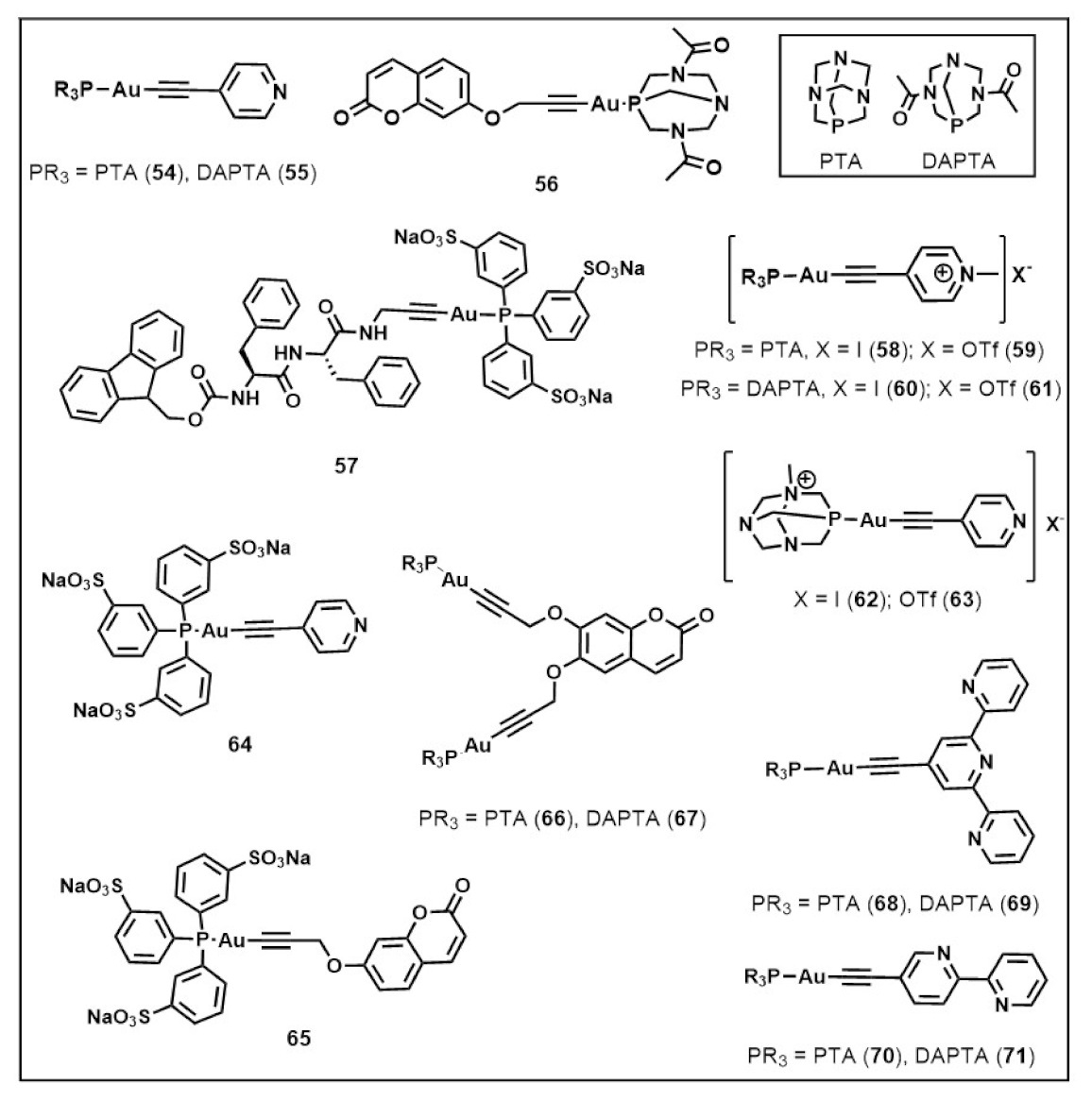
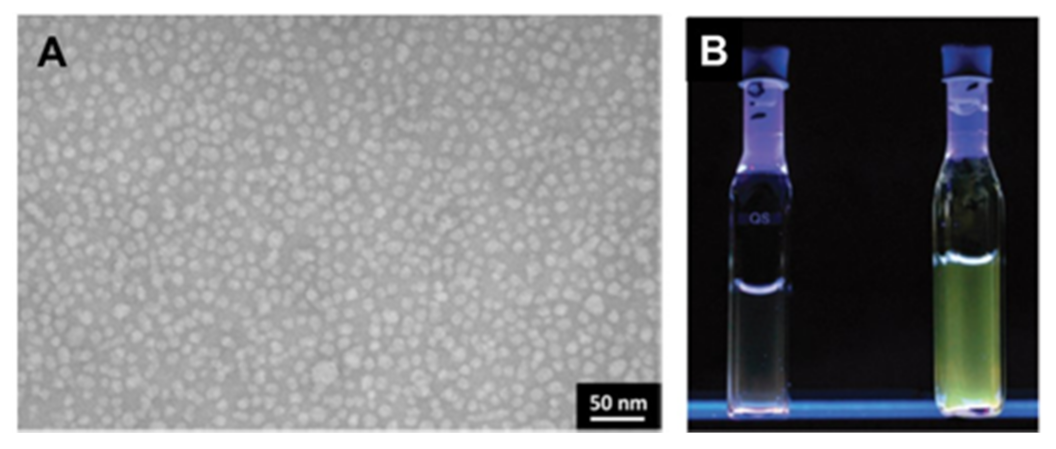
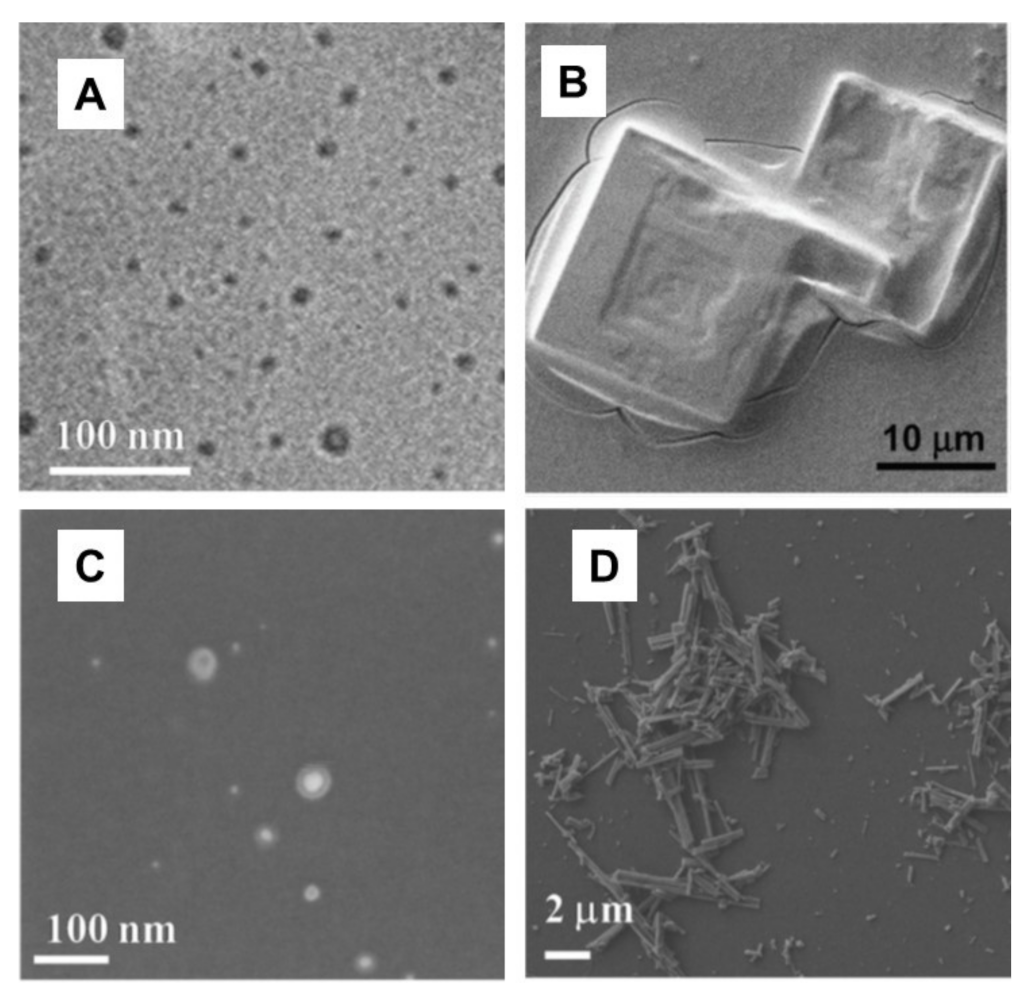

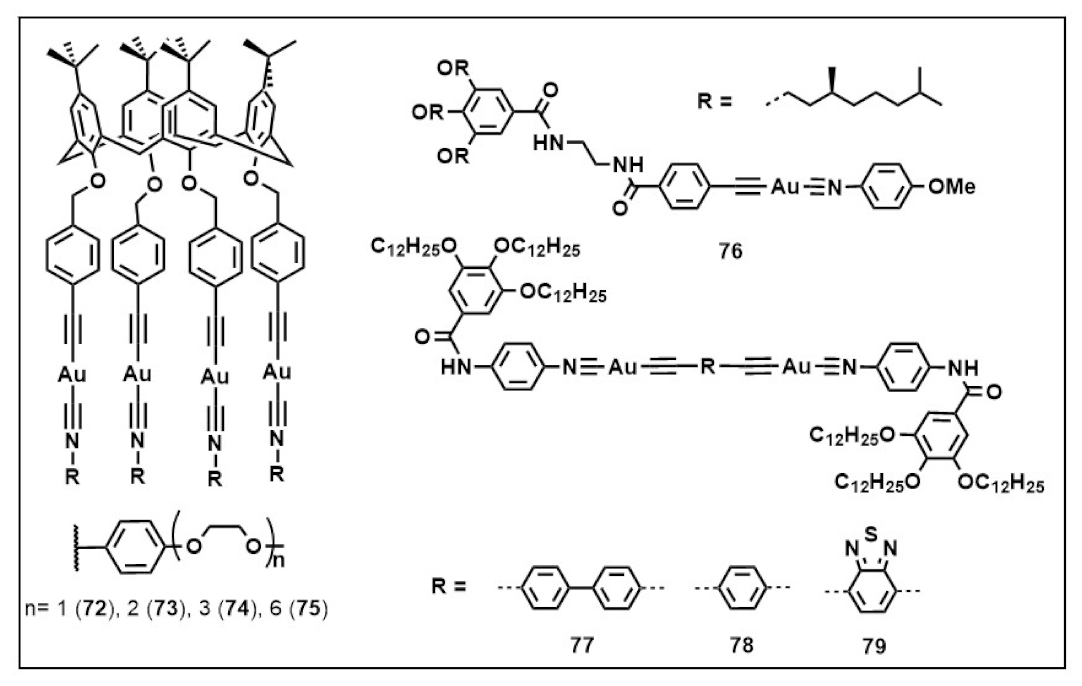
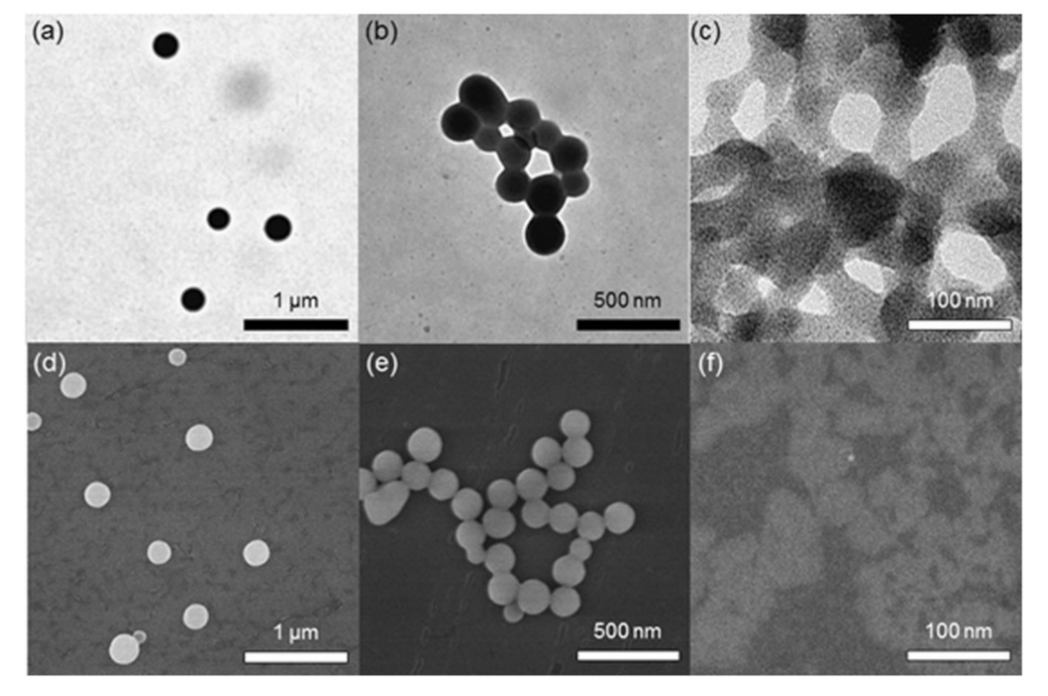

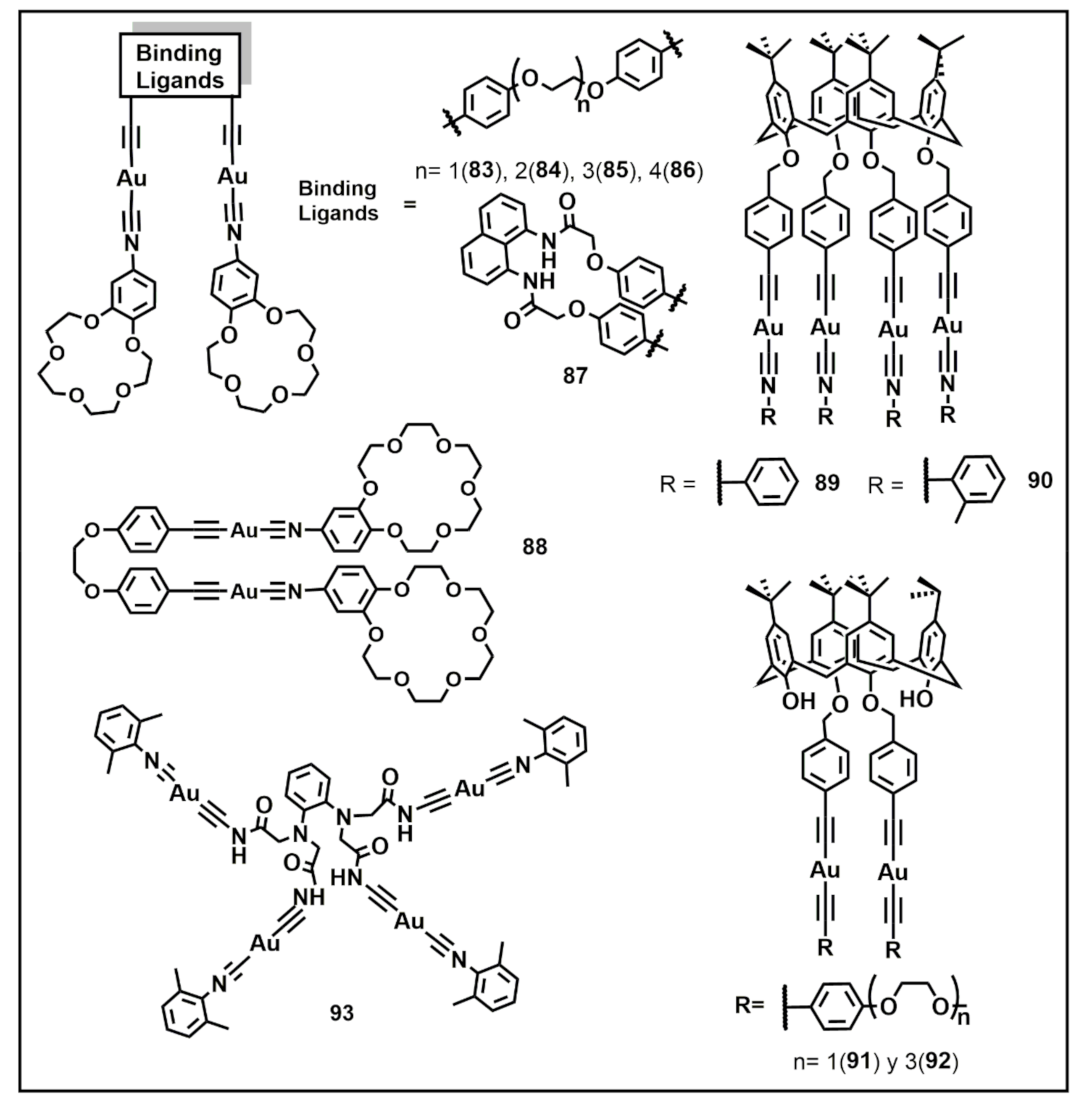

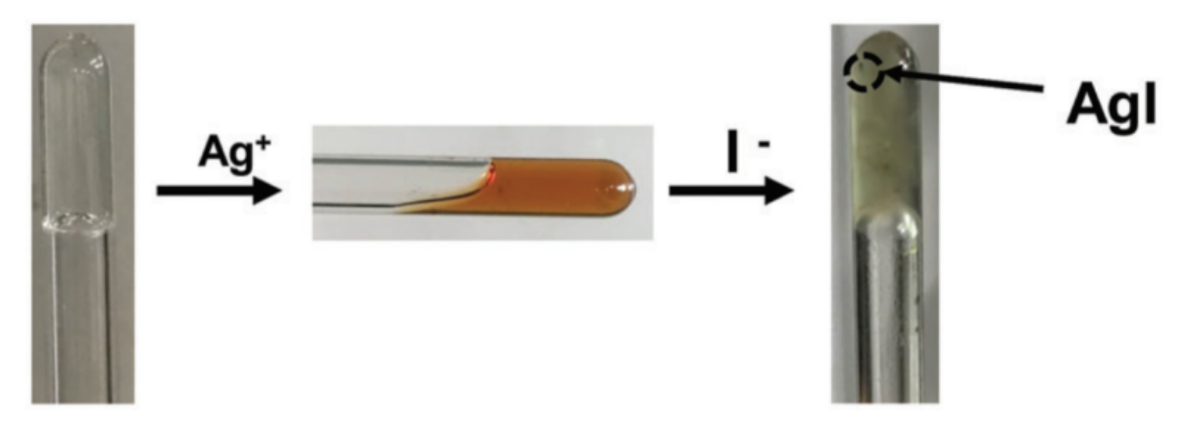

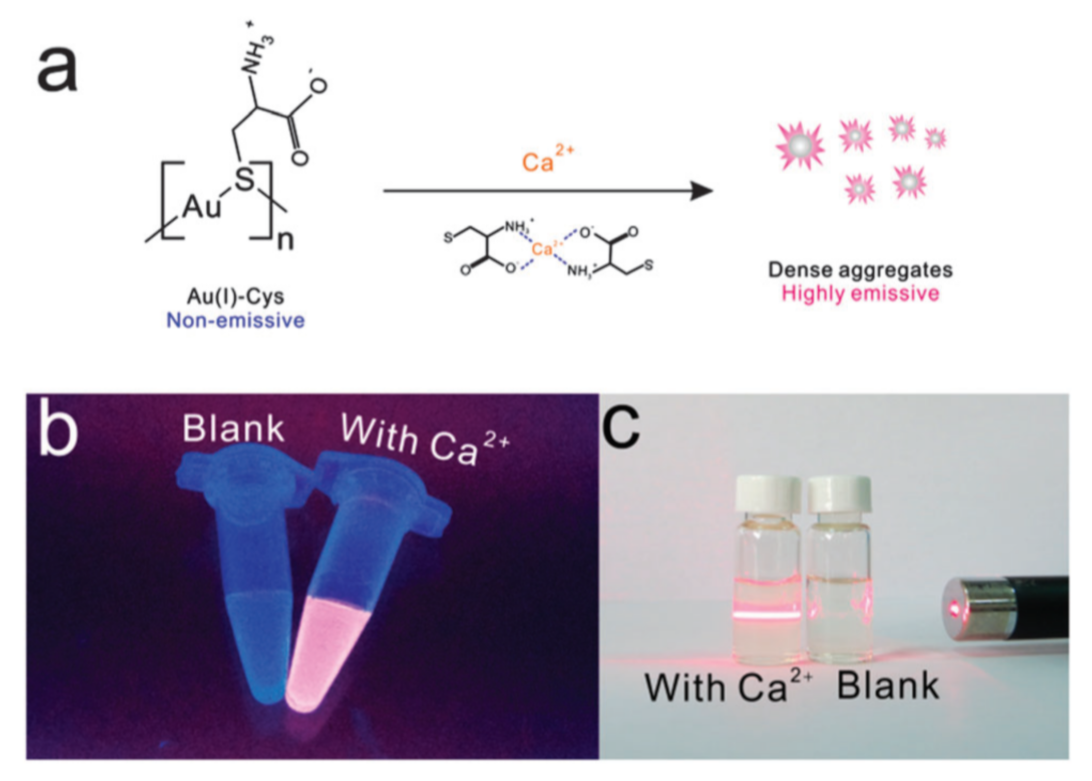
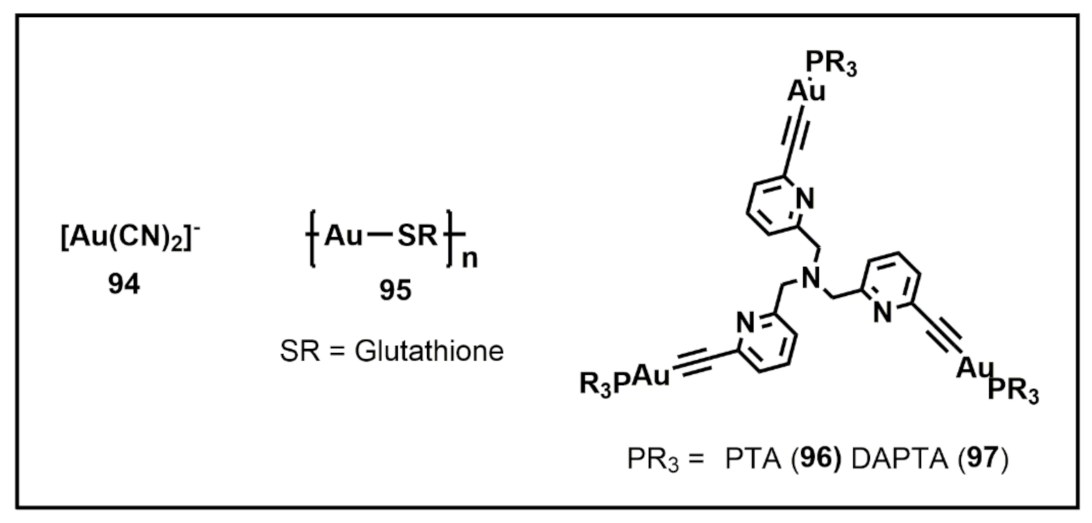

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romo-Islas, G.; Gavara, R. Recent Progress on Supramolecular Luminescent Assemblies Based on Aurophilic Interactions in Solution. Inorganics 2021, 9, 32. https://doi.org/10.3390/inorganics9050032
Romo-Islas G, Gavara R. Recent Progress on Supramolecular Luminescent Assemblies Based on Aurophilic Interactions in Solution. Inorganics. 2021; 9(5):32. https://doi.org/10.3390/inorganics9050032
Chicago/Turabian StyleRomo-Islas, Guillermo, and Raquel Gavara. 2021. "Recent Progress on Supramolecular Luminescent Assemblies Based on Aurophilic Interactions in Solution" Inorganics 9, no. 5: 32. https://doi.org/10.3390/inorganics9050032
APA StyleRomo-Islas, G., & Gavara, R. (2021). Recent Progress on Supramolecular Luminescent Assemblies Based on Aurophilic Interactions in Solution. Inorganics, 9(5), 32. https://doi.org/10.3390/inorganics9050032






