Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2
Abstract
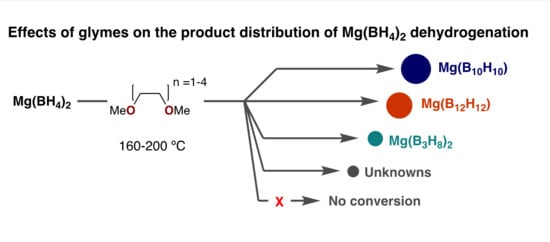
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Considerations.
4.2. General Procedure for Thermolysis Reaction of Mg(BH4)2 with Additives and 11B NMR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and dense magnesium borohydride frameworks: Synthesis, stability, and reversible absorption of guest species. Angew. Chem. Int. Ed. 2011, 50, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrogen Energy 2016, 41, 9885–9892. [Google Scholar] [CrossRef]
- Saldan, I. Decomposition and formation of magnesium borohydride. Int. J. Hydrogen Energy 2016, 41, 11201–11224. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Maekawa, H.; Aoki, M.; Noritake, T.; Matsumoto, M.; Miwa, K.; Towata, S.-i.; Orimo, S.-i. Formation process of [B12H12]2− from [BH4] during the dehydrogenation Reaction of Mg(BH4)2. Mater. Trans. 2011, 52, 1443–1446. [Google Scholar] [CrossRef]
- Chong, M.; Autrey, T.; Jensen, C. Lewis Base Complexes of Magnesium Borohydride: Enhanced Kinetics and Product Selectivity upon Hydrogen Release. Inorganics 2017, 5, 89. [Google Scholar] [CrossRef]
- Chen, J.; Chua, Y.S.; Wu, H.; Xiong, Z.; He, T.; Zhou, W.; Ju, X.; Yang, M.; Wu, G.; Chen, P. Synthesis, structures and dehydrogenation of magnesium borohydride–ethylenediamine composites. Int. J. Hydrogen Energy 2015, 40, 412–419. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Zhang, Y.; Li, Y.; Gao, M.; Pan, H. Hydrogen storage properties and mechanisms of Mg(BH4)2·2NH3–xMgH2 combination systems. J. Alloys Compd. 2014, 585, 674–680. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, B.; Sun, N.; Sun, Z.; Zeng, Y.; Meng, L. Improvement in dehydrogenation performance of Mg(BH4)2·2NH3 doped with transition metal: First principles investigation. Int. J. Hydrogen Energy 2015, 40, 8721–9731. [Google Scholar] [CrossRef]
- Bell, R.T.; Strange, N.A.; Leick, N.; Stavila, V.; Bowden, M.E.; Autrey, T.S.; Gennett, T. Mg(BH4)2-Based hybrid metal-organic borohydride system exhibiting enhanced chemical stability in melt. ACS Appl. Energy Mater. 2021, 4, 1704–1713. [Google Scholar] [CrossRef]
- Bardají, E.G.; Zhao-Karger, Z.; Boucharat, N.; Nale, A.; van Setten, M.J.; Lohstroh, W.; Röhm, E.; Catti, M.; Fichtner, M. LiBH4−Mg(BH4)2: A Physical Mixture of Metal Borohydrides as Hydrogen Storage Material. J. Phys. Chem. C 2011, 115, 6095–6101. [Google Scholar] [CrossRef]
- Nale, A.; Catti, M.; Bardají, E.G.; Fichtner, M. On the decomposition of the 0.6LiBH4-0.4Mg(BH4)2 eutectic mixture for hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 13676–13682. [Google Scholar] [CrossRef]
- Hagemann, H.; D’Anna, V.; Rapin, J.-P.; Černý, R.; Filinchuk, Y.; Kim, K.C.; Sholl, D.S.; Parker, S.F. New fundamental experimental studies on α-Mg(BH4)2 and other borohydrides. J. Alloys Compd. 2011, 509, S688–S690. [Google Scholar] [CrossRef]
- Li, H.W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2. Scr. Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Bardají, E.G.; Hanada, N.; Zabara, O.; Frichtner, M. Effect of several metal chlorides on the thermal decomposition of α-Mg(BH4)2. Int. J. Hydrogen Energy 2011, 36. [Google Scholar] [CrossRef]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.; Zhang, J.Z. Reversibility and Improved Hydrogen Release of Magnesium Borohydride. J. Phys. Chem. C 2010, 114, 5224–5232. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; et al. Hydrogen storage properties of γ–Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrogen Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Chong, M.; Karkamkar, A.; Autrey, T.; Orimo, S.; Jalisatgi, S.; Jensen, C.M. Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem. Commun. 2011, 47, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Rajput, N.N.; Hu, J.; Hu, M.; Liu, T.; Wei, Z.; Gu, M.; Deng, X.; Xu, S.; Han, K.S.; et al. Nanocomposite polymer electrolyte for rechargeable magnesium batteries. Nano Energy 2015, 12, 750–759. [Google Scholar] [CrossRef]
- Tuerxun, F.; Abulizi, Y.; NuLi, Y.; Su, S.; Yang, J.; Wang, J. High concentration magnesium borohydride/tetraglyme electrolyte for rechargeable magnesium batteries. J. Power Sources 2015, 276, 255–261. [Google Scholar] [CrossRef]
- Rajput, N.N.; Qu, X.; Sa, N.; Burrell, A.K.; Persson, K.A. The coupling between stability and ion pair formation in magnesium electrolytes from first-principles quantum mechanics and classical molecular dynamics. J. Am. Chem. Soc. 2015, 137, 3411–3420. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, T.; Li, G.; Gu, M.; Nie, Z.; Engelhard, M.; Xiao, J.; Lv, D.; Wang, C.; Zhang, J.G.; et al. Coordination chemistry in magnesium battery electrolytes: How ligands affect their performance. Sci. Rep. 2013, 3, 3130. [Google Scholar] [CrossRef] [PubMed]
- Deetz, J.D.; Cao, F.; Wang, Q.; Sun, H. Exploring the liquid structure and ion formation in magnesium borohydride electrolyte using density functional theory. J. Electrochem. Soc. 2018, 165, A61–A70. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Wegner, W.; Jaron, T.; Dobrowolski, M.A.; Dobrzycki, L.; Cyranski, M.K.; Grochala, W. Organic derivatives of Mg(BH4)2 as precursors towards MgB2 and novel inorganic mixed-cation borohydrides. Dalton Trans. 2016, 45, 14370–14377. [Google Scholar] [CrossRef]
- Solovev, M.V.; Chashchikhin, O.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Zyubin, A.S.; Zyubina, T.S.; Kravchenko, O.V.; Zaytsev, A.A.; Dobrovolsky, Y.A. Hydrolysis of Mg(BH4)2 and its coordination compounds as a way to obtain hydrogen. J. Power Sources 2018, 377, 93–102. [Google Scholar] [CrossRef]
- Yu, Y.; Baskin, A.; Valero-Vidal, C.; Hahn, N.T.; Liu, Q.; Zavadil, K.R.; Eichhorn, B.W.; Prendergast, D.; Crumlin, E.J. Instability at the Electrode/Electrolyte Interface Induced by Hard Cation Chelation and Nucleophilic Attack. Chem. Mater. 2017, 29, 8504–8512. [Google Scholar] [CrossRef]
- Colombier, M.; Atchekzai, J.; Mongeot, H. Studies of the pyrolysis of tetraethyammonium tetrahydroborate. Inorg. Chimica. Acta 1986, 115, 11–16. [Google Scholar] [CrossRef]
- Makhlouf, J.M.; Hough, M.V.; Hefferan, G.T. Practical synthesis of decahydrodecaborates. Inorg. Chem. 1967, 6, 1196–1198. [Google Scholar] [CrossRef]
- Klanberg, F.; Mutterties, E.L. Chemistry of boranes. XXVII. new polyhedral borane anions, B9H92− and B11H112−. Inorg. Chem. 1966, 5, 1955–1960. [Google Scholar] [CrossRef]
- Miller, H.C.; Miller, N.E.; Muetterties, E.L. Chemistry of boranes. XX. Syntheses of polyhedral boranes. Inorg. Chem. 1964, 3, 1456–1463. [Google Scholar] [CrossRef]
- Titov, L.V.; Eremin, E.R.; Rosolovskii, V.Y. Synthesis and thermal decomposition of the mosolvate of sodium octahydrotriborate with dioxane. Russ. J. Inorg. 1982, 27, 891–895. [Google Scholar]
- Preetz, W.; Peters, G. The hexahydro-closo-hexaborate dianion [B6H6]2− and its derivatives. Eur. J. Inorg. Chem. 1999, 1831–1846. [Google Scholar] [CrossRef]
- Hill, T.G.; Godfroid, R.A., III; White, J.P.; J.P., S.G. Reduction of BH3-THF by alkali metal (K, Rb, Cs) and ytterbium mercury amalgams to form salts of [B3H8]−: A simple procedure for the synthesis of tetraborane(10). Inorg. Chem. 1991, 30, 2952–2954. [Google Scholar] [CrossRef]
- Gigante, A.; Leick, N.; Lipton, A.S.; Tran, B.; Strange, N.A.; Bowden, M.; Martinez, M.B.; Moury, R.; Gennett, T.; Hagemann, H.; et al. Thermal conversion of unsolvated Mg(B3H8)2 to BH4– in the presence of MgH2. ACS Appl. Energy Mater. 2021, 4, 3737–3747. [Google Scholar] [CrossRef]
- Bridges, A.N.; Gaines, D.F. The dianion of nido-decaborane(14), nido-dodecahydrodecaborate(2-), B10H122−, its solution behavior. Inorg. Chem. 1995, 34, 4523–4524. [Google Scholar] [CrossRef]
- David, W.I.F.; Callear, S.K.; Jones, M.O.; Aeberhard, P.C.; Culligan, S.D.; Pohl, A.H.; Johnson, S.R.; Ryan, K.R.; Parker, J.E.; Edwards, P.P.; et al. The structure, thermal properties and phase transformations of the cubic polymorph of magnesium tetrahydroborate. Phys. Chem. Chem. Phys. 2012, 14, 11800–11807. [Google Scholar] [CrossRef] [PubMed]
- Soloveichik, G.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Kniajanski, S.; Bowmanjr, R.; Hwang, S.; Zhao, J. Magnesium borohydride as a hydrogen storage material: Properties and dehydrogenation pathway of unsolvated Mg(BH4)2. Int. J. Hydrogen Energy 2009, 34, 916–928. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Chong, M.; Bowden, M.E.; Wu, H.; Zhou, W.; Nayyar, I.; Ginovska, B.; Gennett, T.; Autrey, T.; Jensen, C.M.; et al. Structural and reorientational dynamics of tetrahydroborate (BH4−) and tetrahydrofuran (THF) in a Mg(BH4)2.3THF adduct: Neutron-scattering characterization. Phys. Chem. Chem. Phys. 2019, 22, 368–378. [Google Scholar] [CrossRef]

| Entry | Additive | Equiv. | T (°C) | B10H102− | B12H122− | B3H8− | Unknown | Conv a | Mass Loss% b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | G1 | 1.0 | 180 | 29 | 1 | 1 | 2 | 33 | 11.5 |
| 2 | G3 | 1.0 | 180 | 10 | 25 | 1 | 3 | 39 | 40.3 |
| 3 | G4 | 1.0 | 180 | 19 | 27 | 1 | 3 | 50 | 53.8 |
| 4 | G1 | 1.5 | 180 | 37 | 14 | − | 2 | 53 | |
| 5 | G1 | 26 | 180 | 16 | 30 | − | 17 | 63 | |
| 6 | G4 | 0.25 | 180 | 15 | 1 | 16 | |||
| 7 | G4 | 54 | 180 | − | − | − | − | 0 | |
| 8 | Me-THF | 1.0 | 180 | 3 | − | 1 | 4 | 7 | |
| 9 | dodecane | 1.0 | 180 | − | − | − | − | 0 | |
| 10 | G1 | 1.0 | 160 | 7 | − | 1 | 3 | 10 | |
| 11 | G4 | 1.0 | 160 | 3 | 8 | 3 | 5 | 19 | |
| 12 | G1 | 1.0 | 200 | 54 | 2 | 2 | 3 | 61 | |
| 13 | G2 | 1.0 | 200 | 5 | 2 | 5 | 5 | 17 | |
| 14 | G4 | 1.0 | 200 | 8 | 36 | 11 | 16 | 61 | |
| 15 | Me-THF | 1.0 | 200 | 22 | 1 | 4 | 8 | 35 | |
| 16 | dodecane | 1.0 | 200 | − | − | − | − | 0 |

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, B.L.; Allen, T.N.; Bowden, M.E.; Autrey, T.; Jensen, C.M. Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2. Inorganics 2021, 9, 41. https://doi.org/10.3390/inorganics9060041
Tran BL, Allen TN, Bowden ME, Autrey T, Jensen CM. Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2. Inorganics. 2021; 9(6):41. https://doi.org/10.3390/inorganics9060041
Chicago/Turabian StyleTran, Ba L., Tamara N. Allen, Mark E. Bowden, Tom Autrey, and Craig M. Jensen. 2021. "Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2" Inorganics 9, no. 6: 41. https://doi.org/10.3390/inorganics9060041
APA StyleTran, B. L., Allen, T. N., Bowden, M. E., Autrey, T., & Jensen, C. M. (2021). Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2. Inorganics, 9(6), 41. https://doi.org/10.3390/inorganics9060041