A Dy(III) Fluorescent Single-Molecule Magnet Based on a Rhodamine 6G Ligand
Abstract
:1. Introduction
2. Results and Discussion
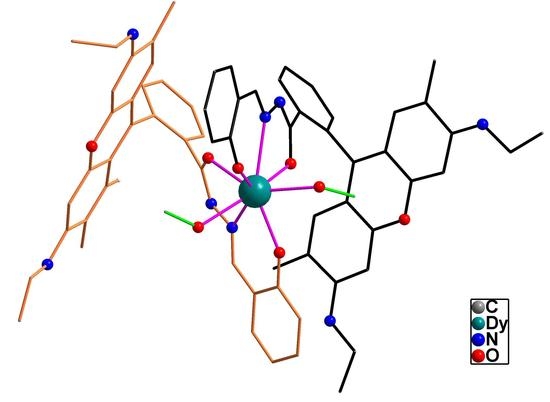
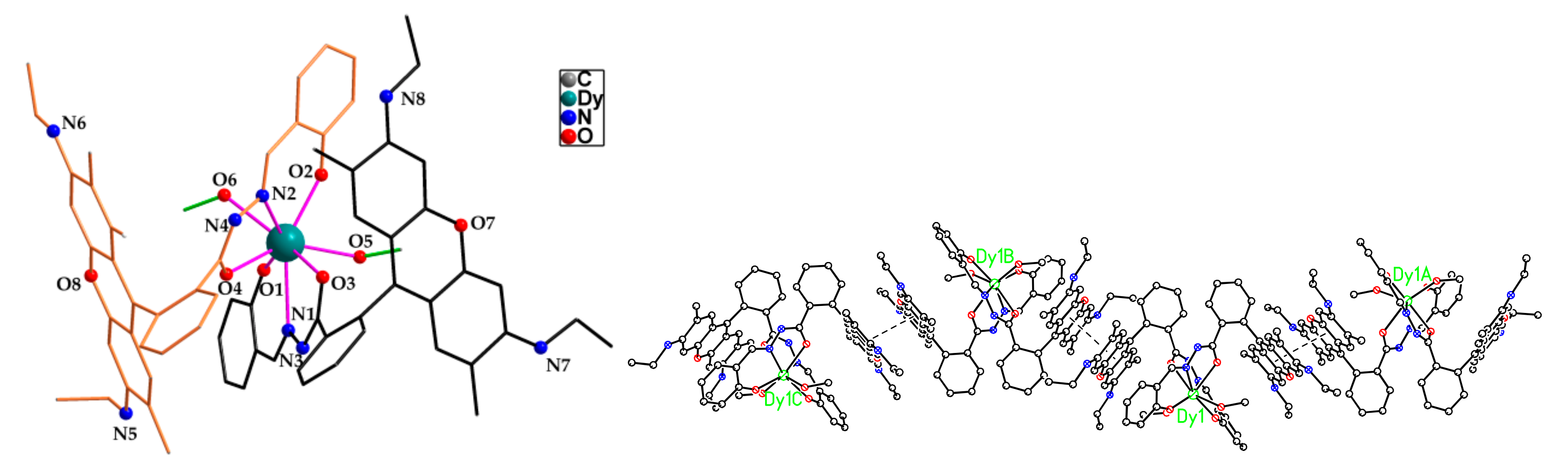
2.1. Crystal Structure
2.2. Solid-State Absorption and Emission Spectra
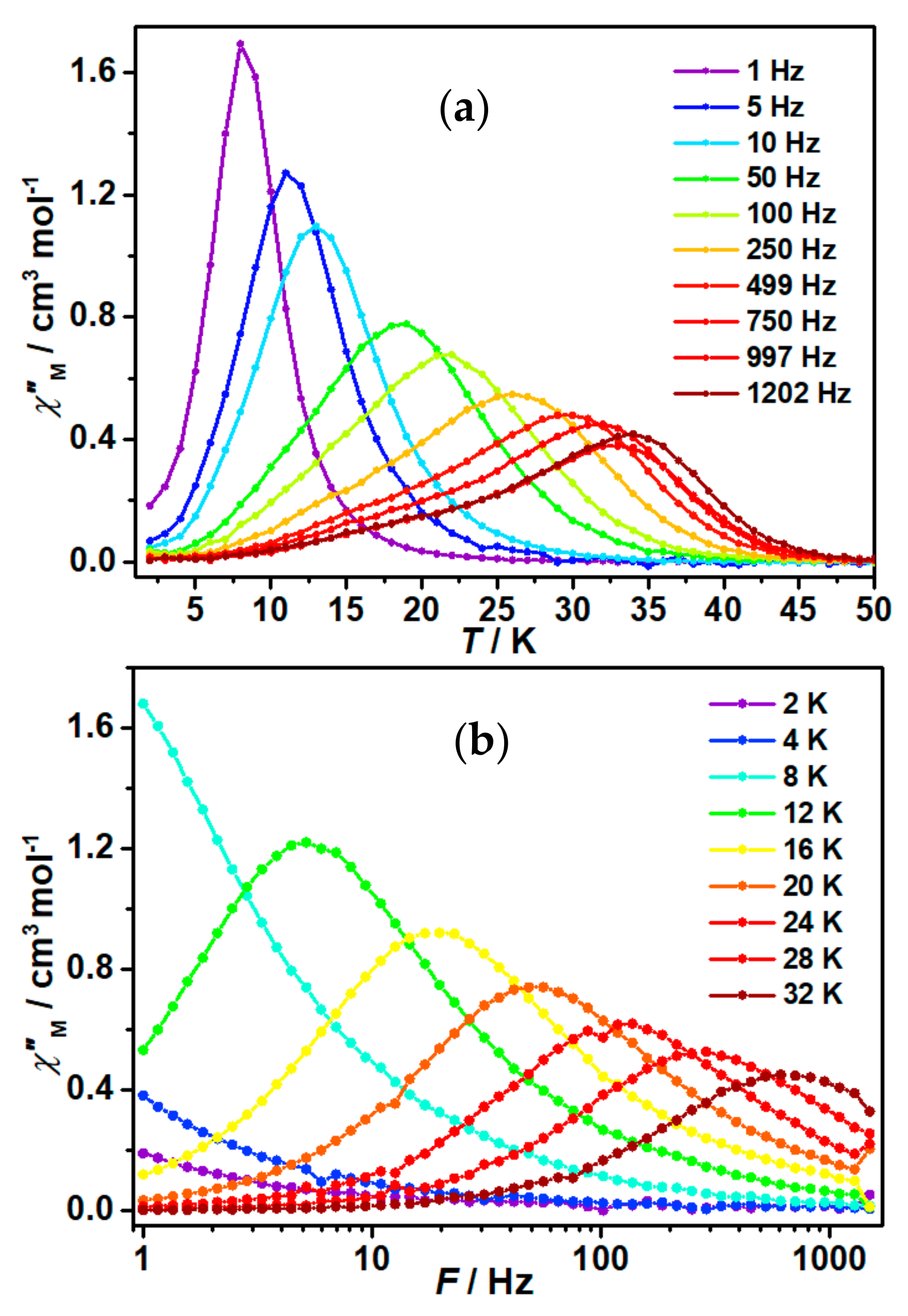
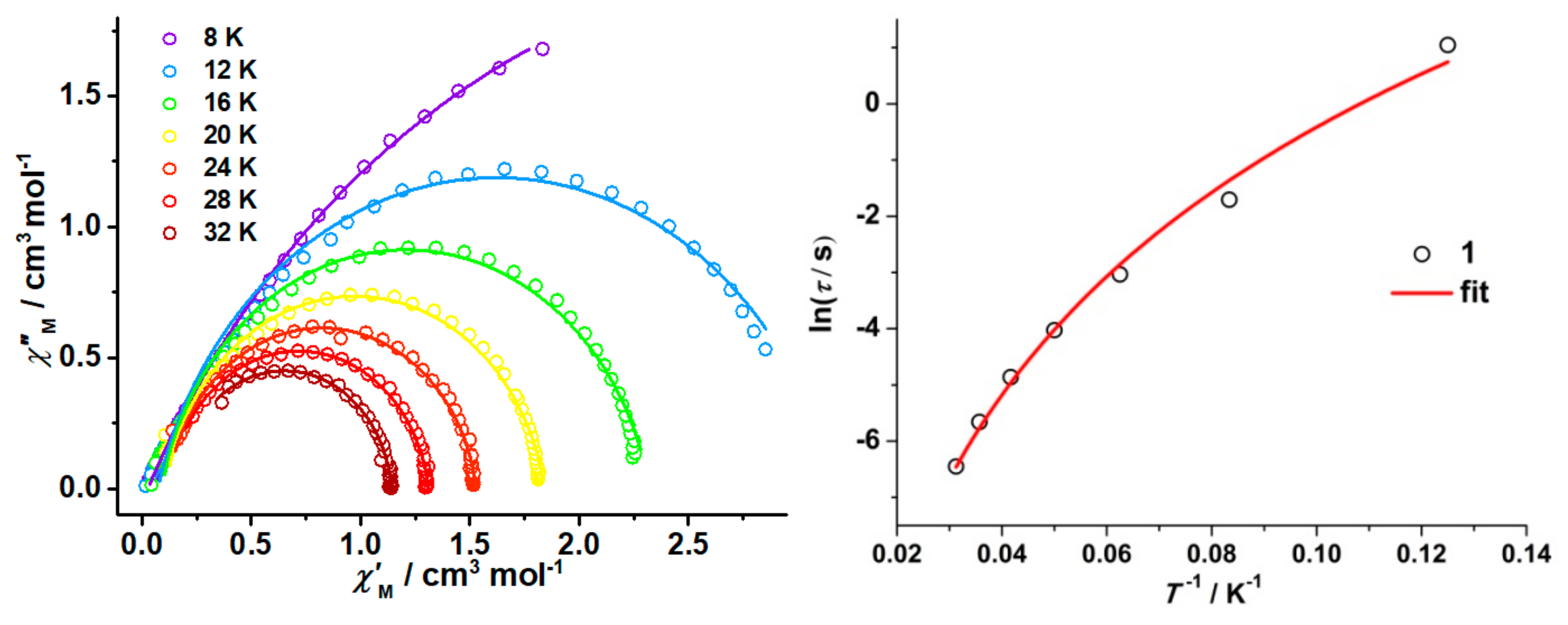
2.3. Magnetic Results
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the [Dy(HL-o)2(MeOH)2](ClO4)3·4.5MeOH (Complex 1)
3.3. Physical Measurements
3.4. Theoretical Computations of Magnetism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leuenberger, M.N.; Loss, D.J.N. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardavan, A.; Rival, O. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett. 2007, 98, 057201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Aubin, S.M.J.; Wemple, M.W.; Adams, D.M.; Tsai, H.-L.; Christou, G.; Hendrickson, D.N. Distorted MnIVMnIII3 Cubane Complexes as Single-Molecule Magnets. J. Am. Chem. Soc. 1996, 118, 7746–7754. [Google Scholar] [CrossRef]
- Feng, M.; Tong, M.-L. Single Ion Magnets from 3d to 5f: Developments and Strategies. Chem. Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Tang, J. Six-Coordinate Ln(III) Complexes with Various Coordination Geometries Showing Distinct Magnetic Properties. Inorganics 2018, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Perlepe, P.S.; Maniaki, D.; Pilichos, E.; Katsoulakou, E.; Perlepes, S.P. Smart Ligands for Efficient 3d-, 4d- and 5d-Metal Single-Molecule Magnets and Single-Ion Magnets. Inorganics 2020, 8, 39. [Google Scholar] [CrossRef]
- Lu, J.; Guo, M.; Tang, J. Recent Developments in Lanthanide Single-Molecule Magnets. Chem. Asian J. 2017, 12, 2772–2779. [Google Scholar] [CrossRef]
- Meng, Y.-S.; Xu, L.; Xiong, J.; Yuan, Q.; Liu, T.; Wang, B.-W.; Gao, S. Low-Coordinate Single-Ion Magnets by Intercalation of Lanthanides into a Phenol Matrix. Angew. Chem. Int. Ed. 2018, 57, 4673–4676. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef]
- Liu, M.-J.; Wu, S.-Q.; Li, J.-X.; Zhang, Y.-Q.; Sato, O.; Kou, H.-Z. Structural Modulation of Fluorescent Rhodamine-Based Dysprosium(III) Single-Molecule Magnets. Inorg. Chem. 2020, 59, 2308–2315. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yuan, J.; Tao, J.; Zhang, Y.-Q.; Liu, C.-M.; Kou, H.-Z. Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes: Fluorescence and Magnetism. Inorg. Chem. 2018, 57, 4061–4069. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, M.-J.; Wu, S.-Q.; Zhu, X.; Zhang, N.; Sato, O.; Kou, H.-Z. Substituent effects on the fluorescent spin-crossover Fe(ii) complexes of rhodamine 6G hydrazones. Inorg. Chem. Front. 2019, 6, 1170–1176. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Luo, Z.-R.; Wang, H.-L.; Zhu, Z.-H.; Liu, T.; Ma, X.-F.; Wang, H.-F.; Zou, H.-H.; Liang, F.-P. Assembly of Dy60 and Dy30 cage-shaped nanoclusters. Commun. Chem. 2020, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Tong, A.; Jin, P.; Ju, Y. New Fluorescent Rhodamine Hydrazone Chemosensor for Cu(II) with High Selectivity and Sensitivity. Org. Lett. 2006, 8, 2863–2866. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Fu, Z.-Y.; Sun, R.; Yuan, J.; Liu, C.-M.; Zou, B.; Wang, B.-W.; Kou, H.-Z. Mechanochromic and Single-Molecule Magnetic Properties of a Rhodamine 6G Dy(III) Complex. ACS Appl. Electron. Mater. 2021, 3, 1368–1374. [Google Scholar] [CrossRef]
- Nakanishi, T.; Hori, Y.; Sato, H.; Wu, S.-Q.; Okazawa, A.; Kojima, N.; Yamamoto, T.; Einaga, Y.; Hayami, S.; Horie, Y.; et al. Observation of Proton Transfer Coupled Spin Transition and Trapping of Photoinduced Metastable Proton Transfer State in an Fe(II) Complex. J. Am. Chem. Soc. 2019, 141, 14384–14393. [Google Scholar] [CrossRef] [PubMed]
- Aquilante, F.; Autschbach, J.; Carlson, R.K.; Chibotaru, L.F.; Delcey, M.G.; De Vico, L.; Galván, I.F.; Ferré, N.; Frutos, L.M.; Gagliardi, L.; et al. Molcas 8: New capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 2016, 37, 506–541. [Google Scholar] [CrossRef] [Green Version]
- Chibotaru, L.F.; Ungur, L.; Soncini, A. The Origin of Nonmagnetic Kramers Doublets in the Ground State of Dysprosium Triangles: Evidence for a Toroidal Magnetic Moment. Angew. Chem. Int. Ed. 2008, 47, 4126–4129. [Google Scholar] [CrossRef] [Green Version]
- Ungur, L.; Van den Heuvel, W.; Chibotaru, L.F. Ab initio investigation of the non-collinear magnetic structure and the lowest magnetic excitations in dysprosium triangles. New J. Chem. 2009, 33, 1224–1230. [Google Scholar] [CrossRef]
- Chibotaru, L.F.; Ungur, L.; Aronica, C.; Elmoll, H.; Pilet, G.; Luneau, D. Structure, Magnetism, and Theoretical Study of a Mixed-Valence CoII3CoIII4 Heptanuclear Wheel: Lack of SMM Behavior despite Negative Magnetic Anisotropy. J. Am. Chem. Soc. 2008, 130, 12445–12455. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Ding, M.M.; Li, J.X.; Wang, B.L.; Zhang, Y.Q. Why lanthanide ErIII SIMs cannot possess huge energy barriers: A theoretical investigation. Dalton Trans. 2020, 49, 14576–14583. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]



| Formula | C72.5H90Cl3DyN8O24.5 |
| Formula weight | 1734.37 |
| T/K | 100 |
| Crystal system | triclinic |
| Space group | P1− |
| a/Å | 14.2953(4) |
| b/Å | 14.6326(4) |
| c/Å | 19.3797(5) |
| α/° | 79.470(2) |
| β/° | 85.124(2) |
| γ/° | 76.571(2) |
| V/Å3 | 3872.73(18) |
| Z | 2 |
| ρcalc/g·cm−3 | 1.487 |
| µ/mm−1 | 6.808 |
| F(000) | 1788 |
| Radiation | CuKα (λ = 1.54184) |
| Data/restraints/parameters | 15,829/12/1012 |
| GOF on F2 | 1.010 |
| Rint, Rsigma | 0.0580, 0.0924 |
| R1,wR2 [I > 2σ(I)] | 0.0670, 0.1712 |
| R1,wR2 (all data) | 0.0824, 0.1849 |
| CCDC | 2,008,107 |
| Dy-O1 | 2.181(4) | Dy-O2 | 2.258(3) |
| Dy-O3 | 2.386(4) | Dy-O4 | 2.384(4) |
| Dy-O5 | 2.398(4) | Dy-O6 | 2.372(4) |
| Dy-N1 | 2.570(5) | Dy-N2 | 2.566(5) |
| O1-Dy-O2 | 115.77(13) | O5-Dy1-O6 | 133.27(13) |
| C8-N3-N1 | 117.7(4) | C41-N4-N2 | 116.0(5) |
| Complexes | Dy-Ophenoxy/Å | Ophenoxy-Dy-Ophenoxy/° | EKD1 (Ueff)/K | Ref. |
|---|---|---|---|---|
| [Dy(L1)3] | 2.224(5) | 82.7(2) | 284.2 (104) | [17] |
| 2.263(5) | 89.45(19) | |||
| 2.244(5) | 148.64(17) | |||
| [Dy(L2)3] | 2.248(3) | 87.69(12) | 23.3 (0) | [17] |
| 2.223(3) | 89.63(13) | |||
| 2.222(4) | 88.12(13) | |||
| [Dy(L-c)2(MeOH)2]ClO4 | 2.170(3) | 96.96(12) | 160.8 (4.8) | [22] |
| 2.209(4) | ||||
| [Dy(HL-o)2(MeOH)2](ClO4)3 (1) | 2.181(4) | 115.77(13) | 365.8 (Raman relaxation) | This Work |
| 2.258(3) | ||||
| [Dy(HL-o)(H2O)4(MeCN)](ClO4)3 | 2.175(3) | No | 320.8 (320) | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, L.; Liu, M.-J.; Ding, M.-M.; Zhang, Y.-Q.; Kou, H.-Z. A Dy(III) Fluorescent Single-Molecule Magnet Based on a Rhodamine 6G Ligand. Inorganics 2021, 9, 51. https://doi.org/10.3390/inorganics9070051
Miao L, Liu M-J, Ding M-M, Zhang Y-Q, Kou H-Z. A Dy(III) Fluorescent Single-Molecule Magnet Based on a Rhodamine 6G Ligand. Inorganics. 2021; 9(7):51. https://doi.org/10.3390/inorganics9070051
Chicago/Turabian StyleMiao, Lin, Mei-Jiao Liu, Man-Man Ding, Yi-Quan Zhang, and Hui-Zhong Kou. 2021. "A Dy(III) Fluorescent Single-Molecule Magnet Based on a Rhodamine 6G Ligand" Inorganics 9, no. 7: 51. https://doi.org/10.3390/inorganics9070051
APA StyleMiao, L., Liu, M.-J., Ding, M.-M., Zhang, Y.-Q., & Kou, H.-Z. (2021). A Dy(III) Fluorescent Single-Molecule Magnet Based on a Rhodamine 6G Ligand. Inorganics, 9(7), 51. https://doi.org/10.3390/inorganics9070051