Home-Applied Dual-Light Photodynamic Therapy in the Treatment of Stable Chronic Periodontitis (HOPE-CP)—Three-Month Interim Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
2.3. Eligibility Criteria for Study Participants
2.3.1. Inclusion Criteria
- Periodontal disease stage I–III, according to criteria the American Academy of Periodontology (AAP) with at least 2 mm interdental clinical attachment level (CAL) in the site of greatest loss;
- Age of 18–85 years;
- Presence of ≥20 teeth;
- Agreement to participate in the study and to sign a written consent form.
2.3.2. Exclusion Criteria
- Untreated/uncontrollable diabetes mellitus (DM) with HbA1c ≥ 7% and HbA1c ≥ 8 if insulin-treated DM.
- Any systemic disease (e.g., wound healing dysfunctions) that could alter the progression of periodontal disease.
- Use of medicine that would affect the periodontal tissue within the last six months (antibiotics, anti-inflammatories, anticonvulsants, immunosuppressants, or calcium channel blockers, including doxycycline, bisphosphonates, and chlorhexidine).
- Periodontal treatment during the previous three months.
- Allergic to photosensitizer.
- Presence of significant physical limitations or restrictions that prohibit the hygiene procedures used in the study protocol.
- Removable major prosthesis or major orthodontic appliance.
- Current smoking or habitual use of smokeless tobacco products.
- Pregnancy or lactation.
- A need for hopeless tooth extraction or open cavities in need of immediate endodontic treatment.
2.4. Randomization
2.5. Intra-Examiner Reproducibility
2.6. Clinical Procedure
2.7. Anti-Infective Treatment
2.8. Clinical Measurements
2.9. Dual-Light aPDT Treatment
2.10. Compliance and Adverse Events Reporting
2.11. Statistical Analysis
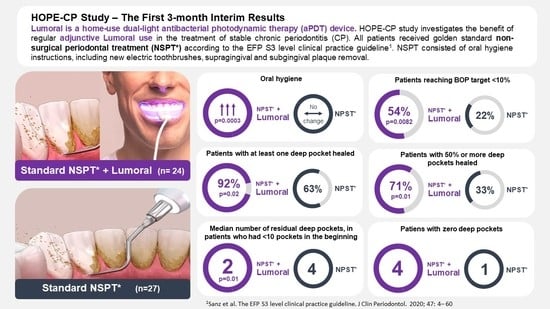
3. Results
3.1. Demographic Characteristics of the Patient Population
3.2. Bleeding on Probing (BOP)
3.3. Plaque Index
3.4. Periodontal Pockets
3.5. Compliance and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S5–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, K.; Goslinski, T. Photodynamic Therapy in Dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef]
- Lins de Sousa, D.; Araújo Lima, R.; Zanin, I.C.; Klein, M.I.; Janal, M.N.; Duarte, S. Effect of Twice-Daily Blue Light Treatment on Matrix-Rich Biofilm Development. PLoS ONE 2015, 10, e0131941. [Google Scholar] [CrossRef]
- Herrera, D. Insufficient evidence for photodynamic therapy use in periodontitis. Evid.-Based Dent. 2011, 12, 46. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.-L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef]
- Lähteenmäki, H.; Pätilä, T.; Räisänen, I.T.; Kankuri, E.; Tervahartiala, T.; Sorsa, T. Repeated Home-Applied Dual-Light Antibacterial Photodynamic Therapy Can Reduce Plaque Burden, Inflammation, and aMMP-8 in Peri-Implant Disease—A Pilot Study. Curr. Issues Mol. Biol. 2022, 44, 1273–1283. [Google Scholar] [CrossRef]
- Giannelli, M.; Materassi, F.; Fossi, T.; Lorenzini, L.; Bani, D. Treatment of severe periodontitis with a laser and light-emitting diode (LED) procedure adjunctive to scaling and root planing: A double-blind, randomized, single-center, split-mouth clinical trial investigating its efficacy and patient-reported outcomes at 1 year. Lasers Med. Sci. 2018, 33, 991–1002. [Google Scholar] [CrossRef]
- Giannelli, M.; Formigli, L.; Lorenzini, L.; Bani, D. Efficacy of Combined Photoablative-Photodynamic Diode Laser Therapy Adjunctive to Scaling and Root Planing in Periodontitis: Randomized Split-Mouth Trial with 4-Year Follow-Up. Photomed. Laser Surg. 2015, 33, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, S.; Alapulli, H.; Auvinen, P.; Vaara, M.; Rantala, J.; Kankuri, E.; Sorsa, T.; Meurman, J.; Pätilä, T. Dual-light photodynamic therapy administered daily provides a sustained antibacterial effect on biofilm and prevents Streptococcus mutans adaptation. PLoS ONE 2020, 15, e0232775. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, S.; Moilanen, N.; Sorsa, T.; Rantala, J.; Alapulli, H.; Kotiranta, A.; Auvinen, P.; Kankuri, E.; Meurman, J.; Pätilä, T. Indocyanine Green-Assisted and LED-Light-Activated Antibacterial Photodynamic Therapy Reduces Dental Plaque. Dent. J. 2021, 9, 52. [Google Scholar] [CrossRef]
- Nikinmaa, S.; Podonyi, A.; Raivio, P.; Meurman, J.; Sorsa, T.; Rantala, J.; Kankuri, E.; Tauriainen, T.; Pätilä, T. Daily Administered Dual-Light Photodynamic Therapy Provides a Sustained Antibacterial Effect on Staphylococcus aureus. Antibiotics 2021, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Trujiilo, K.; Räisänen, I.T.; Sorsa, T.; Pätilä, T. Repeated Daily Use of Dual-Light Antibacterial Photodynamic Therapy in Periodontal Disease—A Case Report. Dent. J. 2022, 10, 163. [Google Scholar] [CrossRef]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing An indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Schär, D.; Ramseier, C.A.; Eick, S.; Mettraux, G.; Salvi, G.E.; Sculean, A. Transgingival photodynamic therapy (tg-aPDT) adjunctive to subgingival mechanical instrumentation in supportive periodontal therapy. A randomized controlled clinical study. Photodiagnosis Photodyn. Ther. 2020, 32, 101971. [Google Scholar] [CrossRef]
- Joshi, K.; Baiju, C.S.; Khashu, H.; Bansal, S. Clinical effectiveness of indocyanine green mediated antimicrobial photodynamic therapy as an adjunct to scaling root planing in treatment of chronic periodontitis-A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2019, 29, 101591. [Google Scholar] [CrossRef]
- Monzavi, A.; Chinipardaz, Z.; Mousavi, M.; Fekrazad, R.; Moslemi, N.; Azaripour, A.; Bagherpasand, O.; Chiniforush, N. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: A randomized clinical trial. Photodiagn. Photodyn. Ther. 2016, 14, 93–97. [Google Scholar] [CrossRef]
- Chiang, C.-P.; Hsieh, O.; Tai, W.-C.; Chen, Y.-J.; Chang, P.-C. Clinical outcomes of adjunctive indocyanine green-diode lasers therapy for treating refractory periodontitis: A randomized controlled trial with in vitro assessment. J. Formos. Med. Assoc. 2019, 119, 652–659. [Google Scholar] [CrossRef]
- Gandhi, K.K.; Pavaskar, R.; Cappetta, E.G.; Drew, H.J. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int. J. Periodontics Restor. Dent. 2019, 39, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Aass, A.M.; Aimetti, M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Hentilä, J.; Laakamaa, N.; Sorsa, T.; Meurman, J.; Välimaa, H.; Nikinmaa, S.; Kankuri, E.; Tauriainen, T.; Pätilä, T. Dual-Light Photodynamic Therapy Effectively Eliminates Streptococcus Oralis Biofilms. J. Pharm. Pharm. Sci. 2021, 24, 484–487. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, M.G.; de Carvalho, V.F.; Godoy-Miranda, B.A.; Kassa, C.T.; Horliana, A.C.R.T.; Prates, R.A. Efficacy of antimicrobial photodynamic therapy (aPDT) for nonsurgical treatment of periodontal disease: A systematic review. Lasers Med. Sci. 2021, 36, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.Z.; Singh, H.-A.; Virdee, S.S. Indocyanine green–mediated antimicrobial photodynamic therapy as an adjunct to periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 5699–5710. [Google Scholar] [CrossRef]
- Sculean, A.; Deppe, H.; Miron, R.; Schwarz, F.; Romanos, G.; Cosgarea, R. Effectiveness of photodynamic therapy in the treatment of periodontal and peri-implant diseases. Oral Biofilms 2021, 29, 133–143. [Google Scholar] [CrossRef]
- Dalvi, S.; Benedicenti, S.; Hanna, R. Effectiveness of Photobiomodulation as an Adjunct to Nonsurgical Periodontal Therapy in the Management of Periodontitis—A Systematic Review of in vivo Human Studies. Photochem. Photobiol. 2020, 97, 223–242. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Low-Level Laser Therapy for Preventing or Treating Oral Mucositis Caused by Radiotherapy or Chemotherapy. NICE Interventional Procedures Guidance [IPG615]. 2018. Available online: https://www.nice.org.uk/guidance/ipg615 (accessed on 23 May 2018).
- Peng, H.; Chen, B.-B.; Chen, L.; Chen, Y.-P.; Liu, X.; Tang, L.-L.; Mao, Y.-P.; Li, W.-F.; Zhang, Y.; Lin, A.-H.; et al. A network meta-analysis in comparing prophylactic treatments of radiotherapy-induced oral mucositis for patients with head and neck cancers receiving radiotherapy. Oral Oncol. 2017, 75, 89–94. [Google Scholar] [CrossRef]
- Figuero, E.; Roldán, S.; Serrano, J.; Escribano, M.; Martín, C.; Preshaw, P.M. Efficacy of adjunctive therapies in patients with gingival inflammation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 47, 125–143. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakarinen, S.; Saarela, R.K.T.; Välimaa, H.; Heikkinen, A.M.; Kankuri, E.; Noponen, M.; Alapulli, H.; Tervahartiala, T.; Räisänen, I.T.; Sorsa, T.; et al. Home-Applied Dual-Light Photodynamic Therapy in the Treatment of Stable Chronic Periodontitis (HOPE-CP)—Three-Month Interim Results. Dent. J. 2022, 10, 206. https://doi.org/10.3390/dj10110206
Pakarinen S, Saarela RKT, Välimaa H, Heikkinen AM, Kankuri E, Noponen M, Alapulli H, Tervahartiala T, Räisänen IT, Sorsa T, et al. Home-Applied Dual-Light Photodynamic Therapy in the Treatment of Stable Chronic Periodontitis (HOPE-CP)—Three-Month Interim Results. Dentistry Journal. 2022; 10(11):206. https://doi.org/10.3390/dj10110206
Chicago/Turabian StylePakarinen, Saila, Riitta K. T. Saarela, Hannamari Välimaa, Anna Maria Heikkinen, Esko Kankuri, Marja Noponen, Heikki Alapulli, Taina Tervahartiala, Ismo T. Räisänen, Timo Sorsa, and et al. 2022. "Home-Applied Dual-Light Photodynamic Therapy in the Treatment of Stable Chronic Periodontitis (HOPE-CP)—Three-Month Interim Results" Dentistry Journal 10, no. 11: 206. https://doi.org/10.3390/dj10110206
APA StylePakarinen, S., Saarela, R. K. T., Välimaa, H., Heikkinen, A. M., Kankuri, E., Noponen, M., Alapulli, H., Tervahartiala, T., Räisänen, I. T., Sorsa, T., & Pätilä, T. (2022). Home-Applied Dual-Light Photodynamic Therapy in the Treatment of Stable Chronic Periodontitis (HOPE-CP)—Three-Month Interim Results. Dentistry Journal, 10(11), 206. https://doi.org/10.3390/dj10110206