Periodontal Disease in Obese Patients; Interleukin-6 and C-Reactive Protein Study: A Systematic Review
Abstract
:1. Introduction
1.1. Hormones
1.2. Obesity-Related Diseases
2. Methods
2.1. Protocol and Registration
2.2. Search and Selection of Evidence
2.3. Eligibility Criteria
2.4. Data Elements
2.5. Assessment of Methodological Quality and Risk of Bias
2.6. Summary of Measures
2.7. Synthesis of the Results
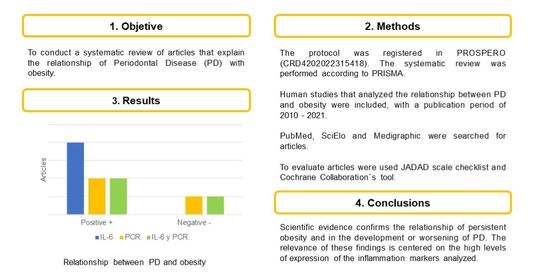
3. Results
3.1. Selection of Studies
3.2. Assessment of Risk of Bias of the Studies
3.3. Characteristics of the Study
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Girano-Castaños, J.; Robello-Malatto, J. Relationship between obesity and periodontal disease: A literature review. Horiz. Med. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Toker, H.; Gorgun, E.P.; Korkmaz, E.M.; Yüce, H.B.; Poyraz, O. The effects of IL-10 gene polymorphism on serum, and gingival crevicular fluid levels of IL-6 and IL-10 in chronic periodontitis. J. Appl. Oral Sci. 2018, 26, e20170232. [Google Scholar] [CrossRef] [PubMed]
- Silva-Boghossian, C.M.; Dezonn, R.S. What Are the Clinical and Systemic Results of Periodontitis Treatment in Obese Individuals? Curr. Oral Health Rep. 2021, 8, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Bascones-Martinez, A.; Figuero-Ruiz, E. Las enfermedades periodontales como infecciones bacterianas. Av. Periodoncia 2005, 17, 147–156. [Google Scholar] [CrossRef]
- Martínez-Aguilar, V.; Carrillo-Ávila, B.A.; Guzmán-Marín, E.; Puerto-Solís, M.; Bermeo-Escalona, J.R.; Pozos-Guillén, A. C reactive protein as an inflammatory marker in periodontal disease. Nova Sci. 2017, 19, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 20, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment ap paratus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, 171–189. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Puetate, J.C.; Garcia-De Carvalho, G.; Spin, J.R. New Classification of Periodontal and Peri-implant Diseases and Conditions: A Brief Review. Dentistry 2018, 20, 70–89. [Google Scholar] [CrossRef]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Periodontol. 2018, 1, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Zerón, A. New classification of periodontal diseases. ADM 2018, 75, 122–124. [Google Scholar]
- Srivastava, M.C.; Srivastava, R.; Verma, P.K.; Gautam, A. Metabolic syndrome and periodontal disease: An overview for physicians. J. Fam. Med. Prim. Care 2019, 8, 3492–3495. [Google Scholar] [CrossRef]
- Izaola, O.; Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflamación y obesidad (lipoinflamación). Nutr. Hosp. 2015, 31, 1–7. [Google Scholar]
- Bray, G.A. Evaluation of obesity. Who are the obese? Postgrad. Med. 2003, 114, 19–27. [Google Scholar] [CrossRef]
- Khaodhiar, L.; Ling, P.R.; Blackburn, G.L.; Bistrian, B.R. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J. Parenter. Enteral. Nutr. 2004, 28, 410–415. [Google Scholar] [CrossRef]
- Cedeño-Morales, R.; Castellanos-González, M.; Benet-Rodríguez, M.; Mass-Sosa, L.; Mora-Hernández, C.; Parada-Arias, J. Anthropometric Indicators to Determine the Obesity and its Relations with the Cardiometabolic Risk. Rev. Enferm. No Transm. Finlay 2015, 5, 12–23. [Google Scholar]
- Moehlecke, M.; Canani, L.H.; Silva, L.O.; Trindade, M.R.; FriedmanR, L.C.B. Determinants of body weight regulation in humans. Arch. Endocrinol. Metab. 2016, 60, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Pausova, Z.; Gossard, F.; Gaudet, T.J.; Cocina, T.A.; Cowley, A.W.; Hamet, P. Heritability estimates of obesity measures in siblings with and without hypertension. Hypertension 2001, 38, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Arner, P.; Atkinson, R.L.; Spiegelman, B.M. Differential Regulation of the p80 Tumor Necrosis Factor Receptor in Human Obesity and Insulin Resistance. Diabetes 1997, 46, 451–455. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ezquerro, S.; Mendez-Gimenez, L.; Becerril, S.; Fruhbeck, G. Revisiting the Adipocyte: A Model for Integration of Cytokine Signaling in the Regulation of Energy Metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, 691–714. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, B.; Gan, X.Q.; Zhang, L.; He, Y.; Liu, B.; Chen, X.; Zhang, S.; Yu, H. Association of circulating leptin and adiponectin with periodontitis: A systematic review and meta-analysis. BMC Oral Health 2017, 17, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, T.E.; Zimmermann, G.S.; Figueiredo, L.C.; Souza Mde, C.; da Cruz, D.F.; Bastos, M.F.; da Silva, H.D.; Duarte, P.M. Local and serum levels of adipokines in patients with obesity after periodontal therapy: One-year follow-up. J. Clin. Periodontol. 2015, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Vielma, S.A.; Klein, R.L.; Levingston, C.A.; Young, M.R. Adipocytes as immune regulatory cells. Int. Immunopharmacol. 2013, 16, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, M.; Van Rijswijk, M.H. Acute phase proteins in monitoring inflammatory disorders. Bailliere’s Clin. Rheumatol. 1994, 8, 531–552. [Google Scholar] [CrossRef]
- Ramírez-Alvarado, M.M.; Sánchez-Roitz, C. Relation of serum levels of C-reactive protein to anthropometric meaurements; a sustematic review of studies in South America. Nutr. Hosp. 2012, 27, 971–977. [Google Scholar] [CrossRef]
- Suvan, J.E.; Finer, N.; D’Aiuto, F. Periodontal complications with obesity. Periodontol. 2000 2018, 78, 98–128. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.; Gonzalez-Juanatey, C.; Piniero, A.; Garcia-Porrua, C.; Testa, A.; Llorca, A. High-grade C-reactive protein elevation correlatos with accelerated atherogenesis in patients with rheumatoid artritis. J. Rheumathol. 2005, 32, 1219–1235. [Google Scholar]
- Deschner, J.; Eick, S.; Damanaki, A.; Nokhbehsaim, M. The role of adipokines in periodontal infection and healing. Mol. Oral Microbiol. 2014, 29, 258–269. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- PROSPERO. International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/logout.php (accessed on 12 April 2022).
- Jadad, A.R.; Moore, R.A.; Carrol, D.; Jenkinson, C.; Reynolds, D.J.; Gavagan, D.J.; HcQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-Of-Bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- De Castilhos, E.D.; Horta, B.L.; Gigante, D.P.; Demarco, F.F.; Peres, K.G.; Peres, M.A. Association between obesity and periodontal disease in young adults: A population-based birth cohort. J. Clin. Periodontol. 2012, 39, 717–724. [Google Scholar] [CrossRef]
- López, N.J.; Quintero, A.; Casanova, P.A.; Ibieta, C.I.; Baelum, V.; López, R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: A controlled clinical trial. J. Periodontol. 2012, 83, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.S.; Bastos, M.F.; Dias Gonçalves, T.E.; Chambrone, L.; Duarte, P.M. Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. J. Periodontol. 2013, 84, 624–633. [Google Scholar] [CrossRef]
- Doğan, G.E.; Toraman, A.; Şebin, S.Ö.; Doğan, Ç.; Güngör, A.; Aksoy, H.; Asutay, H. Salivary IL-6 and IL-10 levels in subjects with obesity and gingivitis. Am. J. Dent. 2016, 29, 261–265. [Google Scholar]
- Altay, U.; Gürgan, C.A.; Ağbaht, K. Changes in inflammatory and metabolic parameters after periodontal treatment in patients with and without obesity. J. Periodontol. 2013, 84, 13–23. [Google Scholar] [CrossRef]
- Buduneli, N.; Bıyıkoğlu, B.; Ilgenli, T.; Buduneli, E.; Nalbantsoy, A.; Saraç, F.; Kinane, D.F. Is obesity a possible modifier of periodontal disease as a chronic inflammatory process? A case-control study. J. Periodontal Res. 2014, 49, 465–471. [Google Scholar] [CrossRef]
- Balli, U.; Ongoz-Dede, F.; Bozkurt-Dogan, S.; Gulsoy, Z.; Sertoglu, E. Chemerin and interleukin-6 levels in obese individuals following periodontal treatment. Oral Dis. 2016, 22, 673–680. [Google Scholar] [CrossRef]
- Li, Z.; Lu, C.; Qiu, J.; Liu, S.; Liu, X.; Ma, S.; Lai, R. Correlation of serum adipocytokine levels with glycolipid metabolism and inflammatory factors in obese patients with periodontal disease. Int. J. Clin. Exp. Pathol. 2018, 11, 1620–1628. [Google Scholar]
- Wanichkittikul, N.; Laohapand, P.; Mansa-Nguan, C.; Thanakun, S. Periodontal Treatment Improves Serum Levels of Leptin, Adiponectin, and C-Reactive Protein in Thai Patients with Overweight or Obesity. Int. J. Dent. 2021, 2021, 6660097. [Google Scholar] [CrossRef]
- Seymour, G.J.; Gemmell, E. Cytokines in periodontal disease: Where to from here? Acta Odontol. Scand. 2001, 59, 167–173. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, S.N. A Population-Based Study on the Association between Periodontal Disease and Major Lifestyle-Related Comorbidities in South Korea: An Elderly Cohort Study from 2002–2015. Medicina 2020, 56, 575. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Carreira, M.C.; Cabia, B.; Andrade, S.; Amil, M.; Casanueva, F.F. Leptin Resistance in Obesity: An Epigenetic Landscape. Life Sci. 2015, 140, 57–63. [Google Scholar] [CrossRef]
- Perlstein, M.I.; Bissada, N.F. Influence of obesity and hypertension on the severity of periodontitis in rats. Oral Surg. Oral Med. Oral Pathol. 1977, 43, 707–719. [Google Scholar] [CrossRef]
- Suvan, J.; D’Aiuto, F.; Moles, D.R.; Petrie, A.; Donos, N. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes. Rev. 2011, 12, 381–404. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Park, J.Y.; Choi, J.K.; Kim, D.W.; Kim, Y.T.; Choi, S.H. Association of Lifestyle-Related Comorbidities With Periodontitis: A Nationwide Cohort Study in Korea. Medicine 2015, 94, e1567. [Google Scholar] [CrossRef]
- Ngoude, J.X.E.; Moor, V.J.A.; Nadia-Flore, T.T.; Agoons, B.B.; Marcelle, G.G.C.; MacBrain, E.E.; Tcheutchoua, D.N.; Nkeck, J.R. Relationship between periodontal diseases and newly-diagnosed metabolic syndrome components in a sub-Saharan population: A cross sectional study. BMC Oral Health 2021, 21, 326. [Google Scholar] [CrossRef]
- Manco, M.; Fernandez-Real, J.M.; Equitani, F.; Vendrell, J.; Valera Mora, M.E.; Nanni, G.; Tondolo, V.; Calvani, M.; Ricart, W.; Castagneto, M.; et al. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J. Clin. Endocrinol. Metab. 2007, 92, 483–490. [Google Scholar] [CrossRef]
- Karthikeyan, B.V.; Pradeep, A.R. Leptin levels in gingival crevicular fluid in periodontal health and disease. J. Periodontal Res. 2007, 42, 300–304. [Google Scholar] [CrossRef]
- Syndergaard, B.; Al-Sabbagh, M.; Kryscio, R.J.; Xi, J.; Ding, X.; Ebersole, J.L.; Miller, C.S. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014, 85, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Teles, R.P.; Likhari, V.; Socransky, S.S.; Haffajee, A.D. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: A cross-sectional study. J. Periodontal Res. 2009, 44, 411–417. [Google Scholar] [CrossRef]



| AUTHOR, YEAR | Was the Study a Controlled Experimental Study? | Was There a Clear Description of the Inclusion and Exclusion Criteria? | Was the Method Used in the Clinical Trial Adequate? | Was There at Least One Control (Comparison) Group? | Were the Statistical Analysis Methods Described? | Were the Outcome Measures Clearly Defined? | Another Bias | JADAD Scale | Methodological Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes = 1 | Not = 0 | Yes = 1 | Not = 0 | Yes = 0 | Not = −1 | Yes = 0 | Not = −1 | Yes = 1 | Not = 0 | Yes = 1 | Not = 0 | Yes = 1 | Not = 0 | Points | Good Quality +3 Poor Quality −3 | |
| 1. De Castilhos ED et al., 2012 [34] | 1 | 0 | 0 | −1 | 1 | 1 | 1 | 3 | Good quality | |||||||
| 2. López NJ et al., 2012 [35] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 3. Zimmermann GS et al., 2013 [36] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 4. Doğan GE et al., 2016 [37] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 5. Altay U et al., 2013 [38] | 1 | 1 | 0 | −1 | 1 | 1 | 1 | 4 | Good quality | |||||||
| 6. Buduneli N et al., 2014 [39] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 7. Gonçalves TED et al., 2015 [22] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 8. Balli U et al., 2016 [40] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 9. Li Z et al., 2018 [41] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Good quality | |||||||
| 10. Wanichkittikul N et al., 2021 [42] | 1 | 1 | 0 | 0 | −1 | 1 | 1 | 1 | 4 | Good quality | ||||||
| Author, Year | Country | Type of Study | Article Title | Journal of Publication | Inflammation Marker |
|---|---|---|---|---|---|
| 1. De Castilhos ED et al., 2012 [34] | Brazil | Cohort | Association between obesity and periodontal disease in young adults: a population-based birth cohort. | J. Clin. Periodontol. | CRP |
| 2. López NJ et al., 2012 [35] | Chile | Randomized double blind | Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. | J. Periodontol. | PCR |
| 3. Zimmermann GS et al., 2013 [36] | Brazil | Cases and controls | Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. | J. Periodontol. | IL-6 |
| 4. Doğan GE et al., 2016 [37] | Turkey | Cases and controls | Salivary IL-6 and IL-10 levels in subjects with obesity and gingivitis. | Am. J. Dent. | IL-6 |
| 5. Altay U et al., 2013 [38] | Turkey | Cases and controls | Changes in inflammatory and metabolic parameters after periodontal treatment in patients with and without obesity. | J. Periodontol. | IL-6 CRP |
| 6. Buduneli N et al., 2014 [39] | Turkey | Cases and controls | Is obesity a possible modifier of periodontal disease as a chronic inflammatory process? A case-control study. | J. Periodontal. Res. | IL-6 CRP |
| 7. Gonçalves TE et al., 2015 [22] | Brazil | Cases and controls | Local and serum levels of adipokines in patients with obesity after periodontal therapy: one-year follow-up. | J. Clin. Periodontol. | IL-6 |
| 8. Balli U et al., 2016 [40] | Turkey | Cases and controls | Chemerin and interleukin-6 levels in obese individuals following periodontal treatment. | Oral Dis. | IL-6 |
| 9. Li Z et al., 2018 [41] | China | Cases and controls | Correlation of serum adipocytokine levels with glycolipid metabolism and inflammatory factors in obese patients with periodontal disease. | Int. J. Clin. Exp. Pathol. | IL-6 CRP |
| 10. Wanichkittikul N et al., 2021 [42] | Thailand | Observational | Periodontal Treatment Improves Serum Levels of Leptin, Adiponectin, and C-reactive Protein in Thai Patients with Overweight or Obesity. | Int. J. Dent. | CRP |
| Articles, Year | Sex (M/F) | Age Range | Cases | Controls | Total Population |
|---|---|---|---|---|---|
| 1. De Castilhos ED et al., 2012 [34] | 36/22 | 23 | 58 | 0 | 58 |
| 2. López NJ et al., 2012 [35] | 46/119 | 35–65 | 82 | 83 | 165 |
| 3. Zimmermann GS et al., 2013 [36] | 21/57 | 31–65 | 58 | 20 | 78 |
| 7. Doğan GE et al., 2016 [37] | 19/21 | 20–55 | 20 | 20 | 40 |
| 4. Altay U et al., 2013 [38] | 14/32 | >25 | 22 | 24 | 46 |
| 5. Buduneli N et al., 2014 [39] | 0/91 | >41 | 60 | 31 | 91 |
| 6. Gonçalves TE et al., 2015 [22] | 21/19 | >30 | 20 | 20 | 40 |
| 8. Balli U et al., 2016 [40] | 41/39 | 30–49 | 60 | 20 | 80 |
| 9. Li Z et al., 2018 [41] | 126/50 | <18 | 126 | 50 | 176 |
| 10. Wanichkittikul N et al., 2021 [42] | 6/23 | >35 | 22 | 7 | 29 |
| Sample Type Used | Number of Items | Data in Percent (%) |
|---|---|---|
| Serum | 6 | 60% |
| Salivary | 1 | 10% |
| GCF | 1 | 10% |
| GCF and Serum | 2 | 20% |
| Author, Year | Type of Sample | Periodontal Disease | BMI (kg/m²) ± SD | Inflammation Marker ± SD | Results |
|---|---|---|---|---|---|
| Zimmermann GS et al., 2013 [36] | GCF Serum | Stage III | Degree of obesity I EG: 33.2 ± 2.8 CG: 23.4 ± 2.0 | IL-6 FCG: EG: 0.47 ± 2.01 CG: 0.63 ± 1.10 Suero: EG: 3.4 ± 1.6 CG: 2.8 ± 2.3 | PP: elevated in obese group (p < 0.05). GCF and Serum: Logistic regression analysis shows serum IL-6 levels (p = 0.04) correlated with obese group individuals. Relationship + between IL-6 level and BMI. |
| Gonçalves TE et al., 2015 [22] | GCF Serum | Stage III | Degree of obesity II EG: 36.1 ± 3.1 CG: 23.4 ± 1.0 | IL-6 FCG: EG: 0.3 ± 0.7 CG: 0.3 ± 0.6 Suero: CG: 2.2 ± 0.9 EG: 2.7 ± 1.6 | PP: elevated in obese group (p < 0.05). GCF and serum: elevated IL-6 values at 3-month follow-up (p < 0.05). Relationship + between IL-6 level and BMI. |
| Doğan GE et al., 2016 [37] | Salivary | Stage II | Degree of obesity I EG: 34.4 ± 3.2 CG: 22.9 ± 2.2 | IL-6 EG: 13.9 ± 11.6CG: 6.1 ± 8.3 | PP: There were no significant differences (p = 0.265), IL-6 levels were higher in obese subjects (p = 0.002). Relationship + between IL-6 level and BMI (p = 0.020). |
| Balli U et al., 2016 [40] | GCF | Stage III | Degree of obesity I EG: 33.80 ± 2.11 CG: 22.75 ± 1.29 | IL-6 EG: 0.506 CG: 0.369 | PP: elevated in obese group (p < 0.05). IL-6 levels were elevated with obesity (p < 0.008). Relationship + of IL-6 with BMI and clinical attachment levels (p < 0.05). |
| De Castilhos ED et al., 2012 [34] | Serum | Stage II | Degree of obesity I 1, 49 (0, 74; 3, 00) | PCR 1.23 (0.26; 5, 85) | PP: elevated in obese group (p < 0.05). The increase in CRP levels had a + correlation with obesity (p < 0.05). |
| López NJ et al., 2012 [35] | Serum | Stage II | Degree of obesity I EG: 29.96 ± 3.89 CG: 30.39 ± 4.26 | PCR EG: 4.43 ± 3.05 CG: 04.39 ± 3.17 | PP: There were no significant differences in the groups (p > 0.05). Elevated CRP levels in both study groups (p < 0.05). |
| Wanichkittikul N et al., 2021 [42] | Serum | Stage IV | Degree of obesity I EG: 25.07 (23.96; 29.20) CG: 19.98 (18.14, 23.32) | PCR EG: 3, 17 (2, 08; 8, 04) CG: 1, 58 (0, 66; 3, 97) | PP: Higher levels in the obese group (p < 0.001). CRP levels were higher in patients in the obese group (p < 0.001). |
| Altay U et al., 2013 [38] | Serum | Stage III | Degree of obesity I 13 (59.1) Degree of obesity II 6 (27.3) Degree of obesity III 3 (136) | IL-6 EG: 1.1 (0.8–1.9) CG: 1 (0.9–1.04) PCR EG: 3.3 (3.2–6.0) CG: 3.3(3.0–4.2) | PP: elevated in favor of the obese group (p < 0.01). No significant differences in inflammation markers. |
| Buduneli N et al., 2014 [39] | Serum | Stage II | Degree of obesity II (35–39.9) EG: 37.90 ± 4.56 CG: 23.00 ± 0.87 | IL-6 EG: 0.59 ± 0.16 CG: 0.10 ± 0.01 PCR EG: 2.69 ± 1.81 CG: 3.19 ± 2.19 | PP: clinical attachment level was significantly higher in the obese group (p < 0.05). Serum IL-6 levels were significantly higher in the obese group (p < 0.05). Serum CRP levels were similar in both study groups (p > 0.05). The BMI was related to elevated IL-6 levels (p < 0.05). |
| Li Z et al., 2018 [41] | Serum | Stage II | Degree of obesity I EG: 2.10 ± 0.34CG: 0.56 ± 0.08 | IL-6 EG: 2.94 ± 0.61 CG: 0.56 ± 0.08 PCR EG: 8.81 ± 3.27 CG: 4.35 ± 2.04 | PP: elevated in obese group (p < 0.05). Elevated levels of IL-6 and CRP in obese group (p < 0.05). The obesity status of the experimental group was related + with elevated serum levels of IL-6 and CRP (p < 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Ávila, J.; Hernández-Pérez, E.; González-González, R.; Bologna-Molina, R.; Molina-Frechero, N. Periodontal Disease in Obese Patients; Interleukin-6 and C-Reactive Protein Study: A Systematic Review. Dent. J. 2022, 10, 225. https://doi.org/10.3390/dj10120225
Cruz-Ávila J, Hernández-Pérez E, González-González R, Bologna-Molina R, Molina-Frechero N. Periodontal Disease in Obese Patients; Interleukin-6 and C-Reactive Protein Study: A Systematic Review. Dentistry Journal. 2022; 10(12):225. https://doi.org/10.3390/dj10120225
Chicago/Turabian StyleCruz-Ávila, Julieta, Elizabeth Hernández-Pérez, Rogelio González-González, Ronell Bologna-Molina, and Nelly Molina-Frechero. 2022. "Periodontal Disease in Obese Patients; Interleukin-6 and C-Reactive Protein Study: A Systematic Review" Dentistry Journal 10, no. 12: 225. https://doi.org/10.3390/dj10120225
APA StyleCruz-Ávila, J., Hernández-Pérez, E., González-González, R., Bologna-Molina, R., & Molina-Frechero, N. (2022). Periodontal Disease in Obese Patients; Interleukin-6 and C-Reactive Protein Study: A Systematic Review. Dentistry Journal, 10(12), 225. https://doi.org/10.3390/dj10120225