Short Narrow Dental Implants versus Long Narrow Dental Implants in Fixed Prostheses: A Prospective Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedures
2.2. Data Collection and Analysis
2.3. Statistical Analysis
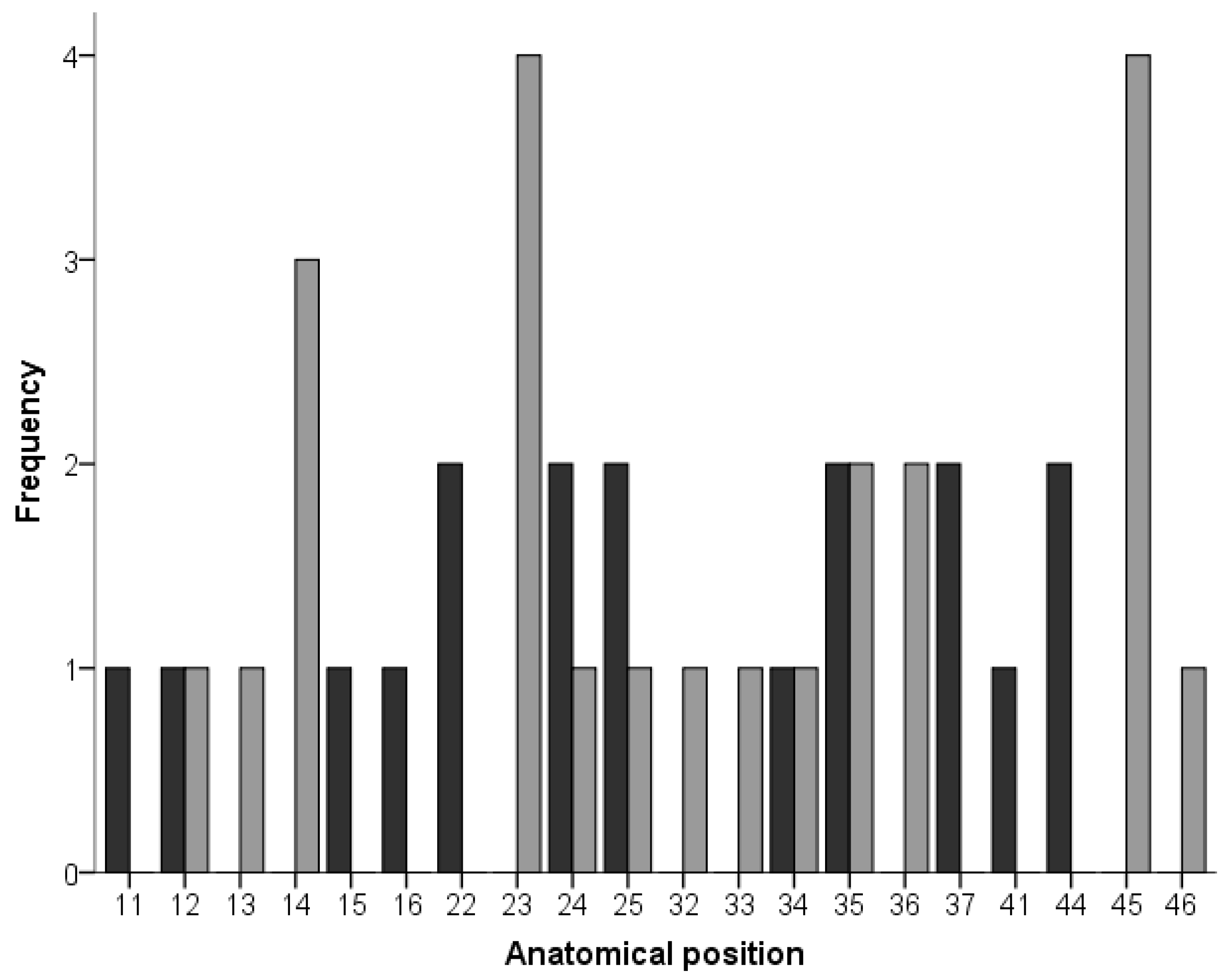
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-analysis. J. Dent. Res. 2014, 93, 20S–28S. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.A. Reduction of residual ridges: A major oral disease entity. J. Prosthet. Dent. 1971, 26, 266–279. [Google Scholar] [CrossRef]
- Kingsmill, V.J. Post-extraction remodeling of the adult mandible. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1999, 10, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.M.; Huber, C.D.; Lippnig, W.R.; Ulm, C.; Watzek, G.; Tangl, S. Atrophy of the residual alveolar ridge following tooth loss in an historical population. Oral Dis. 2011, 17, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Helkimo, E.; Carlsson, G.E.; Helkimo, M. Bite force and state of dentition. Acta Odontol. Scand. 1977, 35, 297–303. [Google Scholar] [CrossRef]
- Akeson, W.H.; Amiel, D.; Abel, M.F.; Garfin, S.R.; Woo, S.L. Effects of immobilization on joints. Clin. Orthop. Relat. Res. 1987, 219, 28–37. [Google Scholar] [CrossRef]
- Kingsmill, V.J.; Boyde, A. Mineralisation density of human mandibular bone: Quantitative backscattered electron image analysis. J. Anat. 1998, 192 Pt 2, 245–256. [Google Scholar] [CrossRef]
- Pudwill, M.L.; Wentz, F.M. Microscopic anatomy of edentulous residual alveolar ridges. J. Prosthet. Dent. 1975, 34, 448–455. [Google Scholar] [CrossRef]
- Sanders, A.E.; Akinkugbe, A.A.; Slade, G.D.; Essick, G.K. Tooth loss and obstructive sleep apnea signs and symptoms in the US population. Sleep Breath 2016, 20, 1095–1102. [Google Scholar] [CrossRef]
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Smith-Palmer, J.; Valentine, W. Evaluating the health economic implications and cost-effectiveness of dental implants: A literature review. Int. J. Oral Maxillofac. Implant. 2013, 28, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H. Is Alveolar Ridge Split a Risk Factor for Implant Survival? J. Oral Maxillofac. Surg. 2016, 74, 2182–2191. [Google Scholar] [CrossRef]
- Torres, J.; Tamimi, F.; Alkhraisat, M.H.; Manchón, Á.; Linares, R.; Prados-Frutos, J.C.; Hernández, G.; López Cabarcos, E. Platelet-rich plasma may prevent titanium-mesh exposure in alveolar ridge augmentation with anorganic bovine bone. J. Clin. Periodontol. 2010, 37, 943–951. [Google Scholar] [CrossRef]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H.; Orive, G. Implant-Guided vertical bone augmentation around extra-short implants for the management of severe bone atrophy. J. Oral Implantol. 2015, 41, 563–569. [Google Scholar] [CrossRef]
- Anitua, E.; Flores, J.; Alkhraisat, M.H. Transcrestal Sinus Lift Using Platelet Concentrates in Association to Short Implant Placement: A Retrospective Study of Augmented Bone Height Remodeling. Clin. Implant. Dent. Relat. Res. 2016, 18, 993–1002. [Google Scholar] [CrossRef]
- Hamdan Alkhraisat, M.; Tamimi Mariño, F.; Rubio Retama, J.; Blanco Jerez, L.; López-Cabarcos, E. Beta-tricalcium phosphate release from brushite cement surface. J. Biomed. Mater. Res.-Part A 2008, 84A, 710–717. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Marchese, S.; Worsaae, N.; Jensen, S.S. The sandwich osteotomy technique to treat vertical alveolar bone defects prior to implant placement: A systematic review. Clin. Oral Investig. 2020, 24, 1073–1089. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Imber, J.-C.; Jensen, S.S. Need for lateral bone augmentation at two narrow-diameter implants: A prospective, controlled, clinical study. Clin. Oral Implant. Res. 2021, 32, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Schiegnitz, E.; Al-Nawas, B. Systematic Review on Success of Narrow-Diameter Dental Implants. Int. J. Oral Maxillofac. Implant. 2014, 29, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Alrabiah, M. Comparison of survival rate and crestal bone loss of narrow diameter dental implants versus regular dental implants: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2019, 10, e12367. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Qi, M.; Zhang, D.; Liu, H. The clinical performance of narrow diameter implants versus regular diameter implants: A systematic review and meta-analysis. J. Oral Implantol. 2019, 45, 503–508. [Google Scholar] [CrossRef]
- Assaf, A.; Saad, M.; Daas, M.; Abdallah, J.; Abdallah, R. Use of narrow-diameter implants in the posterior jaw: A systematic review. Implant. Dent. 2015, 24, 294–306. [Google Scholar] [CrossRef]
- Anitua, E.; Fernandez-de-Retana, S.; Anitua, B.; Alkhraisat, M.H. Long-Term Retrospective Study of 3.0-mm-Diameter Implants Supporting Fixed Multiple Prostheses: Immediate Versus Delayed Implant Loading. Int. J. Oral Maxillofac. Implant. 2020, 35, 1229–1238. [Google Scholar] [CrossRef]
- Anitua, E.; Saracho, J.; Begoña, L.; Alkhraisat, M.H. Long-Term Follow-Up of 2.5-mm Narrow-Diameter Implants Supporting a Fixed Prostheses. Clin. Implant. Dent. Relat. Res. 2016, 18, 769–777. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H. 15-year follow-up of short dental implants placed in the partially edentulous patient: Mandible Vs maxilla. Ann. Anat.-Anat. Anz. 2019, 222, 88–93. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H. Single-unit short dental implants. Would they survive a long period of service? Br. J. Oral Maxillofac. Surg. 2019, 57, 387–388. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H. Fifteen-Year Follow-up of Short Dental Implants in the Completely Edentulous Jaw. Implant. Dent. 2019, 28, 551–555. [Google Scholar] [CrossRef]
- Cruz, R.S.; de Araújo Lemos, C.A.; de Souza Batista, V.E.; Oliveira, H.F.F.E.; de Luna Gomes, J.M.; Pellizzer, E.P.; Verri, F.R. Short implants versus longer implants with maxillary sinus lift. A systematic review and meta-analysis. Braz. Oral Res. 2018, 32, e86. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.D.N.; Pecorari, V.G.A.; Martins, C.B.; Del Fabbro, M.; Casati, M.Z. Short implants versus bone augmentation in combination with standard-length implants in posterior atrophic partially edentulous mandibles: Systematic review and meta-analysis with the Bayesian approach. Int. J. Oral Maxillofac. Surg. 2019, 48, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Burgueño-Barris, G.; Figueiredo, R.; Jung, R.E.; Gay-Escoda, C.; Valmaseda-Castellón, E. Interventions for Dental Implant Placement in Atrophic Edentulous Mandibles: Vertical Bone Augmentation and Alternative Treatments. A Meta-Analysis of Randomized Clinical Trials. J. Periodontol. 2016, 87, 1444–1457. [Google Scholar] [CrossRef]
- Anitua, E.; Flores, C.; Flores, J.; Alkhraisat, M.H. Clinical Effectiveness of 6.5-mm-Long Implants to Support Two-Implant Fixed Prostheses in Premolar-Molar Region: The Influence of Immediate Loading and the Length of Splinting Implant. J. Prosthodont. 2018, 28, e688–e693. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Flores, J.; Flores, C.; Alkhraisat, M.H. Long-term outcomes of immediate loading of short implants: A controlled retrospective cohort study. Int. J. Oral Maxillofac. Implant. 2016, 31, 1360–1366. [Google Scholar] [CrossRef]
- Urban, I.A.; Monje, A.; Lozada, J.L.; Wang, H.L. Long-term Evaluation of Peri-implant Bone Level after Reconstruction of Severely Atrophic Edentulous Maxilla via Vertical and Horizontal Guided Bone Regeneration in Combination with Sinus Augmentation: A Case Series with 1 to 15 Years of Loading. Clin. Implant. Dent. Relat. Res. 2017, 19, 46–55. [Google Scholar] [CrossRef]
- Maló, P.S.; de Araújo Nobre, M.A.; Lopes, A.V.; Ferro, A.S. Retrospective cohort clinical investigation of a dental implant with a narrow diameter and short length for the partial rehabilitation of extremely atrophic jaws. J. Oral Sci. 2017, 59, 357–363. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implants 1999, 14, 529–535. [Google Scholar]
- Anitua, E.; Piñas, L.; Begoña, L.; Alkhraisat, M. Prognosis of Dental Implants Immediately Placed in Sockets Affected by Peri-implantitis: A Retrospective Pilot Study. Int. J. Periodontics Restor. Dent. 2017, 37, 713–719. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. S1), 4–7. [Google Scholar] [CrossRef] [PubMed]
- Favero, R.; Botticelli, D.; Antunes, A.A.; Martinez Sanchez, R.; Caroprese, M.; Salata, L.A. Sequential Healing at Calcium-versus Calcium Phosphate-Modified Titanium Implant Surfaces: An Experimental Study in Dogs. Clin. Implant. Dent. Relat. Res. 2016, 18, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.; Lang, N.P.; Favero, R.; Carneiro Martins Neto, E.; Salata, L.A.; Botticelli, D. Sequential morphometric evaluation at UnicCa® and SLActive® implant surfaces. An experimental study in the dog. Clin. Oral Implant. Res. 2017, 28, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Piñas, L.; Alkhraisat, M.H. Early marginal bone stability of dental implants placed in a transalveolarly augmented maxillary sinus: A controlled retrospective study of surface modification with calcium ions. Int. J. Implant. Dent. 2017, 3, 49. [Google Scholar] [CrossRef]
- Tejero, R.; Rossbach, P.; Keller, B.; Anitua, E.; Reviakine, I. Time-of-flight secondary ion mass spectrometry with principal component analysis of titania-blood plasma interfaces. Langmuir 2013, 29, 902–912. [Google Scholar] [CrossRef]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant. Dent. 2021, 7, 32. [Google Scholar] [CrossRef]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef]
- Bonfante, E.A.; Jimbo, R.; Witek, L.; Tovar, N.; Neiva, R.; Torroni, A.; Coelho, P.G. Biomaterial and biomechanical considerations to prevent risks in implant therapy. Periodontol. 2000 2019, 81, 139–151. [Google Scholar] [CrossRef]
- Anitua, E.; Larrazabal Saez de Ibarra, N.; Morales Martín, I.; Saracho Rotaeche, L. Influence of Dental Implant Diameter and Bone Quality on the Biomechanics of Single-Crown Restoration. A Finite Element Analysis. Dent. J. 2021, 9, 103. [Google Scholar] [CrossRef]
- Anitua, E.; Tapia, R.; Luzuriaga, F.; Orive, G. Influence of implant length, diameter, and geometry on stress distribution: A finite element analysis. Int. J. Periodontics Restor. Dent. 2010, 30, 89. [Google Scholar]
- Pellegrino, G.; Ferri, A.; Del Fabbro, M.; Prati, C.; Gandolfi, M.G.; Marchetti, C. Dynamic Navigation in Implant Dentistry: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2021, 36, e121–e140. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Flores, C.; Pinas, L.; Alkhraisat, M.H. Frequency of Technical Complications in Fixed Implant Prosthesis: The Effect of Prosthesis Screw Emergence Correction by Computer-Aided Design/Computer-Aided Manufacturing. J. Oral Implant. 2018, 44, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Elnayef, B.; Monje, A.; Lin, G.-H.; Gargallo-Albiol, J.; Chan, H.-L.; Wang, H.-L.; Hernández-Alfaro, F. Alveolar ridge split on horizontal bone augmentation: A systematic review. Int. J. Oral Maxillofac. Implant. 2015, 30, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Toledano, M.; Manzano-Moreno, F.J.; Vallecillo, C.; Vallecillo-Rivas, M.; Rodriguez-Archilla, A.; Osorio, R. Alveolar Bone Ridge Augmentation Using Polymeric Membranes: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 1172. [Google Scholar] [CrossRef]
- Jensen, O.; Nock, D. Inferior alveolar nerve repositioning in conjunction with placement of osseointegrated implants: A case report. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 263–268. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H. The adjuvant use of plasma rich in growth factors in the inferior alveolar nerve repositioning technique. Heliyon 2019, 5, e02965. [Google Scholar] [CrossRef]
- Anitua, E.; Padilla, S.; Alkhraisat, M.H. Transalveolar Osteotomy of the Mandibular Canal Wall for the Treatment of Severely Atrophied Posterior Mandible. J. Oral Maxillofac. Surg. 2017, 75, 1392–1401. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Novel technique for the treatment of the severely atrophied posterior mandible. Int. J. Oral Maxillofac. Implant. 2013, 28, 1338–1346. [Google Scholar] [CrossRef]
- Storelli, S.; Del Fabbro, M.; Scanferla, M.; Palandrani, G.; Romeo, E. Implant supported cantilevered fixed dental rehabilitations in partially edentulous patients: Systematic review of the literature. Part I. Clin. Oral Implants Res. 2018, 29 (Suppl. S1), 253–274. [Google Scholar] [CrossRef]
- Schmid, E.; Roccuzzo, A.; Morandini, M.; Ramseier, C.A.; Sculean, A.; Salvi, G.E. Clinical and radiographic evaluation of implant-supported single-unit crowns with cantilever extension in posterior areas: A retrospective study with a follow-up of at least 10 years. Clin. Implant. Dent. Relat. Res. 2021, 23, 189–196. [Google Scholar] [CrossRef]
- Zampelis, A.; Rangert, B.; Heijl, L. Tilting of splinted implants for improved prosthodontic support: A two-dimensional finite element analysis. J. Prosthet. Dent. 2007, 97, S35–S43. [Google Scholar] [CrossRef]
- Stegaroiu, R.; Sato, T.; Kusakari, H.; Miyakawa, O. Influence of restoration type on stress distribution in bone around implants: A three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 1998, 13, 82–90. [Google Scholar]
- Roccuzzo, A.; Jensen, S.S.; Worsaae, N.; Gotfredsen, K. Implant-supported 2-unit cantilevers compared with single crowns on adjacent implants: A comparative retrospective case series. J. Prosthet. Dent. 2020, 123, 717–723. [Google Scholar] [CrossRef]
- Aglietta, M.; Siciliano, V.I.; Zwahlen, M.; Brägger, U.; Pjetursson, B.E.; Lang, N.P.; Salvi, G.E. A systematic review of the survival and complication rates of implant supported fixed dental prostheses with cantilever extensions after an observation period of at least 5 years. Clin. Oral Implant. Res. 2009, 20, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Storelli, S. Systematic review of the survival rate and the biological, technical, and aesthetic complications of fixed dental prostheses with cantilevers on implants reported in longitudinal studies with a mean of 5 years follow-up. Clin. Oral Implants Res. 2012, 23 (Suppl. S6), 39–49. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Morandini, M.; Roccuzzo, A.; Ramseier, C.A.; Sculean, A.; Salvi, G.E. Clinical and radiographic outcomes of implant-supported fixed dental prostheses with cantilever extension. A retrospective cohort study with a follow-up of at least 10 years. Clin. Oral Implant. Res. 2020, 31, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Al-Nawas, B. Narrow-diameter implants: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 21–40. [Google Scholar] [CrossRef]
- Pommer, B.; Mailath-Pokorny, G.; Haas, R.; Buseniechner, D.; Millesi, W.; Fürhauser, R. Extra-short (<7 mm) and extra-narrow diameter (<3.5 mm) implants: A meta-analytic literature review. Eur. J. Oral Implantol. 2018, 11 (Suppl. S1), S137–S146. [Google Scholar]
- Pieri, F.; Forlivesi, C.; Caselli, E.; Corinaldesi, G. Narrow- (3.0 mm) Versus Standard-Diameter (4.0 and 4.5 mm) Implants for Splinted Partial Fixed Restoration of Posterior Mandibular and Maxillary Jaws: A 5-Year Retrospective Cohort Study. J. Periodontol. 2017, 88, 338–347. [Google Scholar] [CrossRef]
- Zinsli, B.; Sägesser, T.; Mericske, E.; Mericske-Stern, R. Clinical evaluation of small-diameter ITI implants: A prospective study. Int. J. Oral Maxillofac. Implant. 2004, 19, 92–99. [Google Scholar]
- Garcez-Filho, J.; Tolentino, L.; Sukekava, F.; Seabra, M.; Cesar-Neto, J.B.; Araújo, M.G. Long-term outcomes from implants installed by using split-crest technique in posterior maxillae: 10 years of follow-up. Clin. Oral Implant. Res. 2015, 26, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Arιsan, V.; Bölükbaşι, N.; Ersanlι, S.; Özdemir, T. Evaluation of 316 narrow diameter implants followed for 5-10 years: A clinical and radiographic retrospective study. Clin. Oral Implant. Res. 2010, 21, 296–307. [Google Scholar] [CrossRef]
- Mangano, F.; Shibli, J.A.; Sammons, R.L.; Veronesi, G.; Piattelli, A.; Mangano, C. Clinical Outcome of Narrow-Diameter (3.3-mm) Locking-Taper Implants: A Prospective Study with 1 to 10 Years of Follow-up. Int. J. Oral Maxillofac. Implant. 2014, 29, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Brunski, J.B. Biomechanical factors affecting the bone-dental implant interface. Clin. Mater 1992, 10, 153–201. [Google Scholar] [CrossRef]
- Ekfelt, A. Incisal and Occlusal Tooth Wear and Wear of Some Prosthodontic Materials. An Epidemiological and Clinical Study. Swed. Dent. J. Suppl. 1989, 65, 62. [Google Scholar]
- Anitua, E.; Alkhraist, M.H.; Piñas, L.; Begoña, L.; Orive, G. Implant survival and crestal bone loss around extra-short implants supporting a fixed denture: The effect of crown height space, crown-to-implant ratio, and offset placement of the prosthesis. Int. J. Oral Maxillofac. Implant. 2014, 29, 682–689. [Google Scholar] [CrossRef]
- Wittneben, J.G.; Millen, C.; Bragger, U. Clinical performance of screw- versus cement-retained fixed implant-supported reconstructions—A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29 (Suppl. S2014), 84–98. [Google Scholar] [CrossRef]
- Lozano-Carrascal, N.; Anglada-Bosqued, A.; Salomó-Coll, O.; Hernández-Alfaro, F.; Wang, H.L.; Gargallo-Albiol, J. Short implants (<8 mm) versus longer implants (≥8 mm) with lateral sinus floor augmentation in posterior atrophic maxilla: A meta-analysis of RCT`s in humans. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e168–e179. [Google Scholar] [CrossRef]
- Chen, S.; Ou, Q.; Wang, Y.; Lin, X. Short implants (5–8 mm) vs long implants (≥10 mm) with augmentation in atrophic posterior jaws: A meta-analysis of randomised controlled trials. J. Oral Rehabil. 2019, 46, 1192–1203. [Google Scholar] [CrossRef]
- Altaib, F.H.; Alqutaibi, A.Y.; Al-Fahd, A.; Eid, S. Short dental implant as alternative to long implant with bone augmentation of the atrophic posterior ridge: A systematic review and meta-analysis of RCTs. Quintessence Int. 2019, 50, 636–650. [Google Scholar] [CrossRef]
- Ravidà, A.; Wang, I.C.; Sammartino, G.; Barootchi, S.; Tattan, M.; Troiano, G.; Laino, L.; Marenzi, G.; Covani, U.; Wang, H.L. Prosthetic Rehabilitation of the Posterior Atrophic Maxilla, Short (≤6 mm) or Long (≥10 mm) Dental Implants? A Systematic Review, Meta-analysis, and Trial Sequential Analysis: Naples Consensus Report Working Group A. Implant. Dent. 2019, 28, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, A.; Arabi, R.; Bidgoli, M.; Yousefi, F.; Poorolajal, J. Association between marginal bone loss and bone quality at dental implant sites based on evidence from cone beam computed tomography and periapical radiographs. Contemp. Clin. Dent. 2019, 10, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.; Berti, C.; Barausse, C.; Badino, M.; Gasparro, R.; Ippolito, D.R.; Felice, P. Clinical Relevance of Bone Density Values from CT Related to Dental Implant Stability: A Retrospective Study. Biomed. Res. Int. 2018, 2018, 6758245. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Marsico, V.; Lehmann, R.B.; de Assis Claro, C.A.; Amaral, M.; Vitti, R.P.; Neves, A.C.C.; da Silva Concilio, L.R. Three-dimensional finite element analysis of occlusal splint and implant connection on stress distribution in implant–supported fixed dental prosthesis and peri-implantal bone. Mater. Sci. Eng. C 2017, 80, 141–148. [Google Scholar] [CrossRef]
- Barewal, R.M.; Stanford, C.; Weesner, T.C. A randomized controlled clinical trial comparing the effects of three loading protocols on dental implant stability. Int. J. Oral Maxillofac. Implant. 2012, 27, 945–956. [Google Scholar]
- Anitua, E.; Prado, R.; Orive, G.; Tejero, R. Effects of calcium-modified titanium implant surfaces on platelet activation, clot formation, and osseointegration. J. Biomed. Mater. Res. A 2015, 103, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Freier, K.; Engel, M.; Krisam, J.; Hoffmann, J.; Freudlsperger, C. Reconstruction of the severely atrophic edentulous maxillae with calvarial bone grafts. Clin. Oral Implant. Res. 2017, 28, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Santagata, M.; Sgaramella, N.; Ferrieri, I.; Corvo, G.; Tartaro, G.; D’Amato, S. Segmental sandwich osteotomy and tunnel technique for three-dimensional reconstruction of the jaw atrophy: A case report. Int. J. Implant. Dent. 2017, 3, 14. [Google Scholar] [CrossRef][Green Version]
- Schulze, R.; Krummenauer, F.; Schalldach, F.; d’Hoedt, B. Precision and accuracy of measurements in digital panoramic radiography. Dentomaxillofac. Radiol. 2000, 29, 52–56. [Google Scholar] [CrossRef]

| Diameter (mm) | Study Groups | p-Value | |
|---|---|---|---|
| Short Implants | Long Implants | ||
| 2.5 | 0 | 2 | 0.087 1 |
| 3.0 | 0 | 1 | |
| 3.3 | 5 | 9 | |
| 3.5 | 13 | 11 | |
| Total: 41 | |||
| Length (mm) | Study Groups | p-Value | |
|---|---|---|---|
| Short Implants | Long Implants | ||
| 6.5 | 5 | 0.000 1 | |
| 7.5 | 13 | ||
| 8.5 | 12 | ||
| 10.0 | 8 | ||
| 11.0 | 1 | ||
| 11.5 | 1 | ||
| 13.0 | 1 | ||
| Total: 41 | |||
| Overall | Test Group | Control Group | p-Value | ||
|---|---|---|---|---|---|
| Number of implants | 41 | 18 | 23 | - | |
| Bone type | Type I | 11 | 7 | 4 | 0.397 1 |
| Type II | 19 | 7 | 12 | ||
| Type III | 10 | 4 | 6 | ||
| Type IV | 1 | 0 | 1 | ||
| Immediate loading (Number of implants) | 25 | 13 | 12 | 0.192 1 | |
| Insertion torque (Ncm) (Median; range) | 40; 5 to 65 | 45; 5 to 60 | 40; 15 to 65 | 0.545 2 | |
| Fixed Prosthesis Type | Single-unit | 3 | 1 | 2 | 0.251 1 |
| Partial | 36 | 15 | 21 | ||
| Complete | 2 | 2 | 0 | ||
| Antagonist type | Tooth | 28 | 11 | 17 | 0.170 1 |
| Implant | 11 | 7 | 4 | ||
| Implant and tooth | 2 | 0 | 2 | ||
| Time of follow-up (Months) (Median; range) | 26; 4 to 114 | 26; 4 to 57 | 26; 10 to 114 | 0.240 2 | |
| MBL (mm) (Median; range) | 0.0; −1.9 to 2.8 | 0.0; −1.1 to 1.0 | 0.0; −1.9 to 2.8 | 0.761 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antiua, E.; Escuer, V.; Alkhraisat, M.H. Short Narrow Dental Implants versus Long Narrow Dental Implants in Fixed Prostheses: A Prospective Clinical Study. Dent. J. 2022, 10, 39. https://doi.org/10.3390/dj10030039
Antiua E, Escuer V, Alkhraisat MH. Short Narrow Dental Implants versus Long Narrow Dental Implants in Fixed Prostheses: A Prospective Clinical Study. Dentistry Journal. 2022; 10(3):39. https://doi.org/10.3390/dj10030039
Chicago/Turabian StyleAntiua, Eduardo, Virginia Escuer, and Mohammad H. Alkhraisat. 2022. "Short Narrow Dental Implants versus Long Narrow Dental Implants in Fixed Prostheses: A Prospective Clinical Study" Dentistry Journal 10, no. 3: 39. https://doi.org/10.3390/dj10030039
APA StyleAntiua, E., Escuer, V., & Alkhraisat, M. H. (2022). Short Narrow Dental Implants versus Long Narrow Dental Implants in Fixed Prostheses: A Prospective Clinical Study. Dentistry Journal, 10(3), 39. https://doi.org/10.3390/dj10030039







