Cemento-Osseous Dysplasia of the Jaw: Demographic and Clinical Analysis of 191 New Cases
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics
Types of COD
3.2. Clinical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COD | cemento-osseous dysplasia |
| FLCOD | florid cemento-osseous dysplasia |
| PCOD | periapical cemento-osseous dysplasia |
| FCOD | focal cemento-osseous dysplasia |
| MRONJ | medication-related osteonecrosis of the jaw |
| UTHSC | University of Tennessee Health Science Center |
| AA | African American |
| C | Caucasian |
| H | Hispanic |
| A | Asian |
| F | female |
| BFOL | benign fibro-osseous lesion |
| OF | ossify fibroma |
| CBCT | cone beam computed tomography |
References
- Waldron, C.A. Fibro-osseous lesions of the jaws. J. Oral Maxillofac. Surg. 1985, 43, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Weathers, D.R.; Waldron, C.A. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas: II. A Clinical and radiologic spectrum of 316 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, D.-J.; Tomich, C.E. Focal cemento-osseous dysplasia: A clinicopathologic study of 221 cases. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 611–620. [Google Scholar] [CrossRef]
- Haefliger, S.; Turek, D.; Andrei, V.; Alborelli, I.; Calgua, B.; Ameline, B.; Harder, D.; Baumhoer, D. Cemento-osseous dysplasia is caused by RAS-MAPK activation. Pathology 2023, 55, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Alsufyani, N.; Lam, E.W.N. Osseous (cemento-osseous) dysplasia of the jaws: Clinical and radiographic analysis. J. Can. Dent. Assoc. 2011, 77, b70. [Google Scholar]
- Owosho, A.A.; Potluri, A.; Bilodeau, E.A. Osseous dysplasia (cemento-osseous dysplasia) of the jaw bones in western Pennsylvania patients: Analysis of 35 cases. Pa. Dent. J. 2013, 80, 25–29. [Google Scholar]
- Kawai, T.; Hiranuma, H.; Kishino, M.; Jikko, A.; Sakuda, M. Cemento-osseous dysplasia of the jaws in 54 Japanese patients: A radiographic study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 87, 107–114. [Google Scholar] [CrossRef]
- Melrose, R.J.; Abrams, A.M.; Mills, B.G. Florid osseous dysplasia: A clinical-pathologic study of thirty-four cases. Oral Surg. Oral Med. Oral Pathol. 1976, 41, 62–82. [Google Scholar] [CrossRef]
- El-Mofty, S.K. Fibro-Osseous Lesions of the Craniofacial Skeleton: An Update. Head Neck Pathol. 2014, 8, 432–444. [Google Scholar] [CrossRef]
- Waldron, C.A. Fibro-osseous lesions of the jaws. J. Oral Maxillofac. Surg. 1993, 51, 828–835. [Google Scholar] [CrossRef]
- Galgano, C.; Samson, J.; Küffer, R.; Lombardi, T. Focal cemento-osseous dysplasia involving a mandibular lateral incisor. Int. Endod. J. 2003, 36, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Patel, K.; Hoskinson, A.E. Periapical cemental dysplasia: A case of misdiagnosis. Br. Dent. J. 1998, 185, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.R. Periapical cemental dysplasia: A case report. N. Z. Dent. J. 1993, 89, 53–54. [Google Scholar] [PubMed]
- Shi, R.-R.; Li, X.-F.; Zhang, R.; Chen, Y.; Li, T.-J. GNAS mutational analysis in differentiating fibrous dysplasia and ossifying fibroma of the jaw. Mod. Pathol. 2013, 26, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-H.; Liu, H.-C.; Liao, G.-Q.; Liang, Y.-J.; Chu, M.; Wan, C.-Q.; Liang, L.-Z.; Zheng, G.-S. Detection of Notch signaling molecules in cemento-ossifying fibroma of the jaws. J. Oral Pathol. Med. 2010, 39, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.D.S.F.; Diniz, M.G.; França, J.A.; Moreira, R.G.; de Menezes, G.H.F.; de Sousa, S.F.; de Castro, W.H.; Gomes, C.C.; Gomez, R.S. The Wnt/beta-catenin pathway is deregulated in cemento-ossifying fibromas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 172–178. [Google Scholar] [CrossRef]
- Tabareau-Delalande, F.; Collin, C.; Gomez-Brouchet, A.; Bouvier, C.; Decouvelaere, A.-V.; de Muret, A.; Pagès, J.-C.; de Pinieux, G. Chromosome 12 long arm rearrangement covering MDM2 and RASAL1 is associated with aggressive craniofacial juvenile ossifying fibroma and extracranial psammomatoid fibro-osseous lesions. Mod. Pathol. 2015, 28, 48–56. [Google Scholar] [CrossRef]
- Newey, P.J.; Bowl, M.R.; Cranston, T.; Thakker, R.V. Cell division cycle protein 73 homolog (CDC73) mutations in the hyperparathyroidism-jaw tumor syndrome (HPT-JT) and parathyroid tumors. Hum. Mutat. 2010, 31, 295–307. [Google Scholar] [CrossRef]
- Bahceci, D.H.; Grenert, J.P.; Jordan, R.C.K.; Horvai, A.E. Genomic Profiling of the Craniofacial Ossifying Fibroma by Next-Generation Sequencing. Head Neck Pathol. 2023, 1–9. [Google Scholar] [CrossRef]
- Cleven, A.H.; Szuhai, K.; van Ijzendoorn, D.G.; Groen, E.; Baelde, H.; Schreuder, W.H.; Bruijn, I.H.B.-D.; van der Meeren, S.W.; Kleijwegt, M.C.; Furth, W.R.; et al. Psammomatoid Ossifying Fibroma Is Defined by SATB2 Rearrangement. Mod. Pathol. 2023, 36, 100013. [Google Scholar] [CrossRef]
- Toferer, A.; Truschnegg, A.; Kashofer, K.; Beham-Schmid, C.; Beham, A. First presentation of a frameshift mutation in the SETD2 gene of a juvenile psammomatoid ossifying fibroma (JPOF) associated with an aneurysmal bone cyst. Diagn. Pathol. 2021, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, T.V.; Tyazhelova, T.V.; Rykalina, V.N.; Gusev, F.E.; Goltsov, A.Y.; Zolotareva, O.I.; Aliseichik, M.P.; Borodina, T.A.; Grigorenko, A.P.; Reshetov, D.A.; et al. Whole exome sequencing links dental tumor to an autosomal-dominant mutation in ANO5 gene associated with gnathodiaphyseal dysplasia and muscle dystrophies. Sci. Rep. 2016, 6, 26440. [Google Scholar] [CrossRef] [PubMed]
- MacDonald-Jankowski, D.S. Florid cemento-osseous dysplasia: A systematic review. Dentomaxillofac. Radiol. 2003, 32, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Baden, E.; Saroff, S.A. Periapical Cemental Dysplasia and Periodontal Disease. A case report with review of the literature. J. Periodontol. 1987, 58, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Groot, R.H.; van Merkesteyn, J.P.; Bras, J. Diffuse sclerosing osteomyelitis and florid osseous dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 333–342. [Google Scholar] [CrossRef]
- Waldron, C.; Giansanti, J.; Browand, B. Sclerotic cemental masses of the jaws (so-called chronic sclerosing osteomyelitis, sclerosing osteitis, multiple enostosis, and gigantiform cementoma). Oral Surg. Oral Med. Oral Pathol. 1975, 39, 590–604. [Google Scholar] [CrossRef]
- Higuchi, Y.; Nakamura, N.; Tashiro, H. Clinicopathologic study of cemento-osseous dysplasia producing cysts of the mandible: Report of four cases. Oral Surg. Oral Med. Oral Pathol. 1988, 65, 339–342. [Google Scholar] [CrossRef]
- Mahomed, F.; Altini, M.; Meer, S.; Coleman, H. Cemento-Osseous Dysplasia with Associated Simple Bone Cysts. J. Oral Maxillofac. Surg. 2005, 63, 1549–1554. [Google Scholar] [CrossRef]
- Brannon, R.B.; Fowler, C.B. Benign Fibro-Osseous Lesions: A Review of Current Concepts. Adv. Anat. Pathol. 2001, 8, 126–143. [Google Scholar] [CrossRef]
- Titinchi, F.; Morkel, J. Ossifying Fibroma: Analysis of Treatment Methods and Recurrence Patterns. J. Oral Maxillofac. Surg. 2016, 74, 2409–2419. [Google Scholar] [CrossRef]
- McDonnell, D. Dense bone island. A review of 107 patients. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Green, T.; Walton, R.E.; Clark, J.M.; Maixner, D. Histologic Examination of Condensing Osteitis in Cadaver Specimens. J. Endod. 2013, 39, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Leider, A.S.; Garbarino, V.E. Generalized hypercementosis. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Brannon, R.B.; Fowler, C.B.; Carpenter, W.M.; Corio, R.L. Cementoblastoma: An innocuous neoplasm? A clinicopathologic study of 44 cases and review of the literature with special emphasis on recurrence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 311–320. [Google Scholar] [CrossRef]
- Gumru, B.; Akkitap, M.P.; Deveci, S.; Idman, E. A retrospective cone beam computed tomography analysis of cemento-osseous dysplasia. J. Dent. Sci. 2021, 16, 1154–1161. [Google Scholar] [CrossRef]
- Cavalcanti, P.H.P.; Nascimento, E.H.L.; Pontual, M.L.D.A.; Pontual, A.D.A.; de Marcelos, P.G.C.L.; Perez, D.E.D.C.; Ramos-Perez, F.M.D.M. Cemento-Osseous Dysplasias: Imaging Features Based on Cone Beam Computed Tomography Scans. Braz. Dent. J. 2018, 29, 99–104. [Google Scholar] [CrossRef]
- Kato, C.D.N.A.D.O.; Barra, S.G.; Amaral, T.M.P.; Silva, T.A.; Abreu, L.G.; Brasileiro, C.B.; Mesquita, R.A. Cone-beam computed tomography analysis of cemento-osseous dysplasia-induced changes in adjacent structures in a Brazilian population. Clin. Oral Investig. 2020, 24, 2899–2908. [Google Scholar] [CrossRef]
- Daviet-Noual, V.; Ejeil, A.-L.; Gossiome, C.; Moreau, N.; Salmon, B. Differentiating early stage florid osseous dysplasia from periapical endodontic lesions: A radiological-based diagnostic algorithm. BMC Oral Health 2017, 17, 161. [Google Scholar] [CrossRef]
- Pick, E.; Schäfer, T.; Al-Haj Husain, A.; Rupp, N.J.; Hingsammer, L.; Valdec, S. Clinical, Radiological, and Pathological Diagnosis of Fibro-Osseous Lesions of the Oral and Maxillofacial Region: A Retrospective Study. Diagnostics 2022, 12, 238. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Jacobs, R.; Bornstein, M.M. Novel low-dose protocols using cone beam computed tomography in dental medicine: A review focusing on indications, limitations, and future possibilities. Clin. Oral Investig. 2019, 23, 2573–2581. [Google Scholar] [CrossRef]
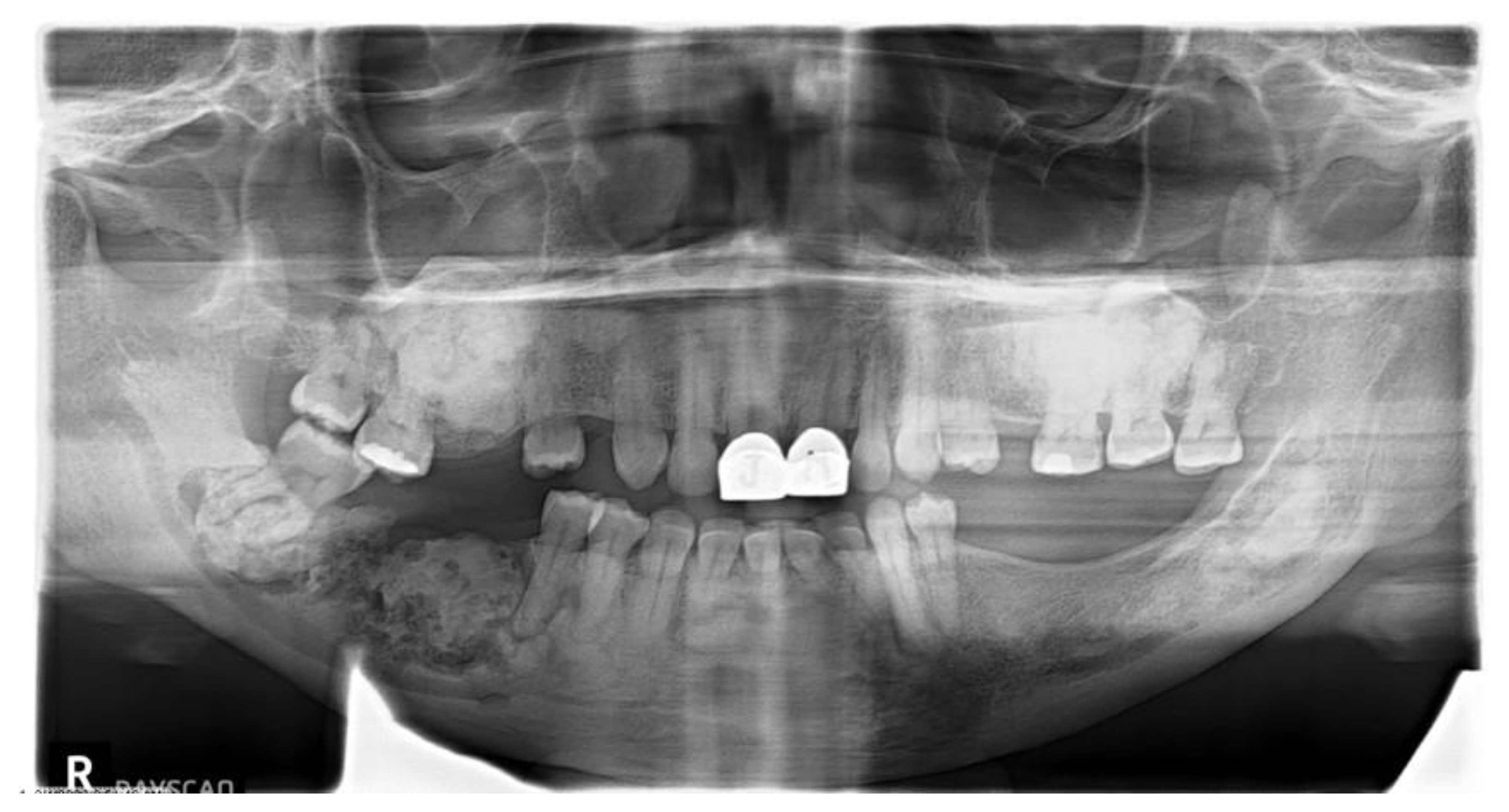
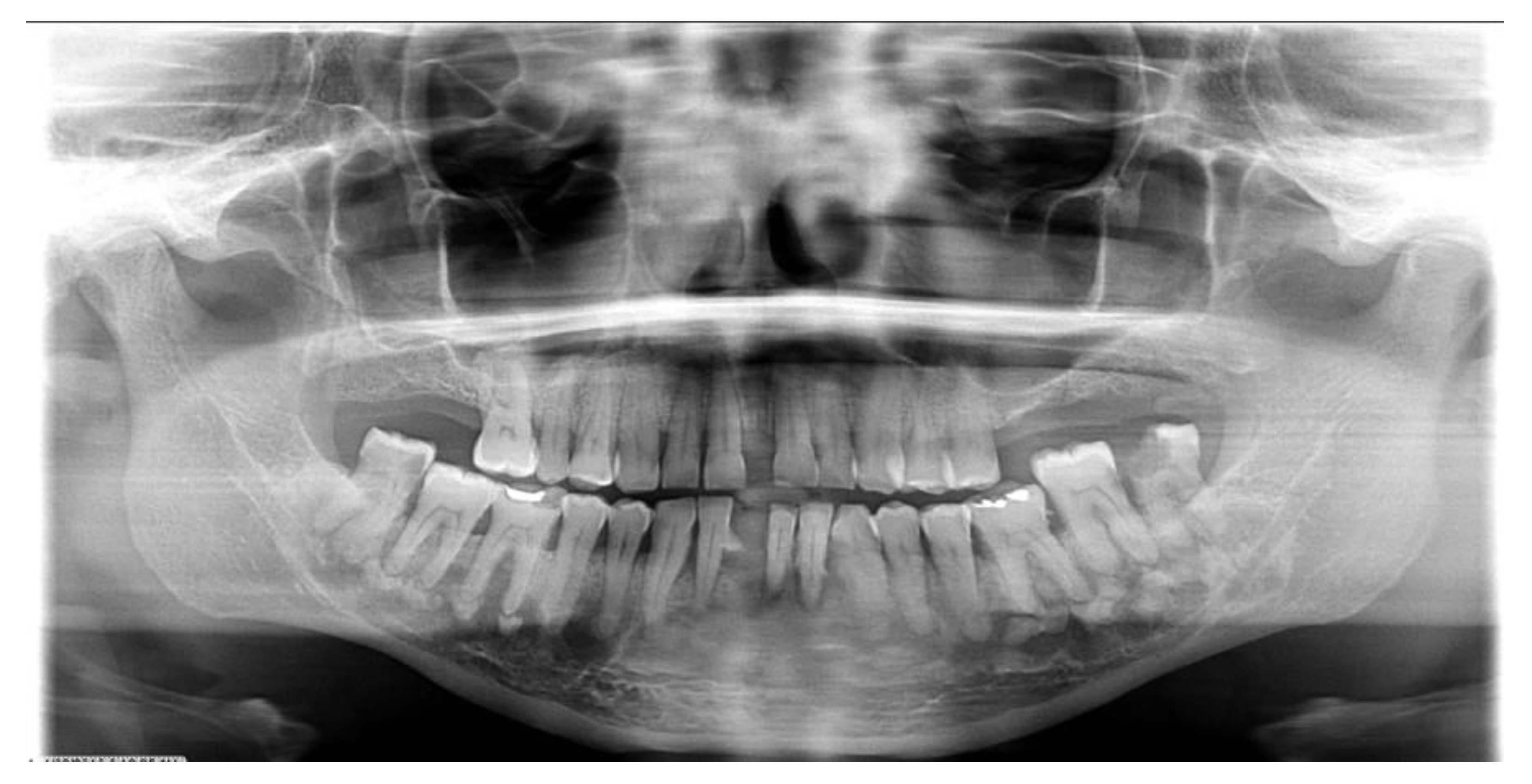
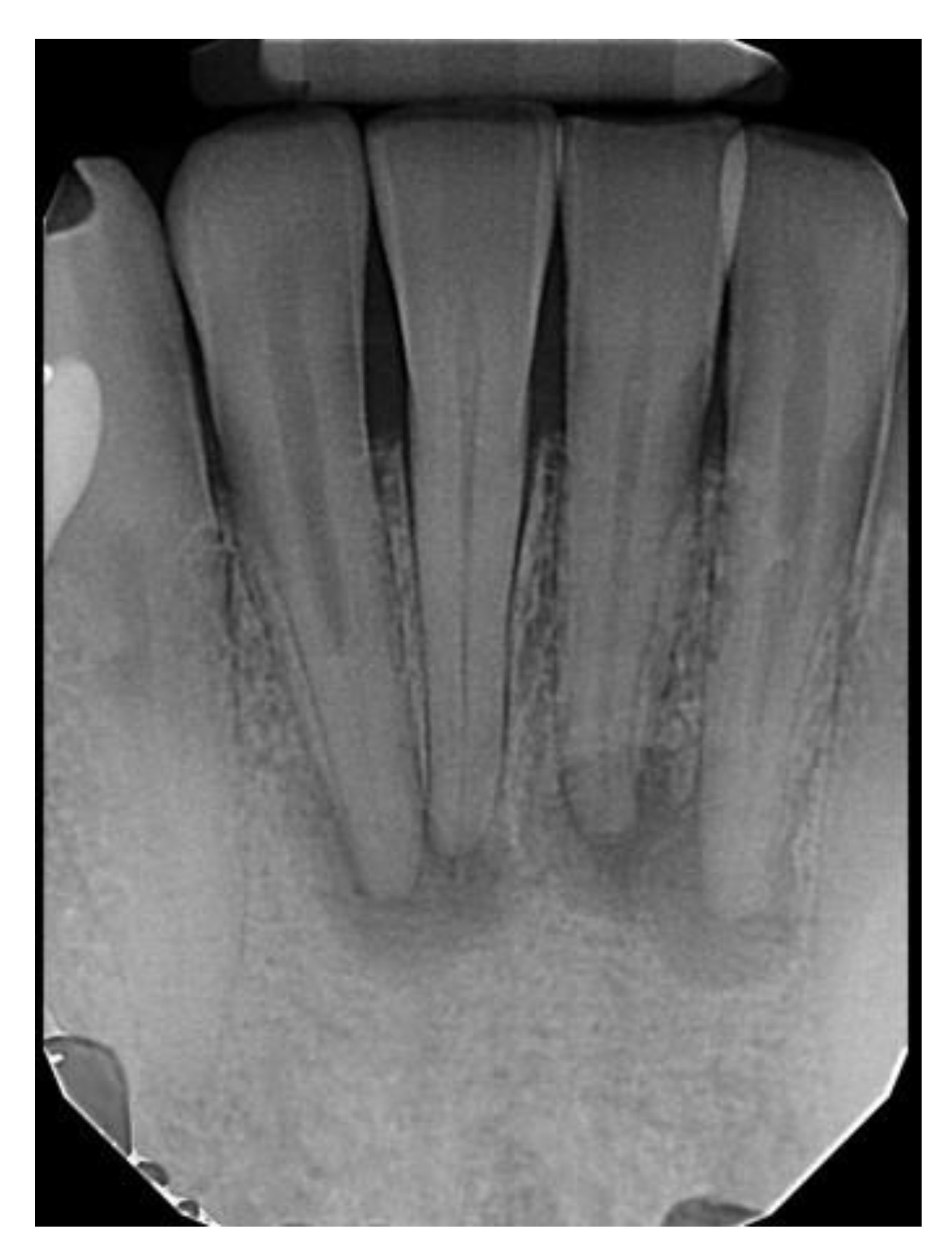
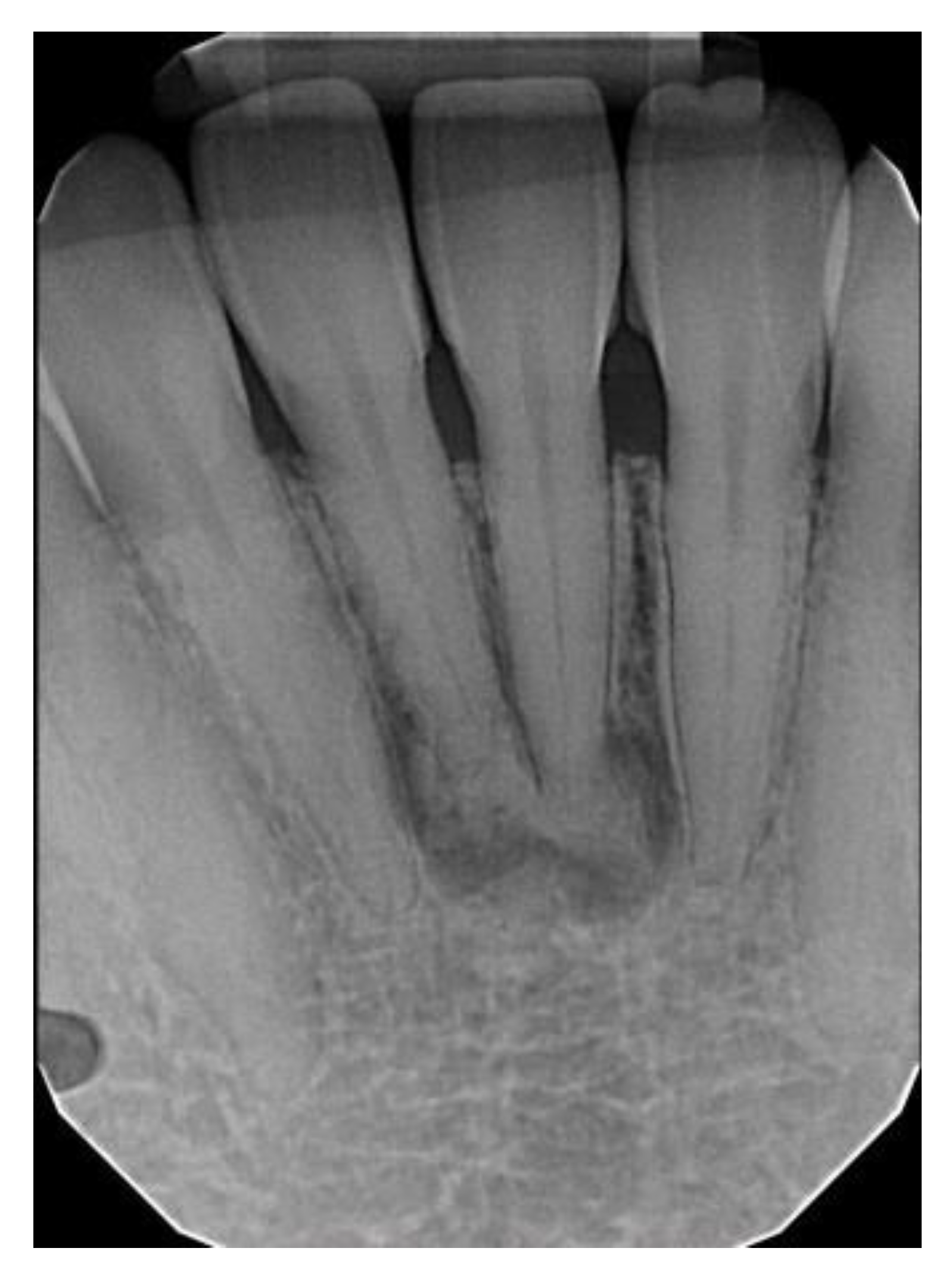
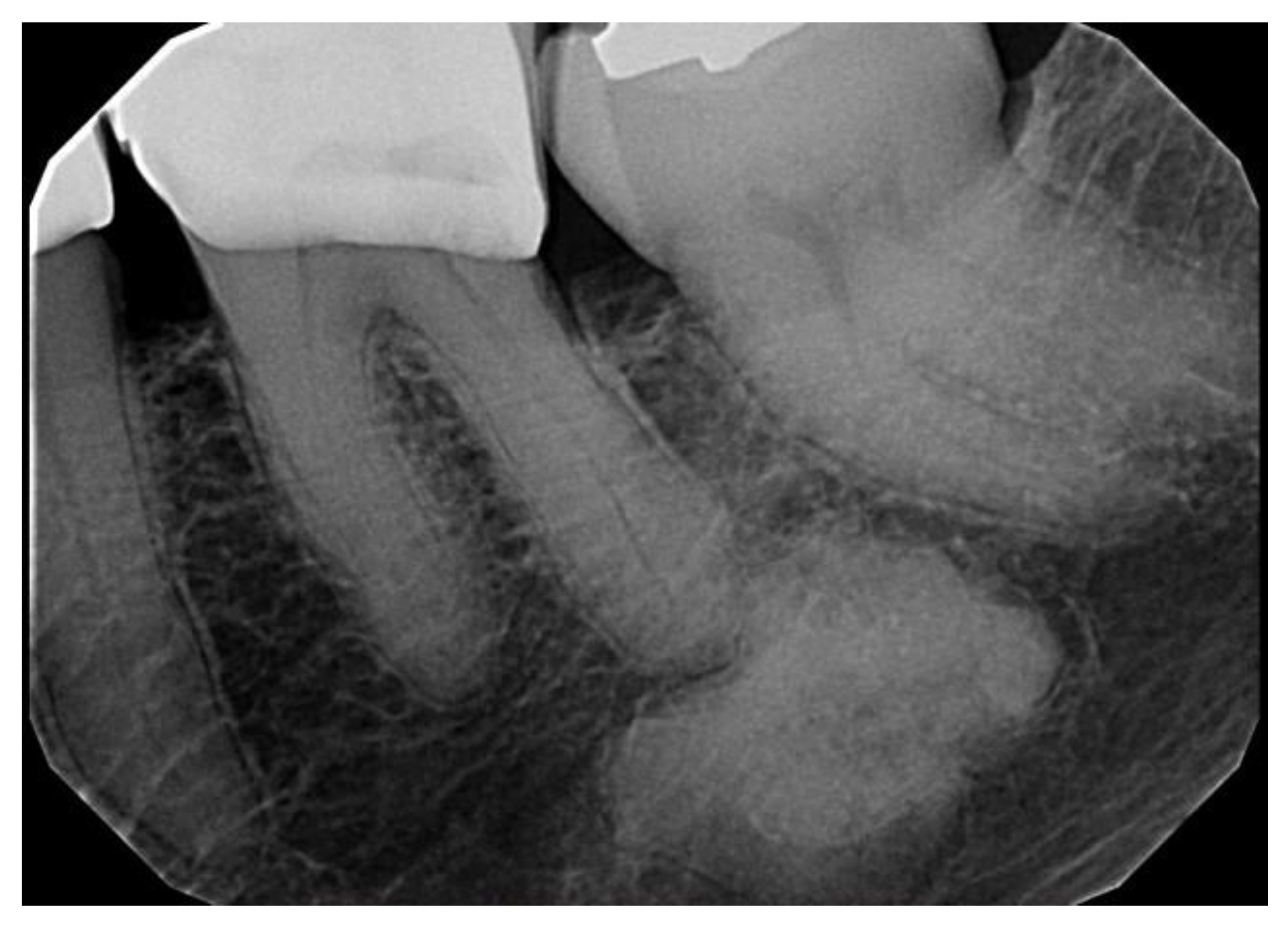
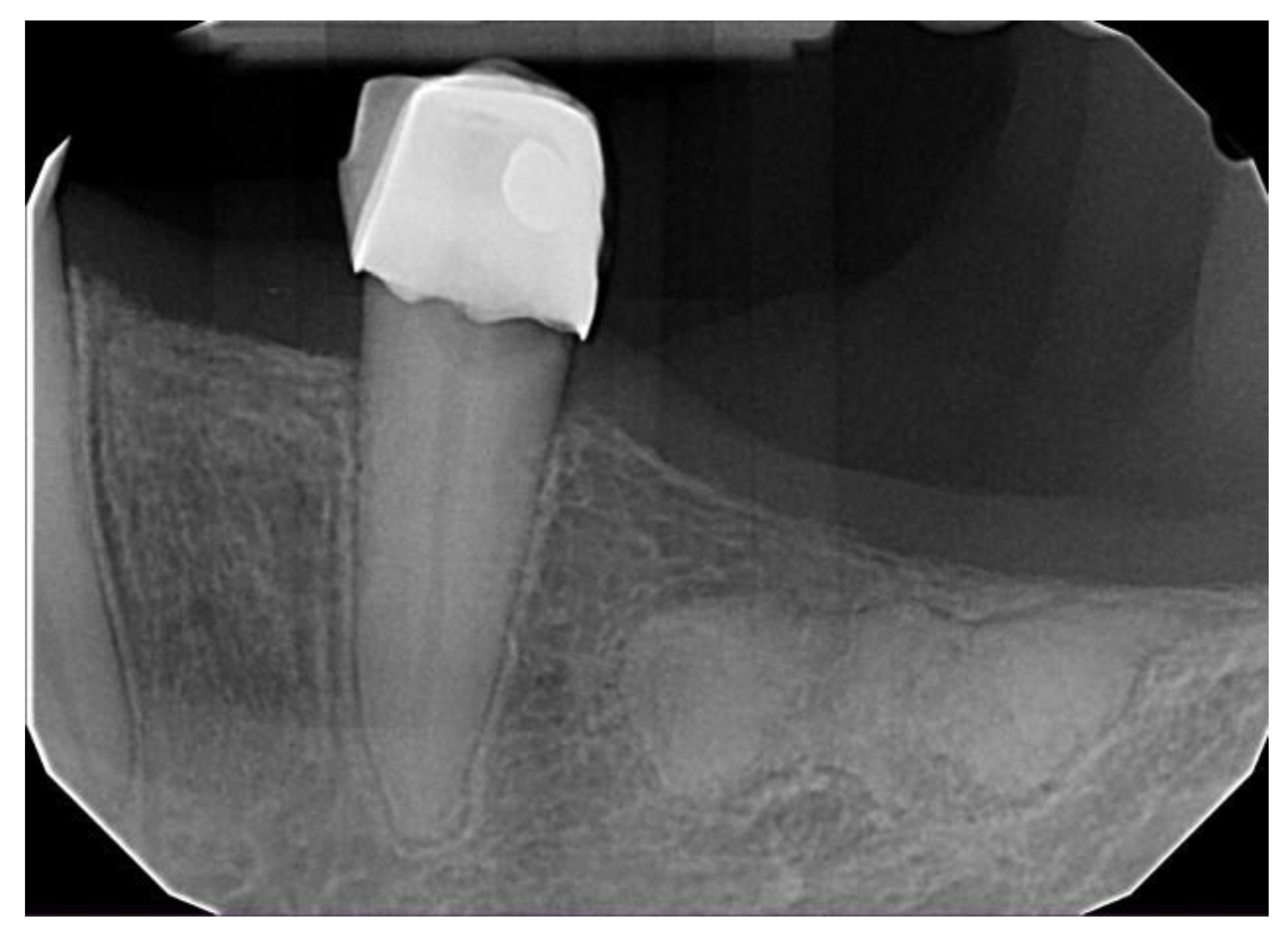
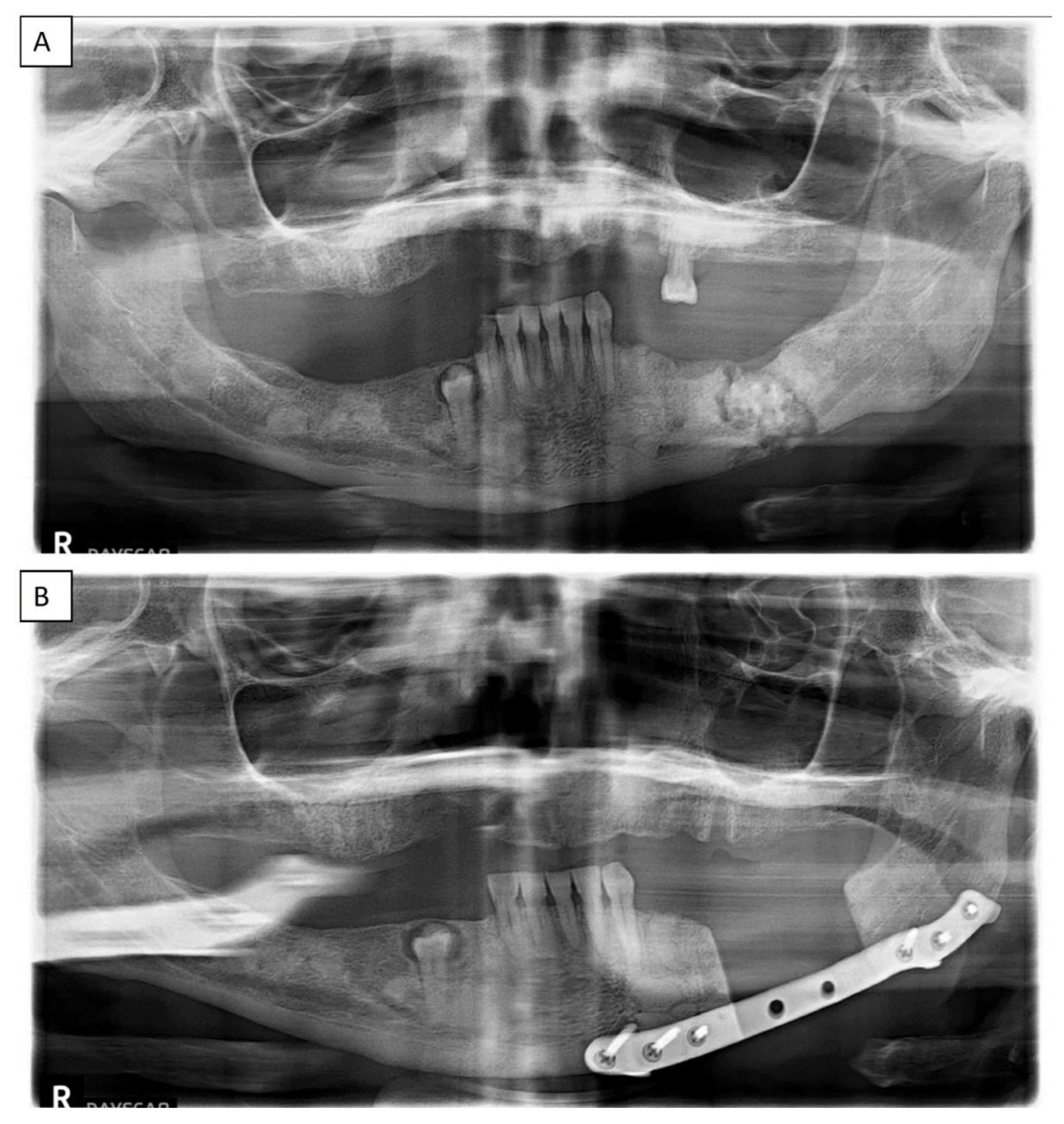

| All COD | Florid COD | Periapical COD | Focal COD | |
|---|---|---|---|---|
| Gender | ||||
| Male | 178 (93.2%) | 82 (96.5%) | 55 (87.3%) | 41 (95.3%) |
| Female | 13 (6.8%) | 3 (3.5%) | 8 (12.7%) | 2 (4.7%) |
| Age distribution | ||||
| 10–19 | 2 | - | - | 2 |
| 20–29 | 9 | 2 | 3 | 4 |
| 30–39 | 36 | 5 | 22 | 9 |
| 40–49 | 31 | 18 | 6 | 7 |
| 50–59 | 45 | 17 | 17 | 11 |
| 60–69 | 39 | 23 | 11 | 5 |
| 70–79 | 22 | 16 | 2 | 4 |
| 80–89 | 6 | 3 | 2 | 1 |
| 90–99 | 1 | 1 | - | - |
| Ethnicity | ||||
| African American | 160 (83.8%) | 85 (100%) | 53 (84.1%) | 22 (51.2%) |
| Caucasian | 24 (12.6%) | - | 7 (11.1%) | 17 (39.5%) |
| Hispanic | 4 (2.1%) | - | 2 (3.2%) | 3 (7%) |
| Asian | 3 (1.6%) | - | 1 (1.6%) | 1 (2.3%) |
| Case No. | Age | Gender | Ethnicity | COD Type | Symptom (s) |
|---|---|---|---|---|---|
| 1 | 66 | F | AA | Florid | Draining fistula/discharge |
| 2 | 79 | F | AA | Florid | Exposed bone |
| 3 | 60 | F | AA | Florid | Exposed bone |
| 4 | 63 | F | AA | Florid | Pain, abscess, and failed implant |
| 5 | 41 | F | AA | Florid | Draining fistula/discharge and pain |
| 6 | 52 | F | AA | Florid | Non-healing extraction sites |
| 7 | 66 | F | AA | Florid | Pain |
| 8 | 84 | F | AA | Florid | Pain |
| 9 | 48 | F | AA | Florid | Pain |
| 10 | 44 | F | AA | Florid | Pain |
| 11 | 65 | F | AA | Florid | Pain and swelling |
| 12 | 67 | F | AA | Florid | Pain and bone exposure |
| 13 | 42 | F | AA | Florid | Pain and expansion |
| 14 | 63 | F | AA | Florid | Pain and foul discharge |
| 15 | 57 | F | AA | Florid | Pain and swelling |
| 16 | 64 | F | AA | Florid | Pain |
| 17 | 54 | F | AA | Florid | Pain |
| 18 | 68 | F | AA | Florid | Pain |
| 19 | 59 | F | AA | Florid | Pain and swelling after extractions |
| 20 | 72 | F | C | Focal | Pain and purulent discharge |
| 21 | 73 | F | C | Focal | Pain and swelling |
| 22 | 72 | F | C | Focal | Pain and swelling |
| 23 | 63 | F | AA | Focal | Pain and swelling |
| 24 | 59 | F | C | Focal | Intermittent pain |
| 25 | 60 | F | AA | Focal | Pain and swelling |
| 26 | 62 | F | AA | Focal | Pain |
| 27 | 54 | F | C | Focal | Intermittent pain and pressure |
| 28 | 59 | F | AA | Periapical | Redness and swelling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decolibus, K.; Shahrabi-Farahani, S.; Brar, A.; Rasner, S.D.; Aguirre, S.E.; Owosho, A.A. Cemento-Osseous Dysplasia of the Jaw: Demographic and Clinical Analysis of 191 New Cases. Dent. J. 2023, 11, 138. https://doi.org/10.3390/dj11050138
Decolibus K, Shahrabi-Farahani S, Brar A, Rasner SD, Aguirre SE, Owosho AA. Cemento-Osseous Dysplasia of the Jaw: Demographic and Clinical Analysis of 191 New Cases. Dentistry Journal. 2023; 11(5):138. https://doi.org/10.3390/dj11050138
Chicago/Turabian StyleDecolibus, Katherine, Shokoufeh Shahrabi-Farahani, Anmol Brar, Shane D. Rasner, Sarah E. Aguirre, and Adepitan A. Owosho. 2023. "Cemento-Osseous Dysplasia of the Jaw: Demographic and Clinical Analysis of 191 New Cases" Dentistry Journal 11, no. 5: 138. https://doi.org/10.3390/dj11050138
APA StyleDecolibus, K., Shahrabi-Farahani, S., Brar, A., Rasner, S. D., Aguirre, S. E., & Owosho, A. A. (2023). Cemento-Osseous Dysplasia of the Jaw: Demographic and Clinical Analysis of 191 New Cases. Dentistry Journal, 11(5), 138. https://doi.org/10.3390/dj11050138






