Reliability and Failure Mode of Ti-Base Abutments Supported by Narrow/Wide Implant Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Fatigue Testing
3. Results
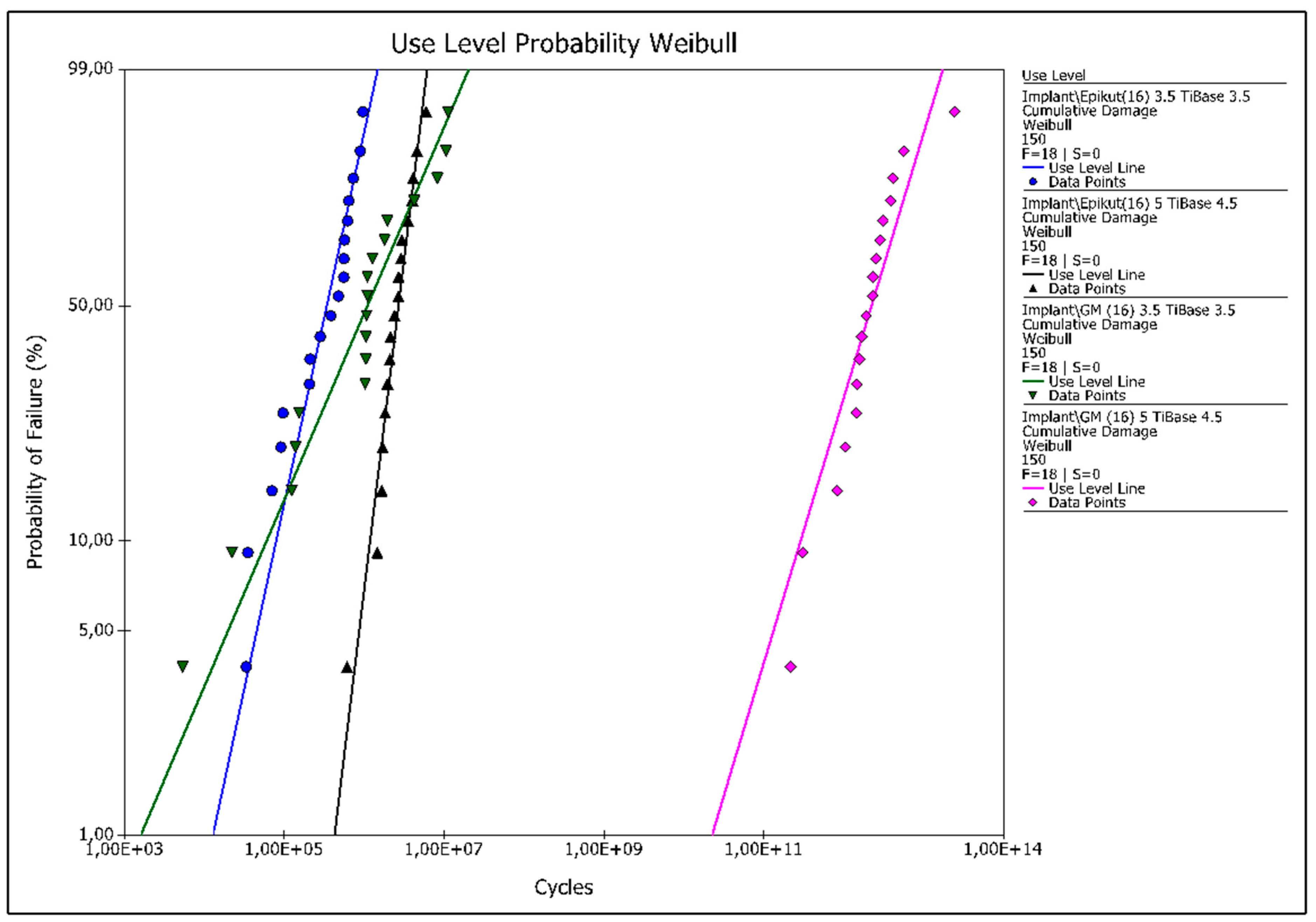
3.1. Reliability
3.2. Failure Modes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hjalmarsson, L.; Gheisarifar, M.; Jemt, T. A systematic review of survival of single implants as presented in longitudinal studies with a follow-up of at least 10 years. Eur. J. Oral Implantol. 2016, 9 (Suppl. S1), S155–S162. [Google Scholar] [PubMed]
- Pieralli, S.; Kohal, R.J.; Rabel, K.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Clinical outcomes of partial and full-arch all-ceramic implant-supported fixed dental prostheses. A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 224–236. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 199–214. [Google Scholar] [CrossRef]
- Rabel, K.; Spies, B.C.; Pieralli, S.; Vach, K.; Kohal, R.J. The clinical performance of all-ceramic implant-supported single crowns: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 196–223. [Google Scholar] [CrossRef]
- Sailer, I.; Strasding, M.; Valente, N.A.; Zwahlen, M.; Liu, S.; Pjetursson, B.E. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 184–198. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surgery. Suppl. 1977, 16, 1–132. [Google Scholar]
- Romero-Millan, J.J.; Aizcorbe-Vicente, J.; Penarrocha-Diago, M.; Galindo-Moreno, P.; Canullo, L.; Penarrocha-Oltra, D. Implants in the Posterior Maxilla: Open Sinus Lift Versus Conventional Implant Placement. A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, e65–e76. [Google Scholar] [CrossRef]
- Al-Johany, S.S. Survival Rates of Short Dental Implants (</= 6.5 mm) Placed in Posterior Edentulous Ridges and Factors Affecting their Survival after a 12-Month Follow-up Period: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, 605–621. [Google Scholar] [CrossRef]
- Cheng, Q.; Su, Y.Y.; Wang, X.; Chen, S. Clinical Outcomes Following Immediate Loading of Single-Tooth Implants in the Esthetic Zone: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 167–177. [Google Scholar] [CrossRef]
- Gallardo, Y.N.R.; da Silva-Olivio, I.R.; Gonzaga, L.; Sesma, N.; Martin, W. A Systematic Review of Clinical Outcomes on Patients Rehabilitated with Complete-Arch Fixed Implant-Supported Prostheses According to the Time of Loading. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2019, 28, 958–968. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R. Osseointegration of metallic devices: Current trends based on implant hardware design. Arch. Biochem. Biophys. 2014, 561, 99–108. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Jimbo, R.; Witek, L.; Tovar, N.; Neiva, R.; Torroni, A.; Coelho, P.G. Biomaterial and biomechanical considerations to prevent risks in implant therapy. Periodontol 2000 2019, 81, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Benalcazar Jalkh, E.B.; Parra, M.; Torroni, A.; Nayak, V.V.; Tovar, N.; Castellano, A.; Badalov, R.M.; Bonfante, E.A.; Coelho, P.G.; Witek, L. Effect of supplemental acid-etching on the early stages of osseointegration: A preclinical model. J. Mech. Behav. Biomed. Mater. 2021, 122, 104682. [Google Scholar] [CrossRef]
- Benic, G.I.; Mokti, M.; Chen, C.J.; Weber, H.P.; Hammerle, C.H.; Gallucci, G.O. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: A clinical and cone beam computed tomography study. Clin. Oral Implant. Res. 2012, 23, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Merheb, J.; Quirynen, M. Critical horizontal dimensions of interproximal and buccal bone around implants for optimal aesthetic outcomes: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 134–145. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Martin, W.; Belser, U.C. Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. Int. J. Oral Maxillofac. Implant. 2004, 19, 43–61. [Google Scholar]
- Walton, J.N.; MacEntee, M.I. Choosing or refusing oral implants: A prospective study of edentulous volunteers for a clinical trial. Int. J. Prosthodont. 2005, 18, 483–488. [Google Scholar] [CrossRef]
- Zinsli, B.; Sagesser, T.; Mericske, E.; Mericske-Stern, R. Clinical evaluation of small-diameter ITI implants: A prospective study. Int. J. Oral Maxillofac. Implant. 2004, 19, 92–99. [Google Scholar]
- Andersen, E.; Saxegaard, E.; Knutsen, B.M.; Haanaes, H.R. A prospective clinical study evaluating the safety and effectiveness of narrow-diameter threaded implants in the anterior region of the maxilla. Int. J. Oral Maxillofac. Implant. 2001, 16, 217–224. [Google Scholar]
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental Implant Length and Diameter: A Proposed Classification Scheme. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2017, 26, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Almeida, E.O.; Lorenzoni, F.C.; Coelho, P.G. Effects of implant diameter and prosthesis retention system on the reliability of single crowns. Int. J. Oral Maxillofac. Implant. 2015, 30, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Freitas, G.P.; Hirata, R.; Bonfante, E.A.; Tovar, N.; Coelho, P.G. Survival Probability of Narrow and Standard-Diameter Implants with Different Implant-Abutment Connection Designs. Int. J. Prosthodont. 2016, 29, 179–185. [Google Scholar] [CrossRef]
- Malo, P.; de Araujo Nobre, M. Implants (3.3 mm diameter) for the rehabilitation of edentulous posterior regions: A retrospective clinical study with up to 11 years of follow-up. Clin. Implant. Dent. Relat. Res. 2011, 13, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Al-Nawas, B. Narrow-diameter implants: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 21–40. [Google Scholar] [CrossRef]
- Klein, M.O.; Schiegnitz, E.; Al-Nawas, B. Systematic review on success of narrow-diameter dental implants. Int. J. Oral Maxillofac. Implant. 2014, 29, 43–54. [Google Scholar] [CrossRef]
- Allum, S.R.; Tomlinson, R.A.; Joshi, R. The impact of loads on standard diameter, small diameter and mini implants: A comparative laboratory study. Clin. Oral Implant. Res. 2008, 19, 553–559. [Google Scholar] [CrossRef]
- Bordin, D.; Witek, L.; Fardin, V.P.; Bonfante, E.A.; Coelho, P.G. Fatigue Failure of Narrow Implants with Different Implant-Abutment Connection Designs. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2016, 27, 659–664. [Google Scholar] [CrossRef]
- Almeida, E.O.; Freitas, A.C., Jr.; Bonfante, E.A.; Marotta, L.; Silva, N.R.; Coelho, P.G. Mechanical testing of implant-supported anterior crowns with different implant/abutment connections. Int. J. Oral Maxillofac. Implant. 2013, 28, 103–108. [Google Scholar] [CrossRef]
- Bonfante, E.A.; Coelho, P.G. A Critical Perspective on Mechanical Testing of Implants and Prostheses. Adv. Dent. Res. 2016, 28, 18–27. [Google Scholar] [CrossRef]
- Freitas-Junior, A.C.; Rocha, E.P.; Bonfante, E.A.; Almeida, E.O.; Anchieta, R.B.; Martini, A.P.; Assuncao, W.G.; Silva, N.R.; Coelho, P.G. Biomechanical evaluation of internal and external hexagon platform switched implant-abutment connections: An in vitro laboratory and three-dimensional finite element analysis. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, e218–e228. [Google Scholar] [CrossRef]
- Machado, L.S.; Bonfante, E.A.; Anchieta, R.B.; Yamaguchi, S.; Coelho, P.G. Implant-abutment connection designs for anterior crowns: Reliability and failure modes. Implant. Dent. 2013, 22, 540–545. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; de Araujo-Junior, E.N.S.; Lopes, A.C.O.; Coelho, P.G.; Zahoui, A.; Benalcazar Jalkh, E.B.; Bonfante, E.A. Failure Modes and Survival of Anterior Crowns Supported by Narrow Implant Systems. Biomed. Res. Int. 2020, 2020, 1057846. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; Bordin, D.; Ramalho, I.S.; Lopes, A.C.O.; Gomes, R.S.; Kaizer, M.; Witek, L.; Bonfante, E.A.; Coelho, P.G.; Del Bel Cury, A.A. Zirconia-reinforced lithium silicate crowns: Effect of thickness on survival and failure mode. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W. Accelerated Testing: Statistical Models, Test Plans and Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Zhao, W.E.; Elsayed, E.A. A general accelerated life model for step-stress testing. IIE Trans. 2005, 37, 1059–1069. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barao, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 197–212. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y. Clinical complications with implants and implant prostheses. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontol 2000 2022, 88, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gonzalez, J.A.; Kulkarni, P.A.; Nagy, W.W.; Griggs, J.A. Fatigue lifetime prediction of a reduced-diameter dental implant system: Numerical and experimental study. Dent. Mater. 2018, 34, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Hatakeyama, E.; Sasaki, T.; Dan, H.; Azuma, T.; Karita, K. Effects of sample hardness on human chewing force: A model study using silicone rubber. Arch. Oral Biol. 2004, 49, 805–816. [Google Scholar] [CrossRef]
- Moriwaki, H.; Yamaguchi, S.; Nakano, T.; Yamanishi, Y.; Imazato, S.; Yatani, H. Influence of Implant Length and Diameter, Bicortical Anchorage, and Sinus Augmentation on Bone Stress Distribution: Three-Dimensional Finite Element Analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, e84–e91. [Google Scholar] [CrossRef] [PubMed]
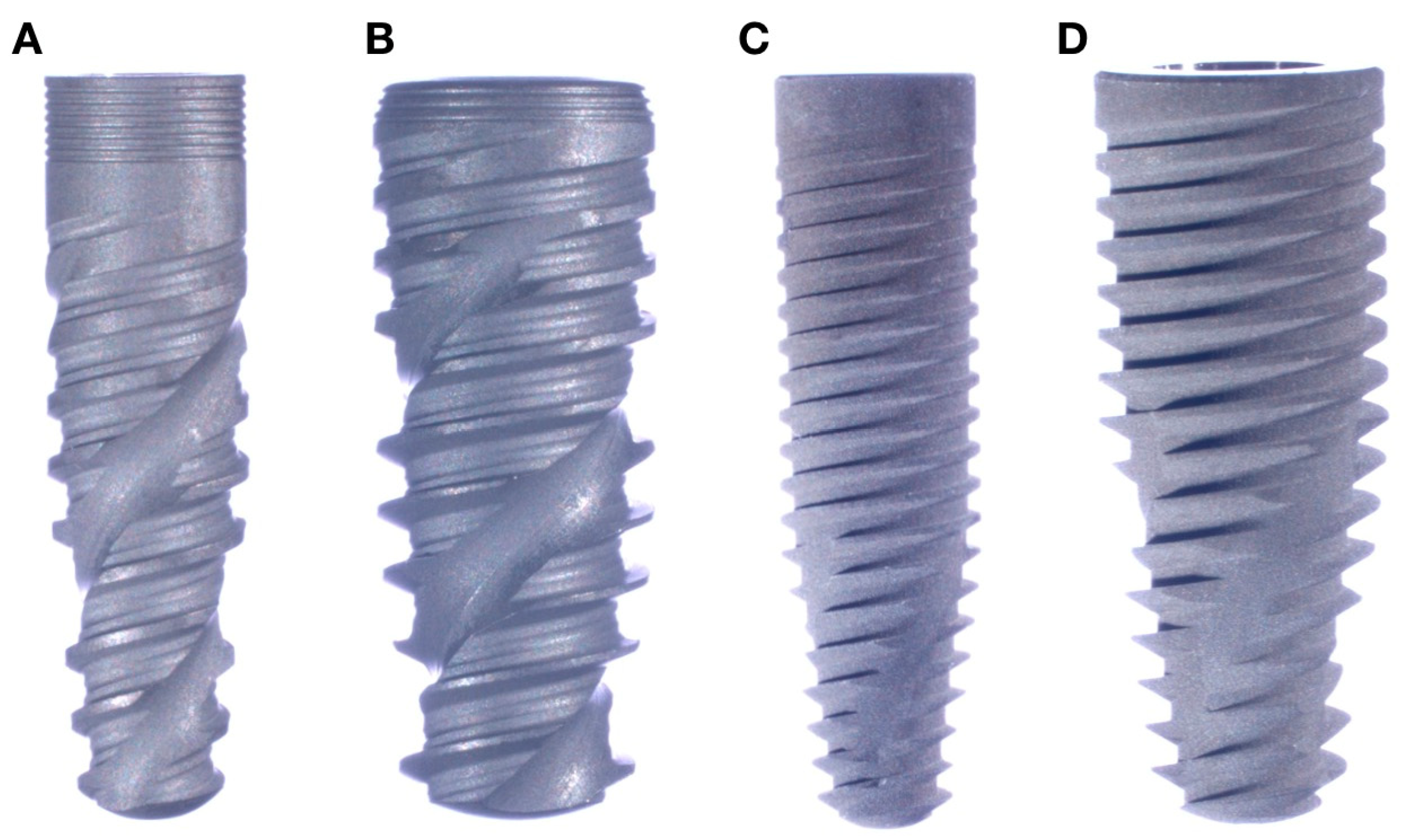



| Epikut 3.5 (3.5 Ti-Base) | GM 3.5 (3.5 Ti-Base) | Epikut 5 (4.5 Ti-Base) | GM 5 (4.5 Ti-Base) | |
|---|---|---|---|---|
| Upper Bound | 0.99 | 0.99 | 1 | 1 |
| Probability of Survival 100 N | 0.99 Aa | 0.99 Aa | 1 Aa | 1 Aa |
| Lower Bound | 0.98 | 0.94 | 0.99 | 1 |
| Upper Bound | 0.95 | 0.95 | 0.99 | 1 |
| Probability of Survival 150 N | 0.86 Bb | 0.86 Ab | 0.99 Aa | 1 Aa |
| Lower Bound | 0.61 | 0.62 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benalcázar-Jalkh, E.B.; de Carvalho, L.F.; Alves, L.M.M.; Campos, T.M.B.; Sousa, E.d.O.; Bergamo, E.T.P.; Coelho, P.G.; Gierthmuehlen, P.C.; Spitznagel, F.A.; Zahoui, A.; et al. Reliability and Failure Mode of Ti-Base Abutments Supported by Narrow/Wide Implant Systems. Dent. J. 2023, 11, 207. https://doi.org/10.3390/dj11090207
Benalcázar-Jalkh EB, de Carvalho LF, Alves LMM, Campos TMB, Sousa EdO, Bergamo ETP, Coelho PG, Gierthmuehlen PC, Spitznagel FA, Zahoui A, et al. Reliability and Failure Mode of Ti-Base Abutments Supported by Narrow/Wide Implant Systems. Dentistry Journal. 2023; 11(9):207. https://doi.org/10.3390/dj11090207
Chicago/Turabian StyleBenalcázar-Jalkh, Ernesto B., Laura F. de Carvalho, Larissa M. M. Alves, Tiago M. B. Campos, Edisa de Oliveira Sousa, Edmara T. P. Bergamo, Paulo G. Coelho, Petra C. Gierthmuehlen, Frank A. Spitznagel, Abbas Zahoui, and et al. 2023. "Reliability and Failure Mode of Ti-Base Abutments Supported by Narrow/Wide Implant Systems" Dentistry Journal 11, no. 9: 207. https://doi.org/10.3390/dj11090207







