Shock Absorption Capacity of High-Performance Polymers for Dental Implant-Supported Restorations: In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
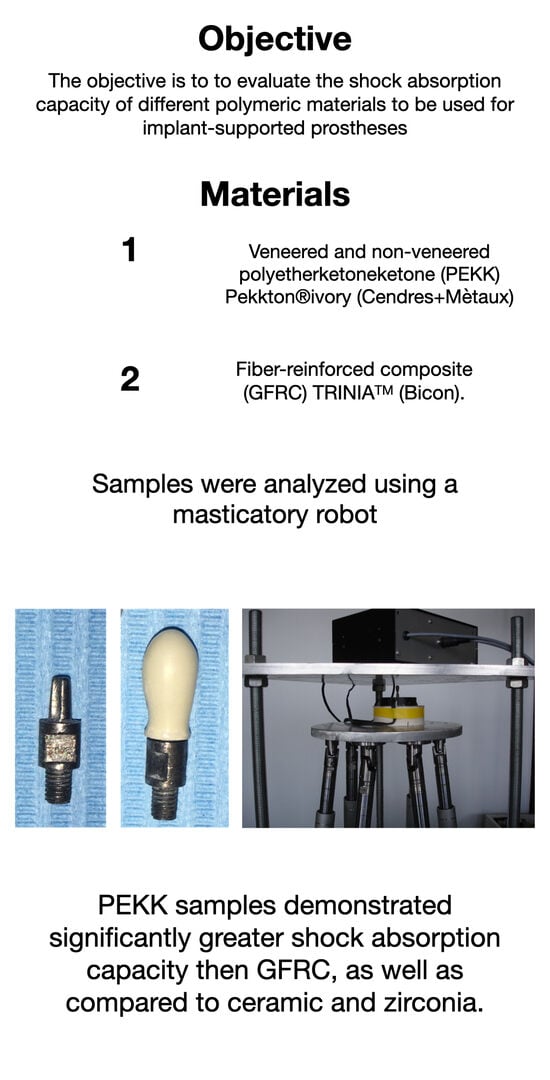
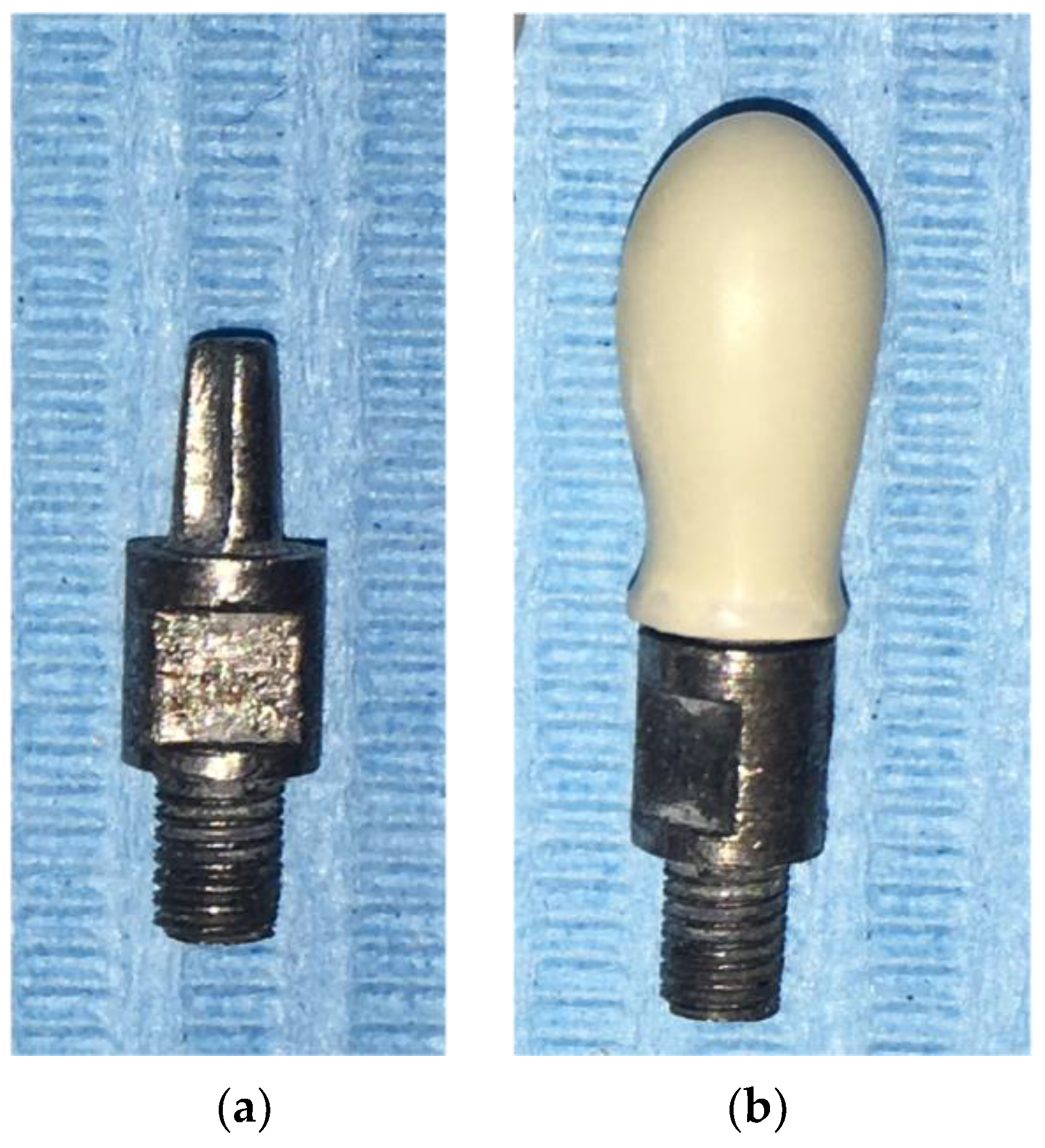
2.1. Sample Crowns
2.2. Test Set-Up
2.3. Statistical Analysis
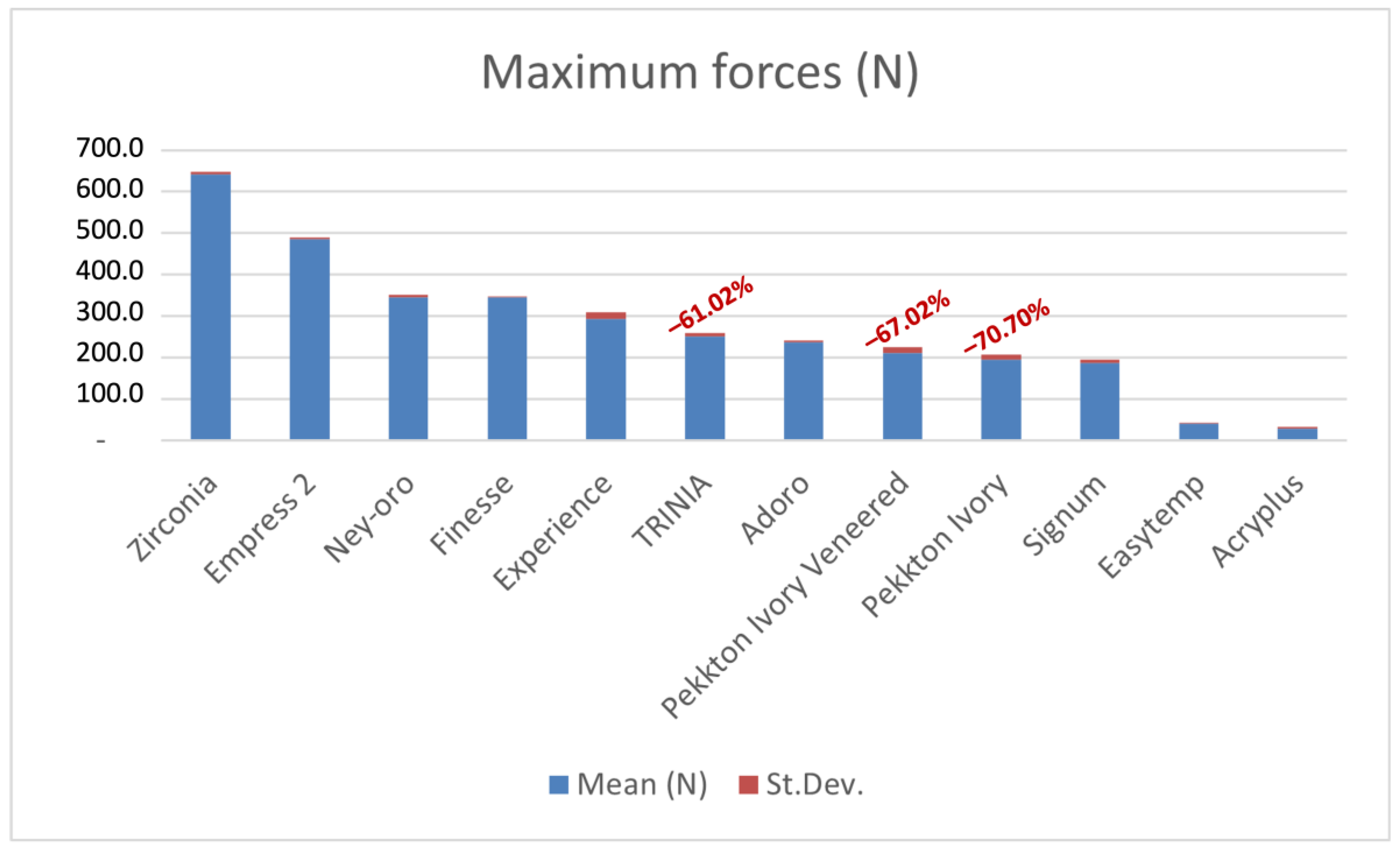
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delucchi, F.; De Giovanni, E.; Pesce, P.; Bagnasco, F.; Pera, F.; Baldi, D.; Menini, M. Framework Materials for Full-Arch Implant-Supported Rehabilitations: A Systematic Review of Clinical Studies. Materials 2021, 14, 3251. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Showva, N.N.; Ahmed, M.; Rashid, A.B.; Sadique, S.E.; El-Bialy, T.; Xu, H. Titanium and titanium alloys in dentistry: Current trends, recent developments, and future prospects. Heliyon 2022, 8, e11300. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.A.; Pinto, P.; Vaz, P.C.S.; Silva, F.S. Design and optimization of zirconia functional surfaces for dental implants applications. Ceram. Int. 2020, 46, 16328–16336. [Google Scholar] [CrossRef]
- Kondo, T.; Komine, F.; Honda, J.; Takata, H.; Moriya, Y. Effect of veneering materials on fracture loads of implant-supported zirconia molar fixed dental prostheses. J. Prosthodont. Res. 2019, 63, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Lal, K. Computer-assisted design/computer-assisted manufacturing zirconia implant fixed complete prostheses: Clinical results and technical complications up to 4 years of function. Clin. Oral Implant. Res. 2013, 24, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Tiossi, R.; Gomes, E.A.; Faria, A.C.L.; Rodrigues, R.C.S.; Ribeiro, R.F. Biomechanical behavior of titanium and zirconia frameworks for implant-supported full-arch fixed dental prosthesis. Clin. Implant. Dent. Relat. Res. 2017, 19, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, A.A.; Lim, K.G.; Narhi, T.O.; Cooper, L.F. Complete-arch implant-supported monolithic zirconia fixed dental prostheses: A systematic review. J. Prosthet. Dent. 2016, 115, 672–677.e1. [Google Scholar] [CrossRef] [PubMed]
- Collaert, B.; De Bruyn, H. Immediate functional loading of TiOblast dental implants in full-arch edentulous maxillae: A 3-year prospective study. Clin. Oral Implant. Res. 2008, 19, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K. An overview of development and status of fiber-reinforced composites as dental and medical biomaterials. Acta Biomater. Odontol. Scand. 2018, 4, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pesce, P.; Bevilacqua, M.; Pera, F.; Tealdo, T.; Barberis, F.; Pera, P. Effect of Framework in an Implant-Supported Full-Arch Fixed Prosthesis: 3D Finite Element Analysis. Int. J. Prosthodont. 2015, 28, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pesce, P.; Pera, F.; Barberis, F.; Lagazzo, A.; Bertola, L.; Pera, P. Biological and mechanical characterization of carbon fiber frameworks for dental implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Matinlinna, J.P. E-Glass Fiber Reinforced Composites in Dental Applications. Silicon 2011, 4, 73–78. [Google Scholar] [CrossRef]
- Maloo, L.M.; Toshniwal, S.H.; Reche, A.; Paul, P.; Wanjari, M.B. A Sneak Peek Toward Polyaryletherketone (PAEK) Polymer: A Review. Cureus 2022, 14, e31042. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Hassantash, S.; Chinian, S.; Sadr, A.; Habibzadeh, S. Fracture strength and three-dimensional marginal evaluation of biocompatible high-performance polymer versus pressed lithium disilicate crowns. J. Prosthet. Dent. 2023, 130, 132.e1–132.e9. [Google Scholar] [CrossRef] [PubMed]
- Elashmawy, Y.; Elshahawy, W.; Seddik, M.; Aboushelib, M. Influence of fatigue loading on fracture resistance of endodontically treated teeth restored with endocrowns. J. Prosthodont. Res. 2021, 65, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Zivic, M.; Milosavljevic, M. A potential application of materials based on a polymer and CAD/CAM composite resins in prosthetic dentistry. J. Prosthodont. Res. 2021, 65, 137–147. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Rashid Habib, S.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Han, K.H.; Lee, J.Y.; Shin, S.W. Implant- and Tooth-Supported Fixed Prostheses Using a High-Performance Polymer (Pekkton) Framework. Int. J. Prosthodont. 2016, 29, 451–454. [Google Scholar] [CrossRef]
- Klur, T.; Hasan, I.; Ottersbach, K.; Stark, H.; Fichte, M.; Dirk, C.; Bourauel, C. PEKK-made indirect temporary crowns and bridges: A clinical pilot study. Clin. Oral Investig. 2019, 23, 771–777. [Google Scholar] [CrossRef]
- Villefort, R.F.; Diamantino, P.J.S.; Zeidler, S.; Borges, A.L.S.; Silva-Concilio, L.R.; Saavedra, G.; Tribst, J.P.M. Mechanical Response of PEKK and PEEK As Frameworks for Implant-Supported Full-Arch Fixed Dental Prosthesis: 3D Finite Element Analysis. Eur. J. Dent. 2022, 16, 115–121. [Google Scholar] [CrossRef]
- Elkabbany, A.; Kern, M.; Elkhadem, A.H.; Wille, S.; Amer, A.A.; Chaar, M.S. Retention of metallic and non-metallic double-crown-retained mandibular overdentures on implants: An in-vitro study. J. Prosthodont. Res. 2020, 64, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Shin, J.H.; Kim, J.E.; Kim, J.H.; Lee, W.C.; Shin, S.W.; Lee, J.Y. Corrigendum to “Biomechanical Evaluation of a Tooth Restored with High Performance Polymer PEKK Post-Core System: A 3D Finite Element Analysis”. BioMed Res. Int. 2017, 2017, 7196847. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Haro Adanez, M.; Att, W. Current status of zirconia implants in dentistry: Preclinical tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Choi, J.W.; Jeon, Y.C.; Jeong, C.M.; Lee, S.H.; Kang, E.S.; Yun, M.J.; Huh, J.B. Comparison of the Microtensile Bond Strength of a Polyetherketoneketone (PEKK) Tooth Post Cemented with Various Surface Treatments and Various Resin Cements. Materials 2018, 11, 916. [Google Scholar] [CrossRef]
- Alsadon, O.; Wood, D.; Patrick, D.; Pollington, S. Fatigue behavior and damage modes of high performance poly-ether-ketone-ketone PEKK bilayered crowns. J. Mech. Behav. Biomed. Mater. 2020, 110, 103957. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Ji, K.; Lu, W.; Wu, G.; Tan, B.; Jing, J.; Ji, J. Polyetherketoneketone, a high-performance polymer for splinting mobile teeth: A clinical report. J. Prosthet. Dent. 2023, in press. [Google Scholar] [CrossRef]
- Salem, M.T.; El-Layeh, M.; El-Farag, S.A.A.; Salem, A.S.; Attia, A. Clinical assessment of different implant-supported esthetic crown systems fabricated with semi-digital workflow: Two-year prospective study. J. Esthet. Restor. Dent. 2022, 34, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Turksayar, A.A.D.; Atsu, S.S. Fracture Resistance of Zirconia, Polyetheretherketone, and Polyetherketoneketone Implant Abutments After Aging. Int. J. Oral Maxillofac. Implant. 2021, 36, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Steiner, M.; Freitag-Wolf, S.; Kern, M. Resin bonding to three types of polyaryletherketones (PAEKs)-durability and influence of surface conditioning. Dent. Mater. 2014, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Moharil, S.; Reche, A.; Durge, K. Polyetheretherketone (PEEK) as a Biomaterial: An Overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef] [PubMed]
- Lommen, J.; Schorn, L.; Sproll, C.; Haussmann, J.; Kubler, N.R.; Budach, W.; Rana, M.; Tamaskovics, B. Reduction of CT Artifacts Using Polyetheretherketone (PEEK), Polyetherketoneketone (PEKK), Polyphenylsulfone (PPSU), and Polyethylene (PE) Reconstruction Plates in Oral Oncology. J. Oral Maxillofac. Surg. 2022, 80, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Skalak, R. Biomechanical considerations in osseointegrated prostheses. J. Prosthet. Dent. 1983, 49, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Pera, P. Invited commentary: On immediately loaded fixed maxillary prostheses. Int. J. Prosthodont. 2014, 27, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Conserva, E.; Tealdo, T.; Bevilacqua, M.; Pera, F.; Signori, A.; Pera, P. Shock absorption capacity of restorative materials for dental implant prostheses: An in vitro study. Int. J. Prosthodont. 2013, 26, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Conserva, E.; Menini, M.; Tealdo, T.; Bevilacqua, M.; Pera, F.; Ravera, G.; Pera, P. Robotic chewing simulator for dental materials testing on a sensor-equipped implant setup. Int. J. Prosthodont. 2008, 21, 501–508. [Google Scholar] [PubMed]
- Liebermann, A.; Wimmer, T.; Schmidlin, P.R.; Scherer, H.; Loffler, P.; Roos, M.; Stawarczyk, B. Physicomechanical characterization of polyetheretherketone and current esthetic dental CAD/CAM polymers after aging in different storage media. J. Prosthet. Dent. 2016, 115, 321–328.e2. [Google Scholar] [CrossRef] [PubMed]
- Omaish, H.H.M.; Abdelhamid, A.M.; Neena, A.F. Comparison of the strain developed around implants with angled abutments with two reinforced polymeric CAD-CAM superstructure materials: An in vitro comparative study. J. Prosthet. Dent. 2022, 127, 634.e1–634.e8. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, N.; Yamaguchi, S.; Hirose, N.; Tanaka, R.; Takahashi, Y.; Imazato, S.; Hayashi, M. Evaluation of physical properties of fiber-reinforced composite resin. Dent. Mater. 2020, 36, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, K.C.; Bhat, V.; Hegde, C. Finite element analysis of the effect of framework materials at the bone-implant interface in the all-on-four implant system. Dent. Res. J. 2021, 18, 1. [Google Scholar]
- Dawson, J.H.; Hyde, B.; Hurst, M.; Harris, B.T.; Lin, W.S. Polyetherketoneketone (PEKK), a framework material for complete fixed and removable dental prostheses: A clinical report. J. Prosthet. Dent. 2018, 119, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Katzenbach, A.; Dorsam, I.; Stark, H.; Bourauel, C.; Keilig, L. Fatigue behaviour of dental crowns made from a novel high-performance polymer PEKK. Clin. Oral Investig. 2021, 25, 4895–4905. [Google Scholar] [CrossRef] [PubMed]
- Shash, Y.H.; El-Wakad, M.T.; El-Dosoky, M.A.A.; Dohiem, M.M. Evaluation of stresses on mandible bone and prosthetic parts in fixed prosthesis by utilizing CFR-PEEK, PEKK and PEEK frameworks. Sci. Rep. 2023, 13, 11542. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Shin, S.W.; Lee, S.P.; Kim, J.E.; Kim, J.H.; Lee, J.Y. Comparative Evaluation of a Four-Implant-Supported Polyetherketoneketone Framework Prosthesis: A Three-Dimensional Finite Element Analysis Based on Cone Beam Computed Tomography and Computer-Aided Design. Int. J. Prosthodont. 2017, 30, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Gracis, S.E.; Nicholls, J.I.; Chalupnik, J.D.; Yuodelis, R.A. Shock-absorbing behavior of five restorative materials used on implants. Int. J. Prosthodont. 1991, 4, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Dhaliwal, S.; Naert, I.; Mine, A.; Kronstrom, M.; Sasaki, K.; Duyck, J. Impact of implant number, distribution and prosthesis material on loading on implants supporting fixed prostheses. J. Oral Rehabil. 2010, 37, 525–531. [Google Scholar] [CrossRef] [PubMed]

| Material Property | TRINIATM | Pekkton®ivory |
|---|---|---|
| Flexural strength | 393 MPa | 200 MPa |
| Flexural modulus of elasticity | 18.8 GPa | 5.1 GPa |
| Tensile strength | 169 MPa | 119 MPa |
| Compressive strength | 339 MPa | 246 MPa |
| Rockwell hardness (R-scale) | 870 HV | 33 HV |
| Density | 1.68 g/cm3 | 1.4 g/cm3 |
| Fracture strength | 9.7 MPa m1/2 | 115 MPa |
| Water absorption | 0.03% | 8.7 μg/mm3 |
| Pekkton®ivory | Pekkton®ivory Veneered | TRINIATM | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD (N) | Min (N) | Max (N) | Mean ± SD (N) | Min (N) | Max (N) | Mean ± SD (N) | Min (N) | Max (N) |
| 194.454 ± 10.544 | 169.962 | 217.816 | 211.640 ± 12.437 | 189.975 | 239.053 | 250.203 ± 7.954 | 237.622 | 286.513 |
| Material 1 | Material 2 | Welch’s t-Test p-Value |
|---|---|---|
| Pekkton Ivory Veneered | Zirconia | <0.0001 |
| Pekkton Ivory Veneered | Empress 2 | <0.0001 |
| Pekkton Ivory Veneered | Ney-oro | <0.0001 |
| Pekkton Ivory Veneered | Finesse | <0.0001 |
| Pekkton Ivory Veneered | Experience | <0.0001 |
| Pekkton Ivory Veneered | TRINIA | <0.0001 |
| Pekkton Ivory Veneered | Adoro | 0.0165 |
| Pekkton Ivory Veneered | Pekkton Ivory Veneered | <0.0001 |
| Pekkton Ivory Veneered | Pekkton Ivory | <0.0001 |
| Pekkton Ivory Veneered | Signum | 0.0167 |
| Pekkton Ivory Veneered | Easytemp | <0.0001 |
| Pekkton Ivory Veneered | Acryplus | <0.0001 |
| Pekkton Ivory | Zirconia | <0.0001 |
| Pekkton Ivory | Empress 2 | <0.0001 |
| Pekkton Ivory | Ney-oro | <0.0001 |
| Pekkton Ivory | Finesse | <0.0001 |
| Pekkton Ivory | Experience | <0.0001 |
| Pekkton Ivory | TRINIA | <0.0001 |
| Pekkton Ivory | Adoro | 0.0012 |
| Pekkton Ivory | Pekkton Ivory Veneered | <0.0001 |
| Pekkton Ivory | Pekkton Ivory | <0.0001 |
| Pekkton Ivory | Signum | 0.2396 |
| Pekkton Ivory | Easytemp | <0.0001 |
| Pekkton Ivory | Acryplus | <0.0001 |
| TRINIA | Zirconia | <0.0001 |
| TRINIA | Empress 2 | <0.0001 |
| TRINIA | Ney-oro | <0.0001 |
| TRINIA | Finesse | <0.0001 |
| TRINIA | Experience | 0.0002 |
| TRINIA | TRINIA | <0.0001 |
| TRINIA | Adoro | 0.018 |
| TRINIA | Pekkton Ivory Veneered | <0.0001 |
| TRINIA | Pekkton Ivory | <0.0001 |
| TRINIA | Signum | <.0001 |
| TRINIA | Easytemp | <0.0001 |
| TRINIA | Acryplus | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menini, M.; Delucchi, F.; Bagnasco, F.; Baldi, D.; Canullo, L.; Setti, P.; Migliorati, M.; Simetti, E.; Pesce, P. Shock Absorption Capacity of High-Performance Polymers for Dental Implant-Supported Restorations: In Vitro Study. Dent. J. 2024, 12, 111. https://doi.org/10.3390/dj12040111
Menini M, Delucchi F, Bagnasco F, Baldi D, Canullo L, Setti P, Migliorati M, Simetti E, Pesce P. Shock Absorption Capacity of High-Performance Polymers for Dental Implant-Supported Restorations: In Vitro Study. Dentistry Journal. 2024; 12(4):111. https://doi.org/10.3390/dj12040111
Chicago/Turabian StyleMenini, Maria, Francesca Delucchi, Francesco Bagnasco, Domenico Baldi, Luigi Canullo, Paolo Setti, Marco Migliorati, Enrico Simetti, and Paolo Pesce. 2024. "Shock Absorption Capacity of High-Performance Polymers for Dental Implant-Supported Restorations: In Vitro Study" Dentistry Journal 12, no. 4: 111. https://doi.org/10.3390/dj12040111
APA StyleMenini, M., Delucchi, F., Bagnasco, F., Baldi, D., Canullo, L., Setti, P., Migliorati, M., Simetti, E., & Pesce, P. (2024). Shock Absorption Capacity of High-Performance Polymers for Dental Implant-Supported Restorations: In Vitro Study. Dentistry Journal, 12(4), 111. https://doi.org/10.3390/dj12040111