Enamel Remineralisation with a Novel Sodium Fluoride-Infused Bristle Toothbrush
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Grouping and Study Design
2.2. Toothbrushes
2.3. Demineralising Solution
2.4. Specimen Surface Testing
2.4.1. Microhardness (Vickers)
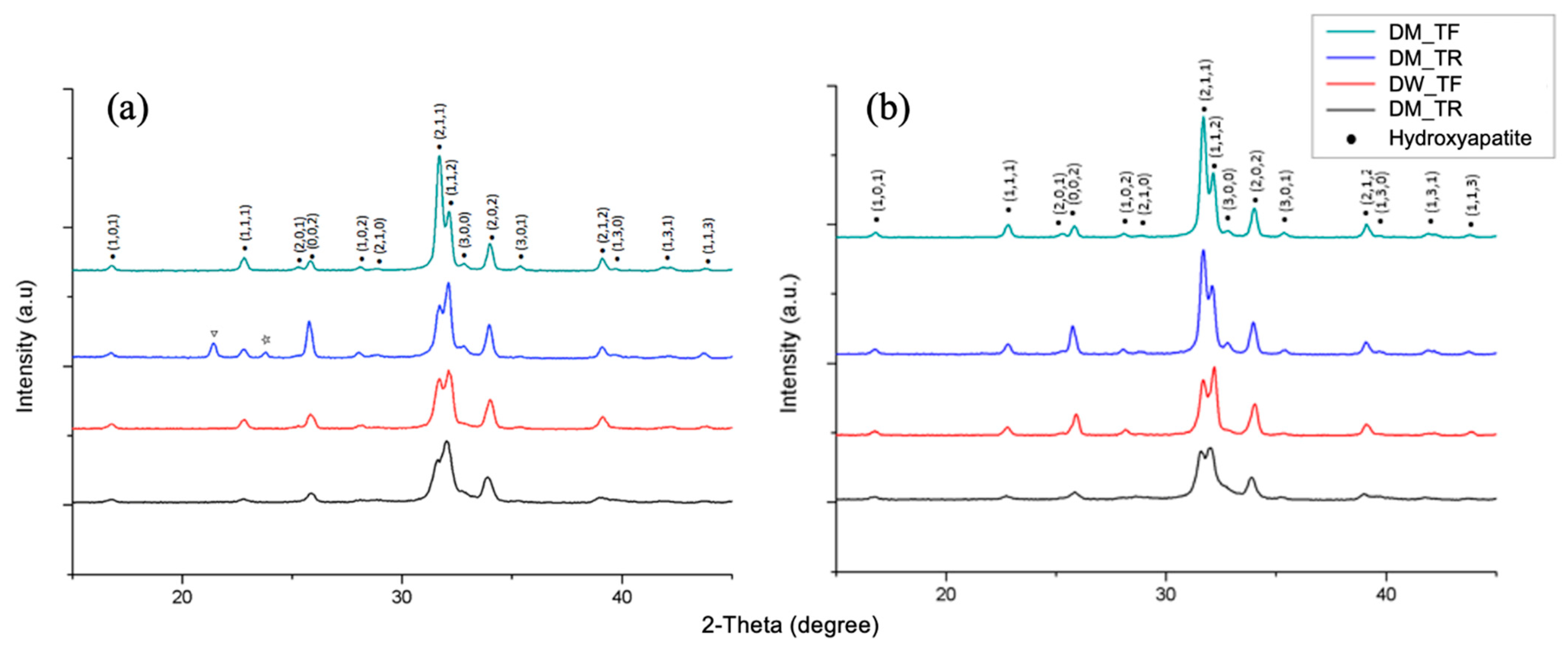
2.4.2. X-ray Diffraction (XRD)
2.4.3. Energy-Dispersive X-ray Spectroscopy (EDS)
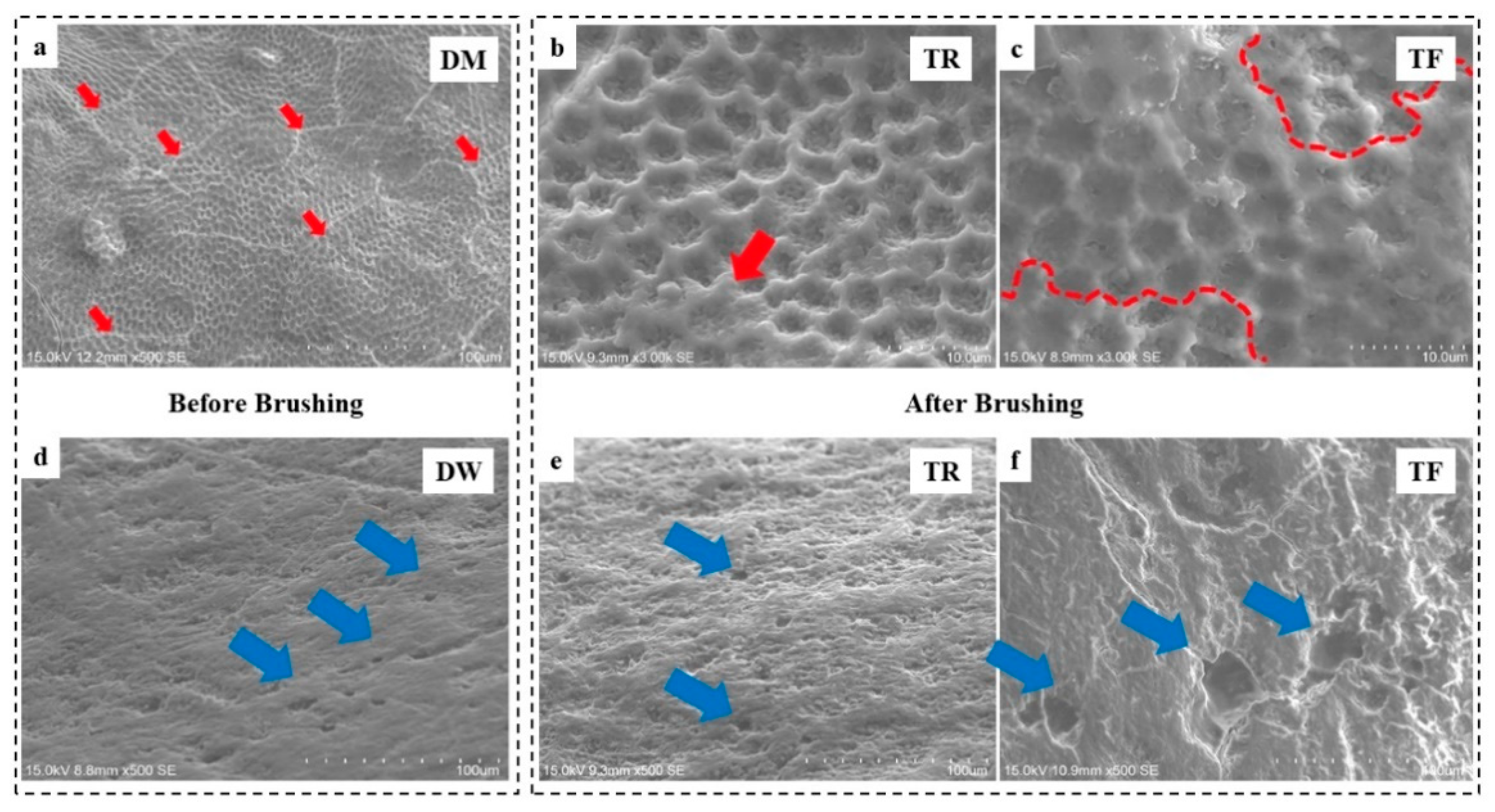
2.4.4. Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results
3.1. Microhardness (Vickers)
3.2. X-ray Diffraction (XRD)
3.3. Energy-Dispersive X-ray Spectroscopy (EDS)
3.4. Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marinho, V.; Higgins, J.; Sheiham, A.; Logan, S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 1, CD002278. [Google Scholar] [CrossRef]
- Adair, S.M. Evidence-based use of fluoride in contemporary pediatric dental practice. Pediatr. Dent. 2006, 28, 133–142. [Google Scholar] [PubMed]
- National Institute of Dental and Craniofacial Research. The Story of Fluoridation. 2018. Available online: https://www.nidcr.nih.gov/health-info/fluoride/the-story-of-fluoridation (accessed on 2 April 2024).
- Bijle, M.N.; Tsoi, J.; Ekambaram, M.; Lo, E.C.M.; Carey, C.M.; Yiu, C.K.Y. Inter-method reliability for determining total and soluble fluorides in child low-fluoride formula dentifrices. Sci. Rep. 2020, 10, 20880. [Google Scholar] [CrossRef]
- Bijle, M.N.; Tsoi, J.; Ekambaram, M.; Lo, E.C.M.; Carey, C.M.; Yiu, C.K.Y. Enhanced Fluoride Bioavailability with Incorporation of Arginine in Child Dentifrices. J. Clin. Pediatr. Dent. 2020, 44, 332–341. [Google Scholar] [CrossRef]
- Ammari, A.; Bloch-Zupan, A.; Ashley, P. Systematic review of studies comparing the anti-caries efficacy of children’s toothpaste containing 600 ppm of fluoride or less with high fluoride toothpastes of 1000 ppm or above. Caries Res. 2003, 37, 85–92. [Google Scholar] [CrossRef]
- O’mullane, D.; Kavanagh, D.; Ellwood, R.; Chesters, R.; Schafer, F.; Huntington, E.; Jones, P. A three-year clinical trial of a combination of trimetaphosphate and sodium fluoride in silica toothpastes. J. Dent. Res. 1997, 76, 1776–1781. [Google Scholar] [CrossRef]
- Narbutaitė, J.; Vehkalahti, M.M.; Milčiuvienė, S. Dental fluorosis and dental caries among 12-yr-old children from high-and low-fluoride areas in Lithuania. Eur. J. Oral Sci. 2007, 115, 137–142. [Google Scholar] [CrossRef]
- Featherstone, J.D. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef]
- Nakai, K.C.A.; Rodrigues, B.M.; Moraes, I.F.D.; Colombo, P.A.R.; Juliana, J.D.A.; Vanessa, T.; Tercilia, G.L.; Pelim, P.J.; Moreira, M.M.A.D.A.; Rabelo, B.M.A. Factors influencing fluoride ingestion from dentifrice by children. Community Dent. Oral Epidemiol. 2011, 39, 426–432. [Google Scholar]
- Panel, M.E. Topical Fluoride Recommendations for High-Risk Children Development of Decision Support Matrix: Recommendations From Maternal and Child Health Bureau (MCHB) Expert Panel; Altarum Institute: Washington, DC, USA, 2007. [Google Scholar]
- Levy, S.M.; Guha-Chowdhury, N. Total fluoride intake and implications for dietary fluoride supplementation. J. Public Health Dent. 1999, 59, 211–223. [Google Scholar] [CrossRef]
- Zohoori, F.V.; Duckworth, R.M.; Omid, N.; O’Hare, W.T.; Maguire, A. Fluoridated toothpaste: Usage and ingestion of fluoride by 4-to 6-yr-old children in England. Eur. J. Oral Sci. 2012, 120, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, M.; Lo, B.; Chan, K.K.F.; Lo, E.C.M.; Tsoi, J.K.H. A Personalised 3D-Printed Dental Plaque Removal Mouthguard for Older Adults. Int. Dent. J. 2023, 73, 828–833. [Google Scholar] [CrossRef]
- Scribante, A.; Pascadopoli, M.; Bergomi, P.; Licari, A.; Marseglia, G.L.; Bizzi, F.M.; Butera, A. Evaluation of two different remineralising toothpastes in children with drug-controlled asthma and allergic rhinitis: A randomised clinical trial. Eur. J. Paediatr. Dent. 2024, 25, 1. [Google Scholar] [PubMed]
- Simon, L.S.; Dash, J.K.; Philip, D.U.S.; Sarangi, S. Management of Post Orthodontic White Spot Lesions Using Resin Infiltration and CPP-ACP Materials—A Clinical Study. J. Clin. Pediatr. Dent. 2022, 46, 70–74. [Google Scholar] [CrossRef]
- Burt, B. The changing patterns of systemic fluoride intake. J. Dent. Res. 1992, 71, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.C.; Hann, H.J.; Williamson, M.F.; Berkowitz, J. Aesthetic concerns of children and parents in relation to different classifications of the Tooth Surface Index of Fluorosis. Community Dent. Oral Epidemiol. 1993, 21, 360–364. [Google Scholar] [CrossRef]
- Mullen, J. History of water fluoridation. Br. Dent. J. 2005, 199, 1. [Google Scholar] [CrossRef]
- Kumar, J. Is water fluoridation still necessary? Adv. Dent. Res. 2008, 20, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, Z.; Wu, L.; Wang, X.; Lu, W.; Liu, S. Effect of high-fluoride water on intelligence in children. Fluoride 2000, 33, 74–78. [Google Scholar]
- Peckham, S.; Lowery, D.; Spencer, S. Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. J. Epidemiol. Community Health 2015, 69, 619–624. [Google Scholar] [CrossRef]
- Chen, L.; Ning, H.; Yin, Z.; Song, X.; Feng, Y.; Qin, H.; Li, Y.; Wang, J.; Ge, Y.; Wang, W. The effects of fluoride on neuronal function occurs via cytoskeleton damage and decreased signal transmission. Chemosphere 2017, 185, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Cantor, K.P. Drinking water and cancer. Cancer Causes Control 1997, 8, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Harvey, P.; Young, W. Chemistry and Bioavailability Aspects of Fluoride in Drinking Water; WRc-NSF: Marlow, UK, 2002. [Google Scholar]
- Maguire, A.; Zohouri, F.; Mathers, J.; Steen, I.; Hindmarch, P.; Moynihan, P. Bioavailability of fluoride in drinking water: A human experimental study. J. Dent. Res. 2005, 84, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Bijle, M.N.; Ekambaram, M.; Lo, E.C.M.; Yiu, C.K.Y. The enamel remineralization potential of fluoride varnishes containing arginine. J. Dent. 2020, 99, 103411. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, J.; Pun, S.Y. Diamine or diammine. Br. Dent. J. 2020, 229, 73. [Google Scholar] [CrossRef]
- Network, F.A. The mystery of declining tooth decay. Nature 1986, 332, 125–129. [Google Scholar]
- Wang, C.; Fang, Y.; Zhang, L.; Su, Z.; Xu, J.; Fu, B. Enamel microstructural features of bovine and human incisors: A comparative study. Ann. Anat. 2021, 235, 151700. [Google Scholar] [CrossRef]
- Tan, C.M.; Tsoi, J.K.; Seneviratne, C.J.; Matinlinna, J.P. Evaluation of the Candida albicans removal and mechanical properties of denture acrylics cleaned by a low-cost powered toothbrush. J. Prosthodont. Res. 2014, 58, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Cate, J.T.; Duijsters, P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982, 16, 201–210. [Google Scholar] [CrossRef]
- Zero, D.T.; Lussi, A. Erosion—Chemical and biological factors of importance to the dental practitioner. Int. Dent. J. 2005, 55, 285–290. [Google Scholar] [CrossRef]
- Devlin, H.; Bassiouny, M.; Boston, D. Hardness of enamel exposed to Coca-Cola® and artificial saliva. J. Oral Rehabil. 2006, 33, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Maupomé, G.; Díez-de-Bonilla, J.; Torres-Villaseñor, G.; del Carmen Andrade-Delgado, L.; Castaño, V.M. In vitro quantitative assessment of enamel microhardness after exposure to eroding immersion in a cola drink. Caries Res. 1998, 32, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Jaeggi, T.; Schärer, S. The influence of different factors on in vitro enamel erosion. Caries Res. 1993, 27, 387–393. [Google Scholar] [CrossRef]
- Clasen, A.B.S.; Øgaard, B. Experimental intra-oral caries models in fluoride research. Acta Odontol. Scand. 1999, 57, 334–341. [Google Scholar] [CrossRef]
- Mellberg, J. Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. J. Dent. Res. 1992, 71, 913–919. [Google Scholar] [CrossRef]
- Gutiérrez-Salazar, M.D.P.; Reyes-Gasga, J. Microhardness and chemical composition of human tooth. Mater. Res. 2003, 6, 367–373. [Google Scholar] [CrossRef]
- Meredith, N.; Sherriff, M.; Setchell, D.; Swanson, S. Measurement of the microhardness and Young’s modulus of human enamel and dentine using an indentation technique. Arch. Oral Biol. 1996, 41, 539–545. [Google Scholar] [CrossRef]
- Attin, T.; Koidl, U.; Buchalla, W.; Schaller, H.; Kielbassa, A.; Hellwig, E. Correlation of microhardness and wear in differently eroded bovine dental enamel. Arch. Oral Biol. 1997, 42, 243–250. [Google Scholar] [CrossRef]
- Tantbirojn, D.; Huang, A.; Ericson, M.; Poolthong, S. Change in surface hardness of enamel by a cola drink and a CPP–ACP paste. J. Dent. 2008, 36, 74–79. [Google Scholar] [CrossRef]
- Arends, J.; Jongebloed, W. Crystallites dimensions of enamel. J. Biol. Buccale 1978, 6, 161–171. [Google Scholar]
- Schilke, R.; Lisson, J.A.; Bauß, O.; Geurtsen, W. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch. Oral Biol. 2000, 45, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Yassen, G.H.; Platt, J.A.; Hara, A.T. Bovine teeth as substitute for human teeth in dental research: A review of literature. J. Oral Sci. 2011, 53, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Skoskiewicz-Malinowska, K.; Mysior, M.; Rusak, A.; Kuropka, P.; Kozakiewicz, M.; Jurczyszyn, K. Application of Texture and Fractal Dimension Analysis to Evaluate Subgingival Cement Surfaces in Terms of Biocompatibility. Materials 2021, 14, 5857. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Kozakiewicz, M.; Jurczyszyn, K. Mechanical Properties, Fractal Dimension, and Texture Analysis of Selected 3D-Printed Resins Used in Dentistry That Underwent the Compression Test. Polymers 2023, 15, 1772. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.G.; Belsher, J.D. A Review of Sodium Fluoride Solubility in Water. J. Chem. Eng. Data 2017, 62, 1743–1748. [Google Scholar] [CrossRef]
- Iijima, M.; Onuma, K. Roles of Fluoride on Octacalcium Phosphate and Apatite Formation on Amorphous Calcium Phosphate Substrate. Cryst. Growth Des. 2018, 18, 2279–2288. [Google Scholar] [CrossRef]
- Cole, A.S.; Eastoe, J.E. Chapter 32—Enamel. In Biochemistry and Oral Biology, 2nd ed.; Cole, A.S., Eastoe, J.E., Eds.; Butterworth-Heinemann: Oxford, UK, 1988; pp. 460–474. [Google Scholar]
- Zhao, D.; Tsoi, J.K.; Wong, H.M.; Chu, C.H.; Matinlinna, J.P. Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel. Dent. J. 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vilchis, L.E.; Contreras-Bulnes, R.; Olea-Mejìa, O.F.; Sánchez-Flores, I.; Centeno-Pedraza, C. Morphological and structural changes on human dental enamel after Er: YAG laser irradiation: AFM, SEM, and EDS evaluation. Photomed. Laser Surg. 2011, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumelis, N.; Balatsoukas, I.; Tzaphlidou, M. Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J. Biol. Phys. 2012, 38, 279–291. [Google Scholar] [CrossRef]
- Nigri, E.M.; Bhatnagar, A.; Rocha, S.D.F. Thermal regeneration process of bone char used in the fluoride removal from aqueous solution. J. Clean. Prod. 2017, 142, 3558–3570. [Google Scholar] [CrossRef]

| Treatment | Condition | Mean * (VHN) | Std. Deviation |
|---|---|---|---|
| Before Brushing | DW | 467.40 A | 94.16 |
| DM | 281.04 C | 48.88 | |
| TF | DW | 528.99 B | 61.29 |
| DM | 341.40 D | 54.75 | |
| TR | DW | 572.27 B | 87.97 |
| DM | 359.63 D | 60.39 |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 1,750,780.258 a | 5 | 350,156.052 | 68.815 | 0.000 |
| Intercept | 26,024,739.699 | 1 | 26,024,739.699 | 5114.536 | 0.000 |
| Treatment (before vs. TF vs. TR) | 252,108.366 | 2 | 126,054.183 | 24.773 | 0.000 |
| Condition (DW vs. DM) | 1,376,346.367 | 1 | 1,376,346.367 | 270.488 | 0.000 |
| Treatment × condition | 5029.674 | 2 | 2514.837 | 0.494 | 0.611 |
| Error | 783,611.567 | 154 | 5088.387 | ||
| Total | 29,745,514.233 | 160 | |||
| Corrected Total | 2,534,391.825 | 159 | |||
| a.R Squared = 0.691 (Adjusted R Squared = 0.681) | |||||
| Treatment | Condition | Ca/P Ratio * | F at% * |
|---|---|---|---|
| Before | DW | 1.24 ± 0.10 A | 1.26 ± 0.25 D |
| DM | 1.26 ± 0.12 A | 1.39 ± 0.30 D | |
| TF | DW | 1.04 ± 0.02 B | 2.21 ± 0.52 E |
| DM | 1.50 ± 0.35 C | 2.02 ± 0.44 E | |
| TR | DW | 1.04 ± 0.00 B | 1.50 ± 0.35 D |
| DM | 1.44 ± 0.30 C | 1.26 ± 0.20 D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lau, C.L.B.; Ding, H.; Matinlinna, J.P.; Tsoi, J.K.H. Enamel Remineralisation with a Novel Sodium Fluoride-Infused Bristle Toothbrush. Dent. J. 2024, 12, 142. https://doi.org/10.3390/dj12050142
Liu X, Lau CLB, Ding H, Matinlinna JP, Tsoi JKH. Enamel Remineralisation with a Novel Sodium Fluoride-Infused Bristle Toothbrush. Dentistry Journal. 2024; 12(5):142. https://doi.org/10.3390/dj12050142
Chicago/Turabian StyleLiu, Xiaotian, Chun Lok Bryan Lau, Hao Ding, Jukka Pekka Matinlinna, and James K. H. Tsoi. 2024. "Enamel Remineralisation with a Novel Sodium Fluoride-Infused Bristle Toothbrush" Dentistry Journal 12, no. 5: 142. https://doi.org/10.3390/dj12050142
APA StyleLiu, X., Lau, C. L. B., Ding, H., Matinlinna, J. P., & Tsoi, J. K. H. (2024). Enamel Remineralisation with a Novel Sodium Fluoride-Infused Bristle Toothbrush. Dentistry Journal, 12(5), 142. https://doi.org/10.3390/dj12050142







