Abstract
Excessive gingival display (EGD) is defined as more than 2 mm of gingiva display above the maxillary incisors at maximum smile. Various skeletal, dental, and soft tissue etiological factors for EGD have been suggested. This study assessed the effectiveness and stability of surgical (SX) and nonsurgical (NSX) interventions for correction of EGD through a systematic review and meta-analysis following PRISMA 2020 guidelines. An electronic search of Ovid MEDLINE, EMBASE, CENTRAL, Scopus, Web of Science, and LILACS was conducted (2010–2023). Results were expressed as mean change in gingival display using the random-effects model at 1, 3, 6, and 12-month follow-up. At 1 month, SX and NSX treatments yielded a comparable mean reduction of 3.50 mm (2.13–4.86) and 3.43 mm (2.67–4.19) in gingival display, respectively. However, by 6 months, NSX treatments showed a reduction of 0.51 mm compared to 2.86 mm with SX treatments. SX outcomes remained stable past 6 months, while NSX outcomes partially relapsed at 6 months and returned to baseline levels at 12 months. Notably, NSX treatments were more effective in cases with mild initial EGD, while SX treatments showed a better outcome in severe cases. To draw more robust conclusions regarding the treatment outcomes, future primary studies of greater rigor are required.
1. Introduction
A smile consists of various elements, including the teeth, the lips, and the appearance of the gingival tissue [1]. The degree of gingival exposure plays a vital role in determining one’s contentment with the esthetics of their smile [2]. Excessive gingival display on smile (EGD) or ‘gummy smile’ is defined as a nonpathological condition that results in disharmony between the maxilla, lips, gingiva, and teeth [1]. There is no agreement about the ideal amount of gingival display; however, Peck and Peck (1995) defined a gingival smile as more than 2 mm of gingiva display above the maxillary central incisors at the maximum smile, which is the limit most commonly used in studies of smile esthetics [3]. Several etiological factors for EGD have been discussed in the literature, including vertical maxillary excess [4], short philtrum height [5], hypermobile upper lip elevator muscles [6,7], altered passive eruption [8], gingival enlargement, retroclination [1] or supra-eruption of maxillary incisors [9]. The recommended treatment modalities for addressing EGD include surgical (SX) and nonsurgical (NSX) treatments. Surgical interventions include orthognathic surgery (with or without V-Y plasty) [5], lip-repositioning surgery (LRS) with or without modifications such as myotomy [10,11], myectomy [12], placement of separator between the lip elevator muscles and nasal spine [13], esthetic crown lengthening (ECL), and gingivectomy. Nonsurgical interventions include injection of botulinum toxin (BTX) and orthodontic intrusion of maxillary teeth [14]. Other uncommonly used surgical or nonsurgical techniques include nasal septum reinforcement using autologous cartilage or an expanded polytetrafluoroethylene implant [15], micro-autologous fat transplantation to the upper lip, nasolabial groove, and ergotrid areas [16], and hyaluronic acid (HA) injection [17,18]. A recent surge in research focused on the treatment of EGD reflects the increasing interest among clinicians and the growing demand from patients to address EGD [19]. There have been systematic reviews conducted on the more common techniques for the reduction of EGD, such as BTX injection [20,21,22,23,24,25,26], LRS [27,28,29,30,31], and the use of skeletal anchorage devices [32]. The majority of studies included in these systematic reviews were case reports and case series with a small patient pool. Consequently, a comprehensive systematic review and meta-analysis of the current literature is required to produce quantifiable results on the outcome and stability of surgical and nonsurgical treatments, identify the limitations, and provide evidence-based indications for clinical practice and insights for future studies [33]. The objective of our systematic review was to appraise the scientific literature, compile the current evidence on outcome and stability of surgical and nonsurgical interventions in adult patients with EGD, and provide evidence-based guidance for clinical practice and insights for future studies.
2. Materials and Methods
2.1. Protocol
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [34]. The protocol for this project was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database under the registration number CRD42022363826.
2.2. Information Sources
An electronic search of literature published from 2010 to 2023 was performed on 30 January 2023, in consultation with a health sciences librarian, in six databases: Ovid MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Web of Science, LILACS, and the bibliography of relevant studies. All relevant search terms were found by identifying word variants of keywords. Medical Subject Headings (MeSh) were used for the conditions and interventions (Table S1). After finalizing the search syntax for Ovid MEDLINE, it was adapted to other databases.
2.3. Eligibility Criteria (Table S2)
- Participants: adult patients 18 years of age or older with a chief complaint of EGD on smiling, with periodontal and systemic health.
- Intervention: surgical and nonsurgical treatments for EGD.
- Comparison: pre-treatment condition.
- Outcome measures: gingival display at maximum smile pre-treatment and at follow-up visits for a minimum of 6 months.
- Study design: randomized controlled trials, non-randomized studies without a control group, prospective, retrospective, comparative cohort, and case–control studies.
Studies with the following criteria were excluded: less than 6 months of follow-up, unpublished and non-peer-reviewed studies, review articles, books, expert opinions, case reports, case series, and clinical guidelines were excluded.
2.4. Study Selection
The retrieved studies were screened by two independent reviewers (M.M. and B.H.) using the inclusion and exclusion criteria. The initial screening was conducted by reviewing the title and abstract. Eligible studies were further screened by full-text review and selected for the final analysis. The disagreements were resolved through discussion to reach a consensus.
2.5. Data Extraction
Two reviewers (M.M. and B.H.) independently extracted data from the included studies using a pre-prepared data extraction form. The following information was extracted: first author, year of publication, region, study design, participants’ demographics, pre- and post-treatment gingival display, etiology, measurement landmarks, type of intervention, outcome, and follow-up period. Inconsistencies were resolved by discussion between the two reviewers.
2.6. Risk of Bias Assessment
The risk of bias for individual studies was assessed using version 2 of the Cochrane risk of bias for randomized trials (RoB 2.0) and risk of bias in non-randomized studies of interventions (ROBINS-1) by two independent reviewers (M.M. and B.H.) [35,36]. Any disagreements were resolved through discussion to reach a consensus.
2.7. Data Analysis
The statistical analysis was conducted with assistance from a statistician (A.S.) using the Meta package (version 6.5.0) [37] in R Studio software (version 2023.09.0) [38]. The studies were divided into two groups, surgical and nonsurgical. The surgical studies included LRS with or without modifications, ECL, gingivectomy, GBR, septal cartilage reinforcement, micro-autologous fat transplantation, and V-Y plasty. The nonsurgical studies included BTX injection and orthodontic intrusion. The studies were further subdivided into three groups based the range of initial EGD: mild (2–3.99 mm), moderate (4–5.99 mm), and severe (6 mm or more) [3,39,40,41]. The post-treatment changes in gingival display at four time points (1, 3, 6, and 12 months) were calculated, pooled by using a random-effects model, and expressed as mean difference with a 95% confidence interval (CI) using a single-cohort meta-analysis. When required, the change in the gingival display was calculated by deducting the final gingival margin level from the baseline level.
The sources of heterogeneity were evaluated through a subgroup analysis of surgical and nonsurgical procedures. The studies in each surgical and nonsurgical group were further subdivided according to the study type (randomized or non-randomized) and initial gingival display (mild, moderate, or severe).
3. Results
The electronic search resulted in 2057 articles for inclusion. Figure 1 outlines the search strategy and the results. After removing the duplicates, 1190 publications were screened by title and abstract for eligibility. A total of 42 articles were included for full-text screening, and 16 articles were excluded due to the following reasons: no measurement of gingival display (n = 5), short follow-up (n = 4), wrong study design (n = 4), wrong patient population (n = 2), or not published (n = 1). A total of 26 studies were included in the data extraction and descriptive analysis, and 21 reports were selected for the meta-analysis. Five articles were excluded from the meta-analysis because the primary outcome could not be compared among them; two did not measure pre-treatment gingival display [42,43], two made measurements at indeterminate timepoints [16,44], and one measured the surface area of gingival exposure [45].
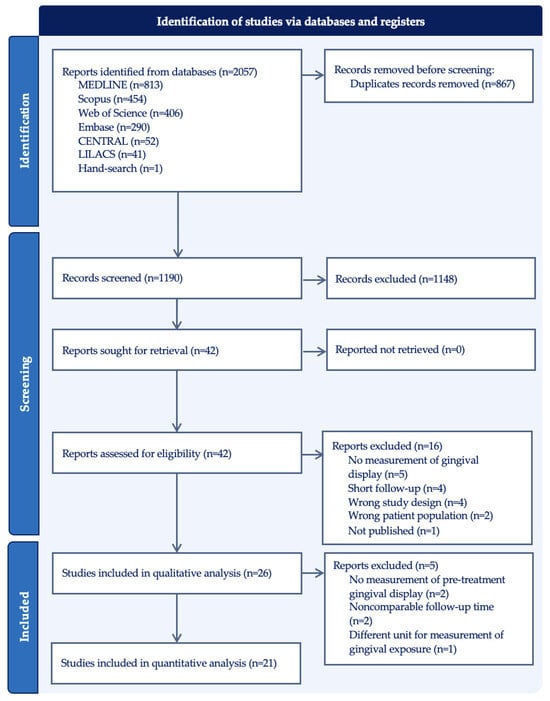
Figure 1.
PRISMA flow diagram of identified studies via databases and registers.
3.1. Study Characteristics
The characteristics of the included studies are presented in Table 1. Our systematic review included 20 non-randomized and 6 randomized clinical trials. Only one study declared a conflict of interest due to ownership of a device patent [16]. Of the 687 participants between ages 16 to 60, gender was not specified for 60 patients, and of the remaining 627, 86.5% were female and 13.5% were male. The surgical studies included 323 and the nonsurgical studies included 364 participants.

Table 1.
Study characteristics.
The etiology of EGD varied among the studies, including hyperactive upper lip (n = 11), short upper lip length (n = 5), altered passive eruption (n = 3), vertical maxillary excess (n = 3), short clinical crown height (n = 1), and nasal septum dysplasia (n = 1). Seven studies excluded VME cases, and six studies did not specify the etiology. Nine studies investigated LRS with different modifications (frenectomy, myotomy, internal dual muscular traction, periosteal suturing, or BTX injection before surgery), two studies used ECL (open-flap, flapless, or laser-assisted), one study investigated gingivectomy with a diode laser, one study used V-Y plasty, one investigated septum cartilage reinforcement, one looked at GBR, one tested fat micro-transplanted into the nasolabial groove, one used orthodontic treatment with extractions and TADs, and ten studies used BTX injection (with different injection sites and dosages and an oral zinc supplement).
The pooled mean pre-treatment gingival display was 5.28 mm, ranging from 2.03 [5] to 7.2 mm [53]. Two studies failed to report the pre-treatment gingival display [42,43]. The shortest follow-up time was 6 months and the longest was 3 years [51]. There were variations among the studies regarding the landmarks used to measure the gingival display.
3.2. Risk of Bias
The overall risk of bias was graded moderate in 16 (61.5%) and serious in 4 (15.4%) studies in the non-randomized category. Among the randomized studies, the overall risk of bias was graded low in 2 (7.7%) and some concerns in 4 (15.4%) studies (Figure 2).
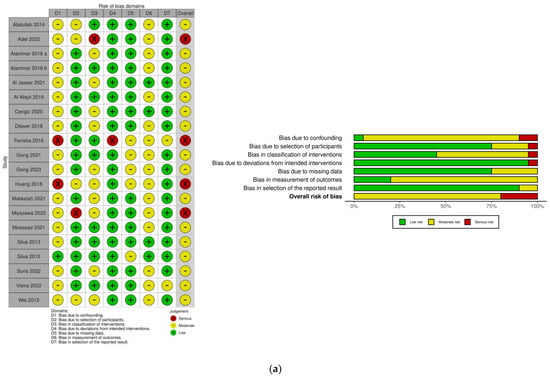

Figure 2.
Risk of bias assessment: (a) ROBINS-I [5,11,15,39,40,44,45,46,47,48,49,51,53,54,56,57,58,59,60]; (b) RoB 2.0. [42,43,50,52,55,61].
3.3. Primary Outcome
We analyzed the data from 633 patients of whom 228 received surgical treatment, 396 received nonsurgical treatments, and 9 received both surgical and nonsurgical treatments. Of the 21 studies included in the meta-analysis, 10 were surgical, 8 were nonsurgical, and 3 investigated both treatment modalities. The pooled mean pre-treatment gingival display was 5.28 mm with a range of 2.03–7.20 mm (95% CI: 4.46–6.09 mm) (Figure S1).
At 1-month post treatment, the studies with nonsurgical treatment reported a mean reduction of 3.43 mm (95% CI: 2.67–4.19 mm) in gingival display, and the studies with surgical treatment reported a mean reduction of 3.50 mm (95% CI: 2.13–4.86 mm) (Figure S2).
At 3 months post treatment, nonsurgical treatments showed a mean reduction of 2.55 mm (95% CI: 1.66–3.44 mm) in gingival display, while surgical treatments showed an average reduction of 2.87 mm (95% CI: 1.99–3.75 mm). No statistically significant difference was observed between the surgical and nonsurgical treatments at 1- and 3-month follow-up (p = 0.93 and p = 0.61) (Figure 3).
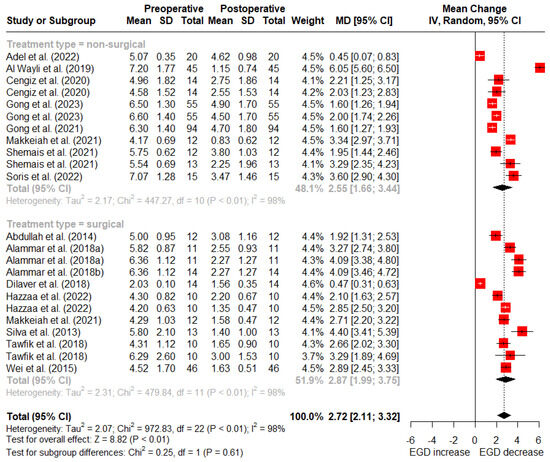
Figure 3.
Random-effects meta-analysis of gingival display reduction with surgical vs. nonsurgical treatments at 3-month follow-up (SD: standard deviation; MD: mean difference; CI: confidence interval) [5,11,15,39,40,46,48,49,50,52,53,54,55,56,57,59].
At 6 months post-treatment, nonsurgical treatments reported a mean reduction of 0.51 mm (95% CI: 0.23–0.79 mm) in gingival display, whereas surgical treatments reported an average reduction of 2.86 mm (95% CI: 2.06–3.65 mm) (Figure 4).
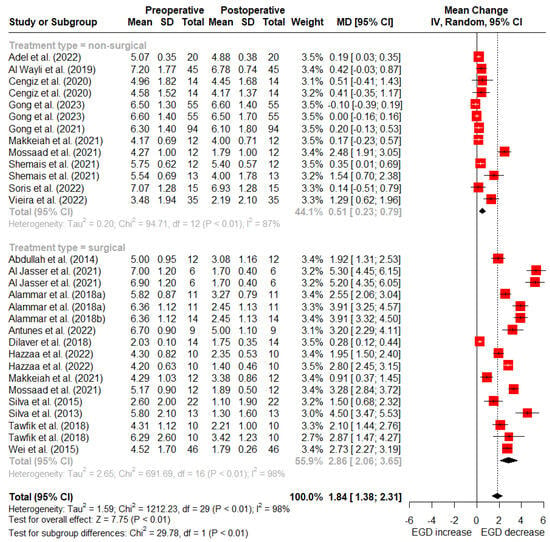
Figure 4.
Random-effects meta-analysis of reduction in gingival display with surgical vs. nonsurgical treatments at 6-month follow-up (SD: standard deviation; MD: mean difference; CI: confidence interval) [5,11,15,39,40,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
At 12 months post-treatment, nonsurgical treatments reported an average reduction of 0.04 mm (95% CI: −0.11–0.19 mm) in gingival display, while surgical treatments reported a mean reduction of 2.81 mm (95% CI: 1.94–3.69 mm) (Figure 5). There was a statistically significant difference between surgical and nonsurgical treatment types at 6- and 12-month follow-up (p < 0.01).
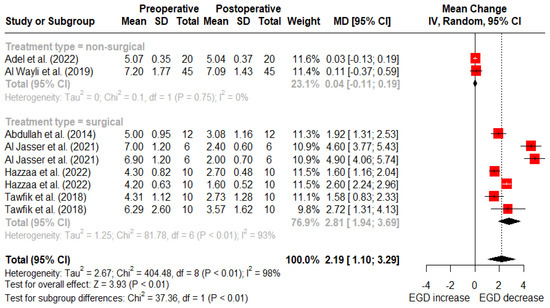
Figure 5.
Random-effects meta-analysis of gingival display reduction with surgical vs. nonsurgical treatments at 12-month follow-up (SD: standard deviation; MD: mean difference; CI: confidence interval) [11,50,51,52,53,56].
3.4. Subgroup Analysis
A subgroup analysis was performed by comparing the randomized and non-randomized surgical treatments at 6 months follow-up. The results showed that the treatment effect was only slightly higher in the non-randomized studies (2.98 mm) compared to the randomized studies (2.50 mm) and the difference was not statistically significant (p = 0.43) (Figure S3). Similarly, the treatment effects at 6 months were comparable between randomized and non-randomized nonsurgical treatments (0.88 and 0.46 mm, respectively) with no statistically significant difference (p = 0.50) (Figure S4).
The studies were categorized based on the pre-treatment gingival display as mild (2–3.99 mm), moderate (4–5.99 mm), and severe (6mm or more) EGD. The random-effects analysis for the surgical treatments at 6 months showed that the treatment effect in severe EGD (4.12 mm) was significantly larger than in moderate (2.48 mm) or mild (0.82 mm) EGD (p < 0.01) (Figure S5). The random-effects analysis for the nonsurgical treatments at 6 months follow-up showed that the treatment effect was smallest in the studies with severe initial gingival display (0.06 mm) compared to moderate (0.78 mm) or mild (1.29 mm) (p < 0.01) (Figure S6).
4. Discussion
In this investigation, the systematic review and meta-analysis included 26 and 21 reports, respectively, published between 2013 and 2023. A total of 633 patients were pooled, of which 228 underwent surgical treatment, 396 nonsurgical, and 9 had a combination of both (botox and LRS). The results of the meta-analysis suggested that there was no significant difference between the two treatment modalities at 1 and 3 months post treatment. The surgical outcomes remained stable at 6 and 12 months, while nonsurgical outcomes partially relapsed at 6 months and returned to baseline at 12 months. The initial severity of gingival exposure and the treatment modality played a role in the treatment effects. Gong et al. (2023) categorized the pre-treatment gingival show into three groups based on severity: mild (3–5 mm), moderate (5–7 mm), and severe (greater than 7 mm) [39]. In this meta-analysis, the studies were divided into three groups based the range of initial EGD: mild (2–3.99 mm), moderate (4–5.99 mm), and severe (6 mm or more). At 6 months, surgical treatments showed significantly greater reduction in gingival display in severe compared to moderate or mild EGD. On the other hand, the treatment effect of nonsurgical treatments was smaller in studies with severe initial gingival display compared to moderate or mild.
4.1. Lip-Repositioning Surgery
There are several variations for LRS with the goal of relapse minimization and improvement of long-term stability by preventing reinsertion of smile muscles to their original position [51]. These variations include frenectomy, use of adjuvants (botox), muscular amputation (myotomy), muscle dissection, muscle containment with insertion of polyester sutures [62], and periosteal suturing [27]. Mendoza-Geng et al. (2022) suggested that the use of periosteal suturing with LRS caused the greatest decrease in EGD, with 5.22 mm (4.23–6.21) at 6 months and 4.94 mm (3.86–6.02) at 12 months post treatment [27]. Similarly, Dos Santo-Pereira et al. (2020) reported a reduction of 2.87 mm (1.91–3.82) after 3 months, which decreased to 2.71 mm (1.95–3.47) at 6 months and 2.10 mm (1.48–2.72) at 12 months, showing a relapse rate of 25% after 12 months [29]. Younespour et al. (2021) showed a reduction range of 2.68–3.22 mm with various LRS modalities [28]. Descriptively, the greatest gingival display reduction was associated with the modality that did not include frenectomy or myotomy [28].
Long-term stability might be one of the most controversial aspects of LRS. Alammar and Heshmeh (2018) explained that relapse may occur due to incomplete stripping of the muscles from the bone during the surgical procedure or muscle memory reattachment to the previous pre-bone base [49]. Nonetheless, due to the lack of follow-ups longer than 12 months, it is not possible to confirm if this decrease continues to the baseline levels.
4.2. Gingivectomy and Crown Lengthening
In cases where there is an excessive amount of keratinized gingiva with short and square-shaped teeth with sufficient distance between the cementoenamel junction (CEJ) and the osseous crest, gingivectomy is indicated [63]. If the osseous crest is found to be close to the level of the CEJ, osseous resection with flap elevation may be required [63]. Where there are clinically visible short teeth with a limited amount of keratinized gingival tissue, treatment involves apical repositioning of the entire dento-gingival complex with or without osseous reduction [63].
Diode 655–980 nm lasers have been used for gingivectomy procedures in patients with EGD [60]. It prevents bleeding by sealing the blood vessels and inhibits pain receptors, contributing to reduced discomfort during the procedure [64]. One article studied the effect of gingivectomy and ostectomy (open-flap and flapless) performed with an Er,Cr–YSGG laser [43]. The outcome measure used was the gingival margin level, and no difference was found between the two techniques.
4.3. V-Y Plasty
V-Y plasty is a method used to cover the increased gingival display that is expected after Le Fort I osteotomy [5]. Dilaver and Uckan (2018) found that the benefits of V–Y plasty following Le Fort I are greater than those of the V-Y plasty applied as a standalone procedure [5]. Muradin et al. (2009) employed a modified alar cinch suture technique, which involved passing sutures through the levator and nasal muscles, including the periosteum, and threading them through the nasal septum [65]. This was combined with a muco-musculo-periosteal V-Y closure following Le Fort 1 osteotomy [65]. Postoperatively, there appeared to be a reduction in the vertical mobility of the corners of the mouth when observing the maximum smile, which could potentially contribute to improving excessive gingival display [65]. Due to the limited number of studies, a conclusion regarding the effect of Le Fort 1 impaction or V-Y plasty on gingival display cannot be drawn.
4.4. Guided Bone Regenration
Guided bone regeneration was suggested by one study as a camouflage treatment for VME [45]. This technique showed an improvement in EGD of 40.7% when performed alone and 60% when combined with a crown-lengthening procedure [45].
4.5. Septum Cartilage Reinforcement
Wei et al. (2015) investigated the role of nasal septal dysplasia in the development of EGD among the Asian populations [15]. The absence of natural antagonism due to nasal septal cartilage dysplasia leads to an upward movement of the upper lip during smiling, resulting in excessive gingival exposure [15]. The outcome showed that there was an average 3.34 mm reduction in EGD at 1 month with an average minimal relapse of 0.61 mm at 6 months post treatment [15]. This was a retrospective study with variable follow-up time among cases.
4.6. Micro-Autologous Fat Transplantation
In a study by Huang et al. (2018), MAFT in the nasolabial groove, ergotrid, and upper lip was used to camouflage EGD by blocking the upper lip elevator muscles and increasing the vertical height of the upper lip [16]. Due to the paucity of evidence on MAFT, there remains a gap in our understanding of the long-term outcomes associated with these procedures [16].
4.7. Botulinum Toxin
4.7.1. Injection Site
The levator labii superioris (LLS), levator labii superioris alaeque nasi (LLSAN), and zygomaticus minor (Zmi) muscles determine the degree of upper lip elevation and converge near a triangular region in the nasolabial fold [66]. The center of this triangle, Yonsei point, has been suggested because the optimal injection site could encompass all three muscles with a single injection [67]. Cengiz et al. (2020) suggested isolated injection into the orbicularis oris (OO) at the junction of the elevator muscles. However, the treatments results with this approach were inferior to those of isolated LLSAN injection (53% versus 61% reduction in gingival exposure) [54]. The OO muscle is involved in many basic facial expressions; therefore, the discomfort associated with the injection, subsequent muscle weakness, or paralysis of this muscle should be considered [54]. Razmaitė and Trakinienė (2021) stated that the different techniques of BTX administration and injection sites did not significantly affect the clinical outcome [25]. A systematic review by Lam and Chan (2022) confirmed that there was no correlation between the number of injection sites and improvement in EGD [21].
4.7.2. Dosage and Initial Severity of EGD
In a study by Gong et al. (2021), it was stated that the effectiveness of an average dose of BTX was influenced by the severity of EGD and the patient’s gender rather than the underlying etiology [40]. For female patients with a baseline anterior gingival exposure of 6 mm or more (or male patients), the BTX dose could be proportionally increased [40]. Additionally, Andriola et al. (2021) confirmed that the initial amount of EGD is an important factor in BTX efficiency [41].
4.7.3. Stability
A systematic review by Chagas et al. (2018) found that the gingival display was considerably reduced at 2 weeks post treatment (4.05 mm) and remained stable until 8 weeks post treatment [25]. Another systematic review by Nasr et al. (2016) showed that the results of BTX lasted within a range of 12 to 24 weeks [22]. Rasteau et al. (2022) found similar results that the improvement in gingival display persisted for 12 to 36 weeks [20]. A meta-analysis by Zengiski et al. (2022) showed a similar decrease in gingival display with a slight decrease in effect size at 12 weeks [23]. After 24 weeks, despite the statistical significance, the observed effect size was very small approaching the initial values, and thus it was not clinically significant [23]. Lam and Chan (2022) confirmed that the results of injection started to disappear after 12 weeks, and normal function returned at 24–30 weeks [21]. As a zinc-dependent metalloprotease, BTX exerts its muscle-paralyzing effect in the presence of a zinc molecule [68]. The clinical efficacy and duration of Botox A injections can fluctuate based on the zinc levels in the body [68]. Shemais et al. (2021) showed that zinc supplementation prolonged the duration of BTX’s effect for more than 6 months. A limitation of this study was the lack of a placebo supplement for the control group [55].
4.8. Orthodontic Treatment
The use of miniscrews offers anchorage for intrusion of anterior teeth in individuals with EGD and deep overbites. Miyazawa et al. (2022) found that orthodontic treatment combined with midpalatal miniscrews can be an effective alternative to orthognathic surgery [44]. However, the degree of lip incompetence, length, and mobility of the upper lip were not assessed in this study [44]. A systematic review by Alshammery et al. (2021) found TSADs to be useful in the correction of a deep bite [32]. The reported dentoalveolar intrusion was 2.25–2.9 mm; however, no soft tissue measurement of the gingival display was reported [32].
4.9. Limtations of the Current Study
The main limitation of our systematic review was the high level of heterogeneity. The ideal study design for a systematic review is a randomized clinical trial; however, our review was based on a sample of mainly non-randomized studies. It has been suggested that well-conducted prospective non-randomized studies can provide complementary evidence [69]. The variable etiology of EGD (and, in some studies, lack of identification of etiology) and the variability of landmarks for the measurement of gingival display can affect the treatment outcome. Furthermore, the reproducibility of maximum smile before and after treatment was not validated. Additionally, the age range of the study participants was wide. It has been shown that the severity of EGD can change with age due to the sagging of perioral soft tissues [70]. The variations in surgical techniques and injection sites and dosages for BTX injection is an important limitation when determining the treatment technique with the best outcome. The aforementioned limitations restrict the generalizability and applicability of the results.
4.10. Future Directions
While it is imperative to regard the results of this meta-analysis with caution due to the high heterogeneity and the aforementioned contributing factors, this review may serve as a starting point for future research endeavors featuring more rigorous research designs. To draw more robust conclusions regarding the short- and long-term treatment outcomes of EGD correction, future primary studies of greater rigor are needed. The studies should adopt a randomized and controlled design and include a detailed diagnosis of EGD etiology, patient characteristics, and quantitative results. In cases in which randomization is not easily employed, future studies should, as a minimum, ensure that the groups compared are matched.
5. Conclusions
This systematic review suggests that both surgical and nonsurgical approaches can successfully address EGD. Surgical treatments seem to be more effective for severe EGD and last up to 12 months, while nonsurgical treatments seem to be more effective for mild EGD with a tendency to relapse after 6 months. Given the limitations of this systematic review including the heterogeneity among included studies, the variability in measurement landmarks for gingival display, the broad age range of participants, and inclusion of randomized and non-randomized studies, the findings of this synthesis must be interpreted with caution. To draw more robust conclusions regarding the short- and long-term treatment outcomes of EGD correction and generalize the findings to various patient populations and different ages, future primary studies of greater rigor are needed. Additionally, management of EGD and selection of appropriate treatment options should be approached with consideration of the underlying etiology, patient education regarding the short- and long-term effects of surgical and nonsurgical treatments, and informed consent. As previously cited in the literature [19], each treatment modality is tailored to address a specific cause of EGD. Orthognathic intervention is recommended for skeletal etiology, while soft tissue etiologies warrant treatments targeted at soft tissues, and dental causes should be addressed accordingly. In certain scenarios, clinicians may encounter patients who decline a recommended procedure due to its invasive nature. Hence, clinicians should be equipped with knowledge and preparedness to suggest alternative treatment options in such instances.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dj12060154/s1, Table S1: Search keywords, synonyms, and related concepts; Table S2: Inclusion and exclusion criteria; Figure S1. Random-effects meta-analysis of pre-treatment gingival display; Figure S2. Random-effects meta-analysis of gingival display reduction with surgical vs. nonsurgical treatments at 1-month follow-up; Figure S3. Random-effects meta-analysis of gingival display reduction with randomized vs. non-randomized surgical treatments at 6-month follow-up; Figure S4. Random-effects meta-analysis of gingival display reduction with randomized vs. non-randomized nonsurgical treatments at 6-month follow-up; Figure S5. Random-effects subgroup analysis of gingival display reduction with surgical treatments based on pre-treatment gingival display at 6-month follow-up; Figure S6. Random-effects subgroup analysis of gingival display reduction with nonsurgical treatments based on pre-treatment gingival display at 6-month follow-up.
Author Contributions
Conceptualization, M.M.; methodology, M.M.; screening, risk of bias assessment, and data extraction, M.M. and B.H.; analysis, M.M.; writing—original draft preparation, M.M.; writing—review and editing, M.M. and Y.F.; supervision, Y.F., M.F.C. and V.C.M.; funding acquisition, M.M. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the American Association of Orthodontists Foundation (AAOF) Research Aid Award; grant number N/A.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Acknowledgments
We would like to thank Anton Svendrovski (B.CompSc, MSc (Math), MBA, IBM SPSS certified) for his assistance with the statistical analysis and Maria Zych for her assistance with the electronic literature search.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pavone, A.F.; Ghassemian, M.; Verardi, S. Gummy Smile and Short Tooth Syndrome–Part 1: Etiopathogenesis, Classification, and Diagnostic Guidelines. Compend. Contin. Educ. Dent. 2016, 37, 102–107. [Google Scholar] [PubMed]
- Van der Geld, P.; Oosterveld, P.; Van Heck, G.; Kuijpers-Jagtman, A.M. Smile Attractiveness. Self-Perception and Influence on Personality. Angle Orthod. 2007, 77, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.; Peck, L. Selected Aspects of the Art and Science of Facial Esthetics. In Seminars in Orthodontics; Elsevier: Amsterdam, The Netherlands, 1995; Volume 1, pp. 105–126. [Google Scholar]
- Willmar, K. On Le Fort I Osteotomy; A Follow-up Study of 106 Operated Patients with Maxillo-Facial Deformity. Scand. J. Plast. Reconstr. Surg. 1974, 12 (Suppl. S12), 1–68. [Google Scholar] [CrossRef] [PubMed]
- Dilaver, E.; Uckan, S. Effect of V-Y Plasty on Lip Lengthening and Treatment of Gummy Smile. Int. J. Oral Maxillofac. Surg. 2018, 47, 184–187. [Google Scholar] [CrossRef]
- Rubin, L.R. The Anatomy of a Smile: Its Importance in the Treatment of Facial Paralysis. Plast. Reconstr. Surg. 1974, 53, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Bhola, M.; Fairbairn, P.J.; Kolhatkar, S.; Chu, S.J.; Morris, T.; de Campos, M. LipStaT: The Lip Stabilization Technique- Indications and Guidelines for Case Selection and Classification of Excessive Gingival Display. Int. J. Periodontics Restor. Dent. 2015, 35, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Evian, C.I.; Cutler, S.A.; Rosenberg, E.S.; Shah, R.K. Altered Passive Eruption: The Undiagnosed Entity. J. Am. Dent. Assoc. 1993, 124, 107–110. [Google Scholar] [CrossRef]
- Kokich, V. Esthetics and Anterior Tooth Position: An Orthodontic Perspective Part II: Vertical Position. J. Esthet. Restor. Dent. 1993, 5, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Ishida, L.H.; Ishida, L.C.; Ishida, J.; Grynglas, J.; Alonso, N.; Ferreira, M.C. Efficiency of Gummy Smile Correction Using the Myotomy of the Elevator of the Upper Lip Muscle. Plast. Reconstr. Surg. 2009, 124, 10–11. [Google Scholar]
- Abdullah, W.A.; Khalil, H.S.; Alhindi, M.M.; Marzook, H. Modifying Gummy Smile: A Minimally Invasive Approach. J. Contemp. Dent. Pract. 2014, 15, 821–826. [Google Scholar] [CrossRef]
- Miskinyar, S.A.C. A New Method for Correcting a Gummy Smile. Plast. Reconstr. Surg. 1983, 72, 397–400. [Google Scholar] [CrossRef]
- Ellenbogen, R.; Swara, N. The Improvement of the Gummy Smile Using the Implant Spacer Technique. Ann. Plast. Surg. 1984, 12, 16–24. [Google Scholar] [CrossRef]
- McEntire, C. Three-Dimensional Soft Tissue Changes upon Smiling, Virginia Commonwealth University. 2013. Available online: https://scholarscompass.vcu.edu/etd/3009/ (accessed on 10 April 2023).
- Wei, J.; Herrler, T.; Xu, H.; Li, Q.; Dai, C. Treatment of Gummy Smile: Nasal Septum Dysplasia as Etiologic Factor and Therapeutic Target. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 1338–1343. [Google Scholar] [CrossRef]
- Huang, S.; Huang, Y.; Lin, Y.; Lee, S.; Chou, C.; Lin, T.; Takahashi, H.; Kuo, Y.; Lai, C.; Lin, S.; et al. Micro-Autologous Fat Transplantation for Treating a Gummy Smile. Aesthet. Surg. J. 2018, 38, 925–937. [Google Scholar] [CrossRef]
- Diaspro, A.; Cavallini, M.; Piersini, P.; Sito, G. Gummy Smile Treatment: Proposal for a Novel Corrective Technique and a Review of the Literature. Aesthet. Surg. J. 2018, 38, 1330–1338. [Google Scholar] [CrossRef]
- Hsien-Li Peng, P.; Peng, J.-H. Treating the Gummy Smile with Hyaluronic Acid Filler Injection. Dermatol. Surg. 2019, 45, 478–480. [Google Scholar] [CrossRef]
- Polo, M. Gummy Smile Treatment: A 40-Year Journey. AJO-DO Clin. Companion 2022, 2, 125–135. [Google Scholar] [CrossRef]
- Rasteau, S.; Savoldelli, C.; Winter, C.; Lerhe, B.; Castillo, L.; Kestemont, P. Botulinum Toxin Type A for the Treatment of Excessive Gingival Display—A Systematic Review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e717–e723. [Google Scholar] [CrossRef]
- Lam, F.; Chan, M.Y.S. The Role of Botulinum Toxin A in the Management of Different Types of Excessive Gingival Display: A Systematic Review. Br. Dent. J. 2022, 233, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.W.; Jabbour, S.F.; Sidaoui, J.A.; Haber, R.N.; Kechichian, E.G. Botulinum Toxin for the Treatment of Excessive Gingival Display: A Systematic Review. Aesthet. Surg. J. 2016, 36, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zengiski, A.C.S.; Basso, I.B.; Cavalcante-Leão, B.L.; Stechman-Neto, J.; Santos, R.S.; Guariza-Filho, O.; Zeigelboim, B.S.; Taveira, K.V.M.; de Araujo, C.M. Effect and Longevity of Botulinum Toxin in the Treatment of Gummy Smile: A Meta-Analysis and Meta-Regression. Clin. Oral Investig. 2022, 26, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Razmaitė, A.; Trakinienė, G. The Effect of Botox for the Correction of the Gummy Smile: A Systematic Review. Stomatologija 2021, 23, 63–68. [Google Scholar]
- Chagas, T.F.; de Almeida, N.V.; Lisboa, C.O.; Ferreira, D.M.T.P.; Mattos, C.T.; Mucha, J.N. Duration of Effectiveness of Botulinum Toxin Type A in Excessive Gingival Display: A Systematic Review and Meta-Analysis. Braz. Oral Res. 2018, 32, e30. [Google Scholar] [CrossRef]
- Duruel, O.; Ataman-Duruel, E.T.; Tözüm, T.F.; Berker, E. Ideal Dose and Injection Site for Gummy Smile Treatment with Botulinum Toxin-A: A Systematic Review and Introduction of a Case Study. Int. J. Periodontics Restor. Dent. 2019, 39, e167–e173. [Google Scholar] [CrossRef]
- Mendoza-Geng, A.; Gonzales-Medina, K.; Meza-Mauricio, J.; Muniz, F.W.M.G.; Vergara-Buenaventura, A. Clinical Efficacy of Lip Repositioning Technique and Its Modifications for the Treatment of Gummy Smile: Systematic Review and Meta-Analysis. Clin. Oral Investig. 2022, 26, 4243–4261. [Google Scholar] [CrossRef] [PubMed]
- Younespour, S.; Yaghobee, S.; Aslroosta, H.; Moslemi, N.; Pourheydar, E.; Ghafary, E.S. Effectiveness of Different Modalities of Lip Repositioning Surgery for Management of Patients Complaining of Excessive Gingival Display: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 9476013. [Google Scholar] [CrossRef]
- dos Santos-Pereira, S.A.; Cicareli, A.J.; Idalgo, F.A.; Nunes, A.G.; Kassis, E.N.; Castanha Henriques, J.F.; Bellini-Pereira, S.A. Effectiveness of Lip Repositioning Surgeries in the Treatment of Excessive Gingival Display: A Systematic Review and Meta-Analysis. J. Esthet. Restor. Dent. 2021, 33, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, O.K.; El-Nahass, H.E.; Shipman, P.; Looney, S.W.; Cutler, C.W.; Brunner, M. Lip Repositioning for the Treatment of Excess Gingival Display: A Systematic Review. J. Esthet. Restor. Dent. 2018, 30, 101–112. [Google Scholar] [CrossRef]
- Ardakani, M.T.; Moscowchi, A.; Valian, N.K.; Zakerzadeh, E. Lip Repositioning with or without Myotomy: A Systematic Review. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Alshammery, D.; Alqhtani, N.; Alajmi, A.; Dagriri, L.; Alrukban, N.; Alshahrani, R.; Alghamdi, S. Non-Surgical Correction of Gummy Smile Using Temporary Skeletal Mini-Screw Anchorage Devices: A Systematic Review. J. Clin. Exp. Dent. 2021, 13, 717–723. [Google Scholar] [CrossRef]
- Moher, D.; Tetzlaff, J.; Tricco, A.C.; Sampson, M.; Altman, D.G. Epidemiology and Reporting Characteristics of Systematic Reviews. PLoS Med. 2007, 4, e78. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Chapter 25: Assessing Risk of Bias in a Non-Randomized Study. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 28 August 2023).
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2023; Available online: www.Training.Cochrane.org/handbook (accessed on 28 August 2023).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. BMJ Ment Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC. Version 2023.09.0. Available online: https://www.rstudio.com/ (accessed on 24 November 2023).
- Gong, X.; Yuan, M.; Gu, C.; Yan, B.; Li, J.; Zou, L.; An, Y.; Tang, Z.; Han, X. Effects of Dose and Injection Site on Gingival Smile Treatment with Botulinum Toxin Type A: A Prospective Study. Plast. Reconstr. Surg. 2023, 151, 56e–67e. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, H.; Gu, C.; Li, F.; Zou, L.; An, Y.; Han, X.; Tang, Z. Individual Factors of Botulinum Type A in Treatment of Gummy Smile: A Prospective Study. Aesthet. Surg. J. 2021, 41, NP842–NP850. [Google Scholar] [CrossRef] [PubMed]
- Andriola, F.d.O.; Chieza, G.S.; Cavagni, J.; Freddo, A.L.; Corsetti, A. Management of Excessive Gingival Display Using Botulinum Toxin Type A: A Descriptive Study. Toxicon 2021, 196, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.V.; Hirata, D.Y.; Reis, A.F.; Santos, V.R.; Miranda, T.S.; Faveri, M.; Duarte, P.M. Open-Flap versus Flapless Esthetic Crown Lengthening: 12-Month Clinical Outcomes of a Randomized Controlled Clinical Trial. J. Periodontol. 2014, 85, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Altayeb, W.; Arnabat-Dominguez, J.; Low, S.; Abdullah, A.; Romanos, G. Laser-Assisted Esthetic Crown Lengthening: Open-Flap Versus Flapless. Int. J. Periodontics Restor. Dent. 2022, 42, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Tamada, Y.; Tabuchi, M.; Kawaguchi, M.; Shibata, M.; Sato, T.; Okamoto, K.; Saito, N.; Goto, S. Effective Approach for Improving a Gummy Smile: Upward Movement of the Maxillary Occlusal Plane Using Midpalatal Miniscrews and a Modified Transpalatal Arch. J. Orofac. Orthop. 2022, 85, 167–180. [Google Scholar] [CrossRef]
- Ferreira, C.E.; Brandao, R.C.; Martinelli, C.B.; Pignaton, T.B. Improving Gingival Smile by Means of Guided Bone Regeneration Principles. Dent. Press. J. Orthod. 2016, 21, 116–125. [Google Scholar] [CrossRef]
- Silva, C.O.; Ribeiro-Junior, N.V.; Campos, T.V.S.; Rodrigues, J.G.; Tatakis, D.N. Excessive gingival display: Treatment by a modified lip repositioning technique. J. Clin. Periodontol. 2013, 40, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Soumaille, J.M.S.; Marson, F.C.; Progiante, P.S.; Tatakis, D.N. Aesthetic crown lengthening: Periodontal and patient-centred outcomes. J. Clin. Periodontol. 2015, 42, 1126–1134. [Google Scholar] [CrossRef]
- Alammar, A.; Heshmeh, O.; Mounajjed, R.; Goodson, M.; Hamadah, O. A comparison between modified and conventional surgical techniques for surgical lip repositioning in the management of the gummy smile. J. Esthet. Restor. Dent. 2018, 30, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.M.; Heshmeh, O.A. Lip Repositioning with a Myotomy of the Elevator Muscles for the Management of a Gummy Smile. Dent. Med. Probl. 2018, 55, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, O.K.; Naiem, S.N.; Tawfik, L.K.; Yussif, N.; Meghil, M.M.; Cutler, C.W.; Darhous, M.; El-Nahass, H.E. Lip repositioning with or without myotomy: A randomized clinical trial. J. Periodontol. 2018, 89, 815–823. [Google Scholar] [CrossRef]
- Al Jasser, R.N.; AlSarhan, M.A.; Alotaibi, D.H.; Bhola, M. A Modified Approach in Lip Repositioning Surgery: A Prospective Study in a Twin Population with a 3-Year Follow-Up. Int. J. Periodontics Restor. Dent. 2021, 41, e243–e253. [Google Scholar] [CrossRef] [PubMed]
- Hazzaa, H.H.; Elwakeel, N.M.; Abdulhady, E.M.; Abdel-Aziz, L.M. Evaluation of the internal dual muscle traction approach as an adjunct to the modified surgical lip repositioning method: A randomized clinical report. J. Oral Maxillofac. Surg. Med. Pathol. 2022, 34, 12–18. [Google Scholar] [CrossRef]
- Al Wayli, H. Versatility of Botulinum Toxin at the Yonsei Point for the Treatment of Gummy Smile. Int. J. Esthet. Dent. 2019, 14, 86–95. [Google Scholar] [PubMed]
- Cengiz, A.F.; Goymen, M.; Akcali, C. Efficacy of Botulinum Toxin for Treating a Gummy Smile. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 50–58. [Google Scholar] [CrossRef]
- Shemais, N.; Elarab, A.E.; ElNahass, H. The Effect of Botulinum Toxin A in Patients with Excessive Gingival Display with and without Zinc Supplementation: Randomized Clinical Trial. Clin. Oral Investig. 2021, 25, 6403–6417. [Google Scholar] [CrossRef]
- Adel, N. A Standardized Technique for Gummy Smile Treatment Using Repeated Botulinum Toxins: A 1-year Follow-up Study. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4281. [Google Scholar] [CrossRef] [PubMed]
- Soris, B.A.T.; Shenoy, K.V.; Ramadorai, A.; Kumar, C.S.C.S.; Marimuthu, L. Botulinum Toxin-A in the Treatment of Excessive Gingival Display: A Clinical Study. J. Maxillofac. Oral Surg. 2022, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.E.A.; de Almeida, W.R.; Cotrin, P.; de Oliveira, R.C.G.; de Oliveira, R.C.G.; Valarelli, F.P.; Zamuner, J.W.; de Freitas, K.M.S. Evaluation of the botulinum toxin effects in the correction of gummy smile 32 weeks after application. ABCS Health Sci. 2022, 47, e022201. [Google Scholar] [CrossRef]
- Makkeiah, M.O.; Harfoush, M.; Makkiah, A.; Saneeva, L.; Tuturov, N.; Katbeh, I. Comparative efficacy of Botox and surgical lip repositioning in the correction of gummy smile. Stomatologiia 2021, 100, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mossaad, A.M.; Abdelrahman, M.A.; Kotb, A.M.; Alolayan, A.B.; Elsayed, S.A.-H. Gummy Smile Management Using Diode Laser Gingivectomy Versus Botulinum Toxin Injection—A Prospective Study. Ann. Maxillofac. Surg. 2021, 11, 70–74. [Google Scholar] [PubMed]
- Antunes, K.B.; Dias, A.; Kahn, S.; Schneider, L.F.J.; Cavalcante, L.M. Use of Botulinum Toxin Before Surgical Lip Repositioning: A Randomized Clinical Trial. Int. J. Periodontics Restor. Dent. 2022, 42, e175–e183. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.O.R.; Elias, C.N.; Joly, J.C. A Lip Repositioning Technique Using Polyester Threads for Gummy Smile Treatment. Int. J. Dent. 2022, 2022, 3972150. [Google Scholar] [CrossRef] [PubMed]
- Garber, D.A.; Salama, M.A. The Aesthetic Smile: Diagnosis and Treatment. Periodontology 2000 1996, 11, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Akram, H.M.; Ali, O.H.; Omran, N.K.; Ali, A.O. Diode Laser versus Scalpel Gingivectomy. Biomed. Pharmacol. J. 2017, 10, 1799–1804. [Google Scholar] [CrossRef]
- Muradin, M.; Rosenberg, A.; van der Bilt, A.; Stoelinga, P.; Koole, R. The Effect of Alar Cinch Sutures and V-Y Closure on Soft Tissue Dynamics after Le Fort I Intrusion Osteotomies. J. Cranio-Maxillo-Facial Surg. 2009, 37, 334–340. [Google Scholar] [CrossRef]
- Rubin, L.R.; Mishriki, Y.; Lee, G. Anatomy of the Nasolabial Fold: The Keystone of the Smiling Mechanism. Plast. Reconstr. Surg. 1989, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.-S.; Hur, M.-S.; Hu, K.-S.; Song, W.-C.; Koh, K.-S.; Baik, H.-S.; Kim, S.-T.; Kim, H.-J.; Lee, K.-J. Surface Anatomy of the Lip Elevator Muscles for the Treatment of Gummy Smile Using Botulinum Toxin. Angle Orthod. 2009, 79, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Koshy, J.C.; Sharabi, S.E.; Feldman, E.M.; Hollier, L.H.; Patrinely, J.R.; Soparkar, C.N.S. Effect of Dietary Zinc and Phytase Supplementation on Botulinum Toxin Treatments. J. Drugs Dermatol. 2012, 11, 507–512. [Google Scholar] [PubMed]
- Papageorgiou, S.N.; Xavier, G.M.; Cobourne, M.T. Basic Study Design Influences the Results of Orthodontic Clinical Investigations. J. Clin. Epidemiol. 2015, 68, 1512–1522. [Google Scholar] [CrossRef]
- Peck, S.; Peck, L.; Kataja, M. The Gingival Smile Line. Angle Orthod. 1992, 62, 91–100; discussion 101–102. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).