A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cellular Models
2.3. Expression and Purification of Cdt, CdtB Mutants, and Cdt Holotoxin
2.4. Treatment with LGM2605
2.5. RNA Isolation and Gene Expression Analysis
2.6. Bacteria and Growth Curve
2.7. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.8. Western Blot Analysis
2.9. Cytokine Analysis
2.10. Confocal Microscopy
2.11. Cell Viability
2.12. Statistical Analysis
3. Results
3.1. LGM2605 Treatment Downregulates Expression of Inflammatory Cytokines
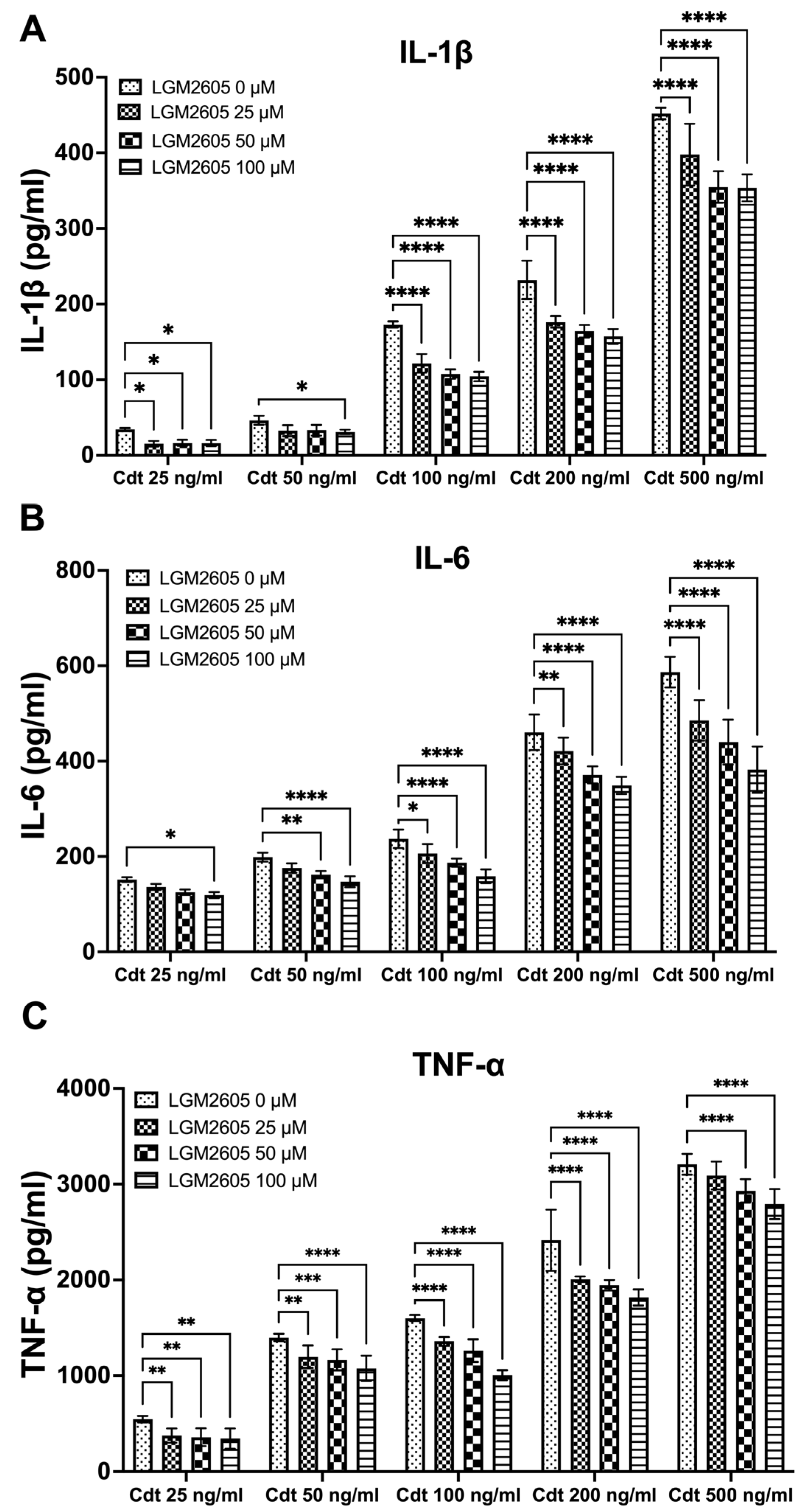
3.2. LGM2605 Diminishes Cdt-Induced Pro-Inflammatory Cytokine Release
3.3. LGM2605 Diminishes Aa-Induced Pro-Inflammatory Cytokine Release or Secretion
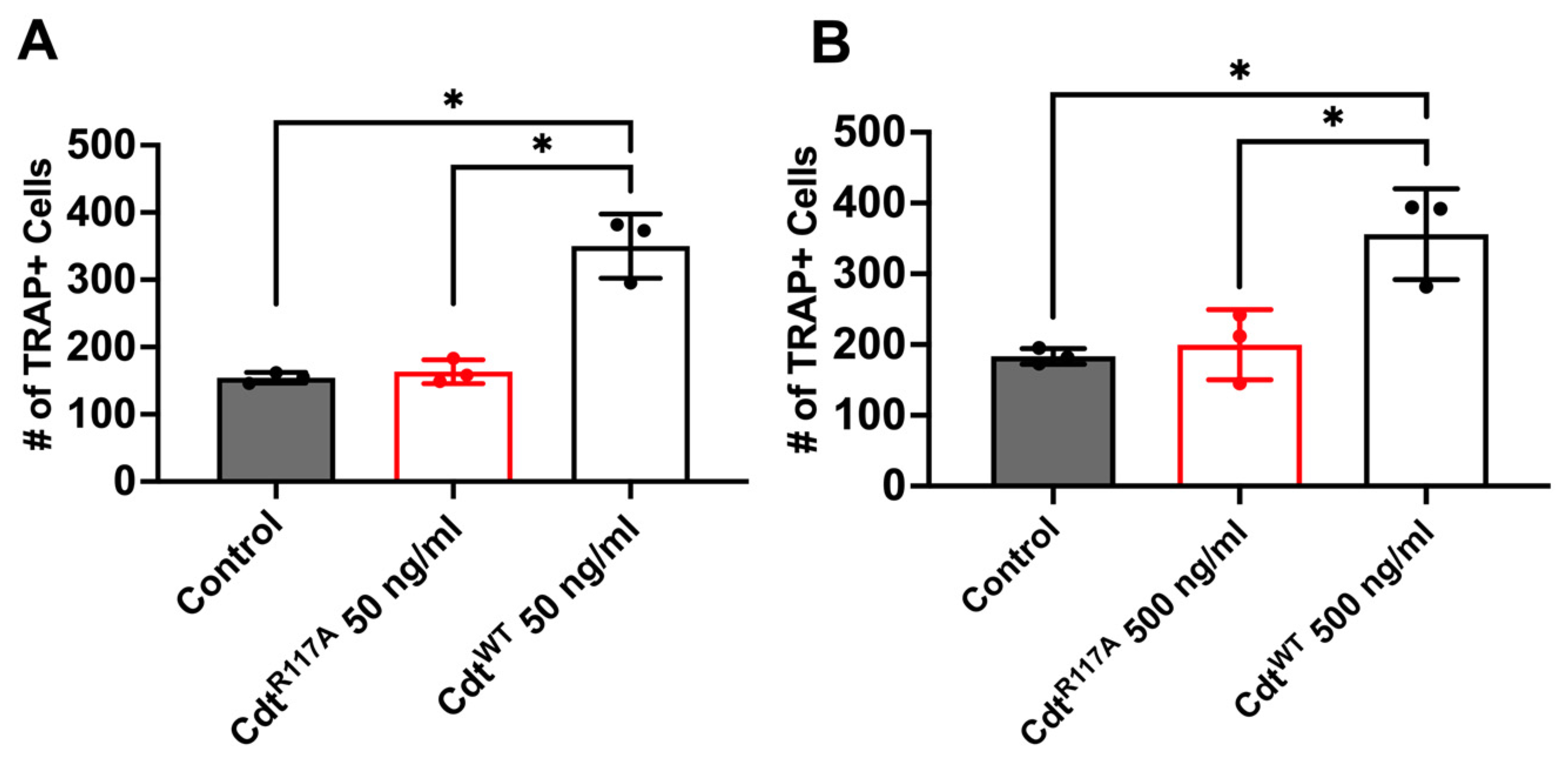
3.4. Cdt Phosphatase Activity Induced Pro-Inflammatory Environment Leads to Increase in Osteoclast Differentiation
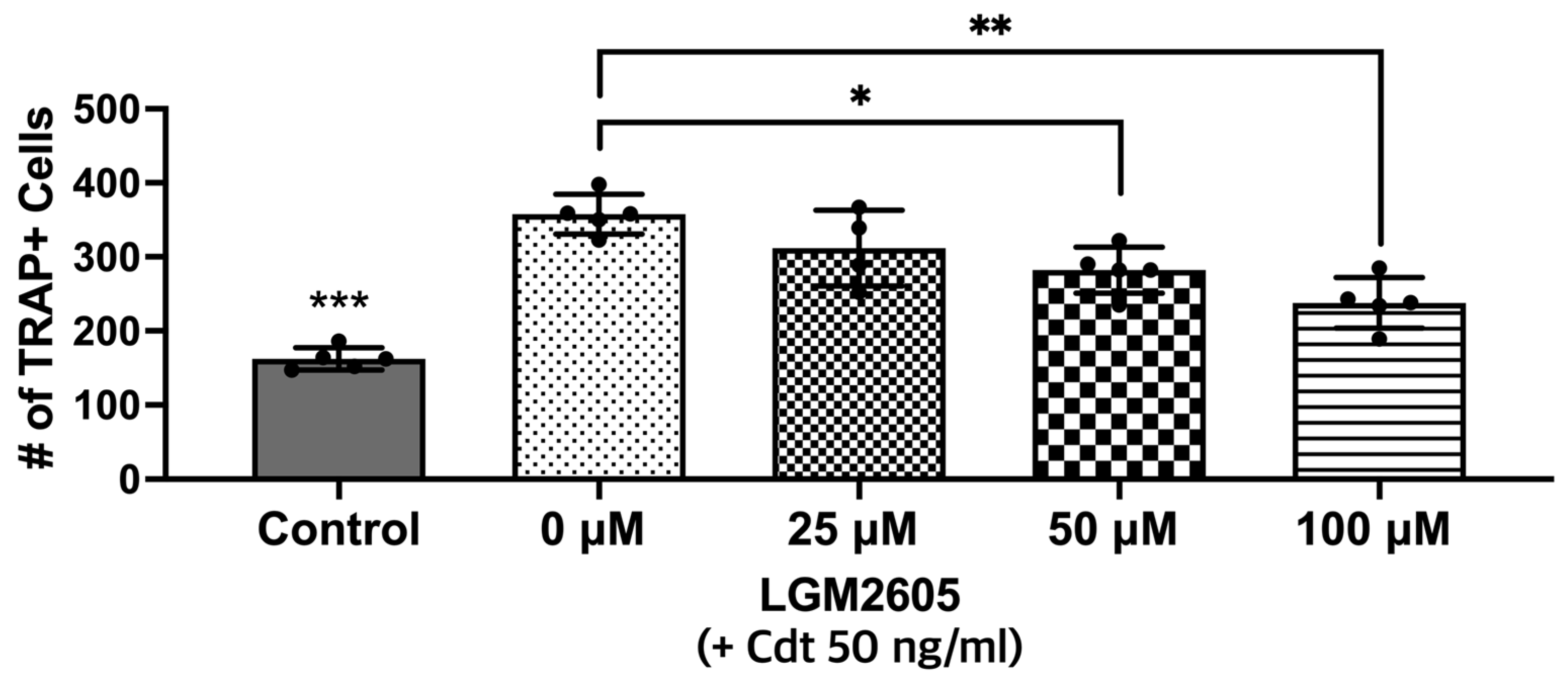
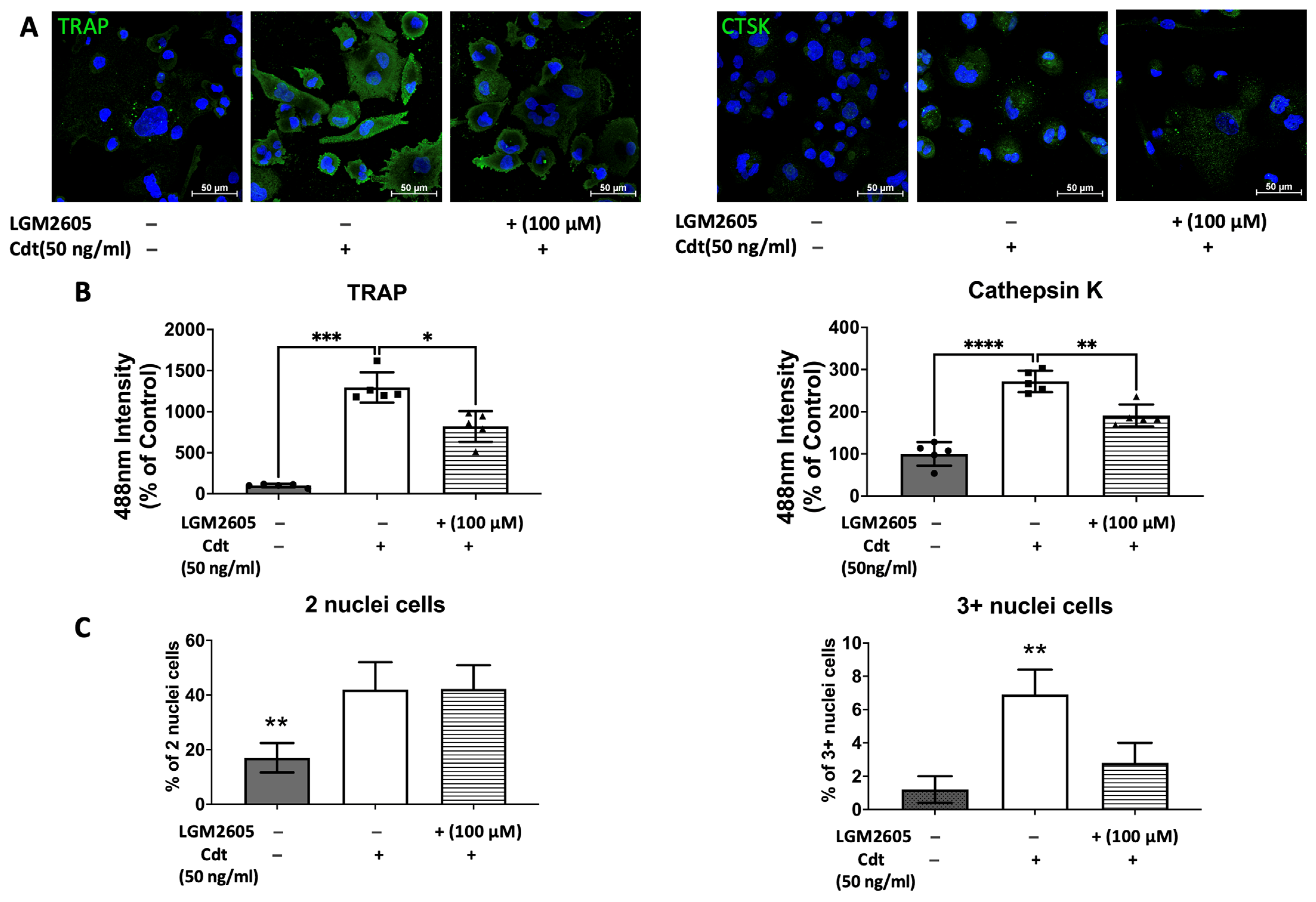
3.5. Cdt-Mediated Increase in Osteoclast Differentiation/Maturation Can Be Mitigated by LGM2605
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Periodontol. 2018, 89 (Suppl. 1), S103–S119. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Dhondt, R.; Dekeyser, C.; Quirynen, M. Treatment of aggressive periodontitis. Periodontol. 2000 2014, 65, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local Delivery and Controlled Release Drugs Systems: A New Approach for the Clinical Treatment of Periodontitis Therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Pons, B.J.; Vignard, J.; Mirey, G. Cytolethal Distending Toxin Subunit B: A Review of Structure-Function Relationship. Toxins 2019, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Schauer, D.B.; Fox, J.G. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell Microbiol. 2008, 10, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Walker, L.P.; Zekavat, A.; Dlakic, M.; Boesze-Battaglia, K. Blockade of the PI-3K signalling pathway by the Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces macrophages to synthesize and secrete pro-inflammatory cytokines. Cell Microbiol. 2014, 16, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Yamano, R.; Ohara, M.; Nishikubo, S.; Fujiwara, T.; Kawamoto, T.; Ueno, Y.; Komatsuzawa, H.; Okuda, K.; Kurihara, H.; Suginaka, H.; et al. Prevalence of cytolethal distending toxin production in periodontopathogenic bacteria. J. Clin. Microbiol. 2003, 41, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.A.; Novince, C.M.; Kirkwood, K.L. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol. Oral Microbiol. 2016, 31, 207–227. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Hotokezaka, H.; Sakai, E.; Ohara, N.; Hotokezaka, Y.; Gonzales, C.; Matsuo, K.; Fujimura, Y.; Yoshida, N.; Nakayama, K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-alpha, lipopolysaccharide, or peptidoglycan. J. Cell Biochem. 2007, 101, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: Longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.L.; Cirelli, J.A.; Rogers, J.E.; Giannobile, W.V. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol. 2000 2007, 43, 294–315. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Pietrofesa, R.A.; Park, K.; Tao, J.Q.; Carabe-Fernandez, A.; Berman, A.T.; Koumenis, C.; Sielecki, T.; Christofidou-Solomidou, M. LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks. Int. J. Mol. Sci. 2019, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Park, K.; Albelda, S.M.; Serve, K.M.; Keil, D.E.; Pfau, J.C. Synthetic secoisolariciresinol diglucoside (LGM2605) inhibits Libby amphibole fiber-induced acute inflammation in mice. Toxicol. Appl. Pharmacol. 2019, 375, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Sharp, R.C.; Kim, T.; Popov, A.V.; Ying, G.S.; Pietrofesa, R.A.; Park, K.; Christofidou-Solomidou, M.; Boesze-Battaglia, K. Assessment of a Small Molecule Synthetic Lignan in Enhancing Oxidative Balance and Decreasing Lipid Accumulation in Human Retinal Pigment Epithelia. Int. J. Mol. Sci. 2021, 22, 5764. [Google Scholar] [CrossRef] [PubMed]
- Kokkinaki, D.; Hoffman, M.; Kalliora, C.; Kyriazis, I.D.; Maning, J.; Lucchese, A.M.; Shanmughapriya, S.; Tomar, D.; Park, J.Y.; Wang, H.; et al. Chemically synthesized Secoisolariciresinol diglucoside (LGM2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Hwang, W.T.; Andrake, M.; Nakamaru-Ogiso, E.; Christofidou-Solomidou, M. Radiation activates myeloperoxidase (MPO) to generate active chlorine species (ACS) via a dephosphorylation mechanism—Inhibitory effect of LGM2605. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129548. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Chatterjee, S.; Kadariya, Y.; Testa, J.R.; Albelda, S.M.; Christofidou-Solomidou, M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Prevents Asbestos-Induced Inflammation and Genotoxic Cell Damage in Human Mesothelial Cells. Int. J. Mol. Sci. 2022, 23, 10085. [Google Scholar] [CrossRef]
- Badger, R.; Aho, K.; Serve, K. Short-term exposure to synthetic flaxseed lignan LGM2605 alters gut microbiota in mice. Microbiologyopen 2021, 10, e1185. [Google Scholar] [CrossRef]
- Kartha, S.; Weisshaar, C.L.; Pietrofesa, R.A.; Christofidou-Solomidou, M.; Winkelstein, B.A. Synthetic Secoisolariciresinol Diglucoside Attenuates Established Pain, Oxidative Stress and Neuroinflammation in a Rodent Model of Painful Radiculopathy. Antioxidants 2020, 9, 1209. [Google Scholar] [CrossRef]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Nakamaru-Ogiso, E.; Andrake, M.; Christofidou-Solomidou, M. Synthetic secoisolariciresinol diglucoside (LGM2605) inhibits myeloperoxidase activity in inflammatory cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Chatterjee, S.; Pietrofesa, R.A.; Koziol-White, C.; Panettieri, R.A.; Lin, L.; Tuttle, S.; Berman, A.; Koumenis, C.; Christofidou-Solomidou, M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. Int. J. Mol. Sci. 2017, 18, 2525. [Google Scholar] [CrossRef]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP-1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef]
- Kim, T.J.; Shenker, B.J.; MacElory, A.S.; Spradlin, S.; Walker, L.P.; Boesze-Battaglia, K. Aggregatibacter actinomycetemcomitans cytolethal distending toxin modulates host phagocytic function. Front. Cell. Infect. Microbiol. 2023, 13, 1220089. [Google Scholar] [CrossRef]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The biology of the cytolethal distending toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef]
- Shenker, B.J.; Walker, L.M.; Zekavat, Z.; Ojcius, D.M.; Huang, P.R.; Boesze-Battaglia, K. Cytolethal distending toxin-induced release of interleukin-1beta by human macrophages is dependent upon activation of glycogen synthase kinase 3beta, spleen tyrosine kinase (Syk) and the noncanonical inflammasome. Cell Microbiol. 2020, 22, e13194. [Google Scholar] [CrossRef]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, D.; Ando-Suguimoto, E.S.; Bueno-Silva, B.; DiRienzo, J.M.; Mayer, M.P. Alteration of Homeostasis in Pre-osteoclasts Induced by Aggregatibacter actinomycetemcomitans CDT. Front. Cell. Infect. Microbiol. 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.K.; Nohutcu, R.M. Mediators of periodontal osseous destruction and remodeling: Principles and implications for diagnosis and therapy. J. Periodontol. 2002, 73, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Chatterjee, S.; Park, K.; Arguiri, E.; Albelda, S.M.; Christofidou-Solomidou, M. Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605) Reduces Asbestos-Induced Cytotoxicity in an Nrf2-Dependent and -Independent Manner. Antioxidants 2018, 7, 38. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Kyritsis, G.; Graves, D.T.; Amar, S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 1999, 67, 4231–4236. [Google Scholar] [CrossRef]
- Ishimi, Y.; Miyaura, C.; Jin, C.H.; Akatsu, T.; Abe, E.; Nakamura, Y.; Yamaguchi, A.; Yoshiki, S.; Matsuda, T.; Hirano, T.; et al. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1990, 145, 3297–3303. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Heulsmann, A.; Tondravi, M.M.; Mukherjee, A.; Abu-Amer, Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 2001, 276, 563–568. [Google Scholar] [CrossRef]
- Raisanen, S.R.; Alatalo, S.L.; Ylipahkala, H.; Halleen, J.M.; Cassady, A.I.; Hume, D.A.; Vaananen, H.K. Macrophages overexpressing tartrate-resistant acid phosphatase show altered profile of free radical production and enhanced capacity of bacterial killing. Biochem. Biophys. Res. Commun. 2005, 331, 120–126. [Google Scholar] [CrossRef]
- Han, J.; Yang, K.; An, J.; Jiang, N.; Fu, S.; Tang, X. The Role of NRF2 in Bone Metabolism—Friend or Foe? Front. Endocrinol. 2022, 13, 813057. [Google Scholar] [CrossRef]
- Hyeon, S.; Lee, H.; Yang, Y.; Jeong, W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013, 65, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Y.; Zhou, X.; Tian, Y.; Miao, Z.; Ko, C.C.; Hu, X. Nrf2 differentially regulates osteoclast and osteoblast differentiation for bone homeostasis. Biochem. Biophys. Res. Commun. 2023, 674, 19–26. [Google Scholar] [CrossRef] [PubMed]





| Antibody (Host) | Source (Catalog #) | Dilution (Application) |
|---|---|---|
| Cathepsin K, CTSK (Rabbit) | Proteintech (11239-1-AP) | 1:500 (Western Blot) 1:100 (Immunocytochemistry) |
| Tartrate-resistant acid phosphatase, TRAP (Rabbit) | Invitrogen (PA5-116970) | 1:1000 (Western Blot) 1:100 (Immunocytochemistry) |
| Glyceraldehyde 3-phosphate dehydrogenase, GAPDH (Rabbit) | Cell Signaling (D16H11) | 1:2500 (Western Blot) |
| Gene ID | Gene Bank | Assay ID | Amplicon Length |
|---|---|---|---|
| IL1B | NM_000576.2 | Hs01555410_m1 | 91 |
| IL6 | NM_000600.4 | Hs00174131_m1 | 95 |
| TNFa | NM_000594.3 | Hs00174128_m1 | 80 |
| GAPDH | NM_001256799.2 | Hs02786624_g1 | 157 |
| Abbreviation | Definition |
|---|---|
| Aa | Aggregatibacter actinomycetemcomitans |
| ARE | Antioxidant response element |
| Cdt | Cytolethal distending toxin |
| CdtR117A | Cdt holotoxin containing phosphatase deficient CdtB subunit |
| CdtWT | Cdt holotoxin containing the wildtype CdtB subunit |
| CFU | Colony forming unit |
| CTSK | Cathepsin K |
| D7S | Wild-type Aa |
| ELISA | Enzyme-linked immunosorbent assay |
| GCF | Gingival crevicular fluid |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| LAP | Localized aggressive periodontitis |
| LGM2605 | SDG synthetic counterpart |
| M-CSF | Macrophage colony-stimulating factor |
| MIPP | Molar/incisor pattern periodontitis |
| NF-κB | Nuclear factor-κB |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| OC | Osteoclast |
| PGE2 | Prostaglandin E2 |
| PI | Phosphatidylinositol |
| PIP2 | Phosphatidylinsoitol-3,4-diphosphate |
| PIP3 | Phosphatidylinositol-3,4,5-triphosphate |
| PMA | Phorbol 12-myristate 13-acetate |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| SDG | Secoisolariciresinol diglucoside |
| TDO | THP-1 cell derived osteoclast |
| TDM | THP-1 cell differentiated macrophage |
| TNF-α | Tumor necrosis factor alpha |
| TRAP | Tartrate-resistant acid phosphatase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.J.; MacElroy, A.S.; Defreitas, A.; Shenker, B.J.; Boesze-Battaglia, K. A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin. Dent. J. 2024, 12, 195. https://doi.org/10.3390/dj12070195
Kim TJ, MacElroy AS, Defreitas A, Shenker BJ, Boesze-Battaglia K. A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin. Dentistry Journal. 2024; 12(7):195. https://doi.org/10.3390/dj12070195
Chicago/Turabian StyleKim, Taewan J., Andrew S. MacElroy, Aleena Defreitas, Bruce J. Shenker, and Kathleen Boesze-Battaglia. 2024. "A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin" Dentistry Journal 12, no. 7: 195. https://doi.org/10.3390/dj12070195
APA StyleKim, T. J., MacElroy, A. S., Defreitas, A., Shenker, B. J., & Boesze-Battaglia, K. (2024). A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin. Dentistry Journal, 12(7), 195. https://doi.org/10.3390/dj12070195







