Direct Oral Anticoagulants and Bleeding Management Following Tooth Extractions—A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Patients Enrolment
2.3. Study Groups
- -
- Group 1: Patients treated with DOACs with no modification of administration regimen for the surgical intervention.
- -
- Group 2: Patients treated with DOACs with postponed, reduced, or interrupted treatment regimens for the surgical intervention. Indication of DOACs suspension was provided by the cardiologist or the general practitioner based on the clinical conditions of the patient.
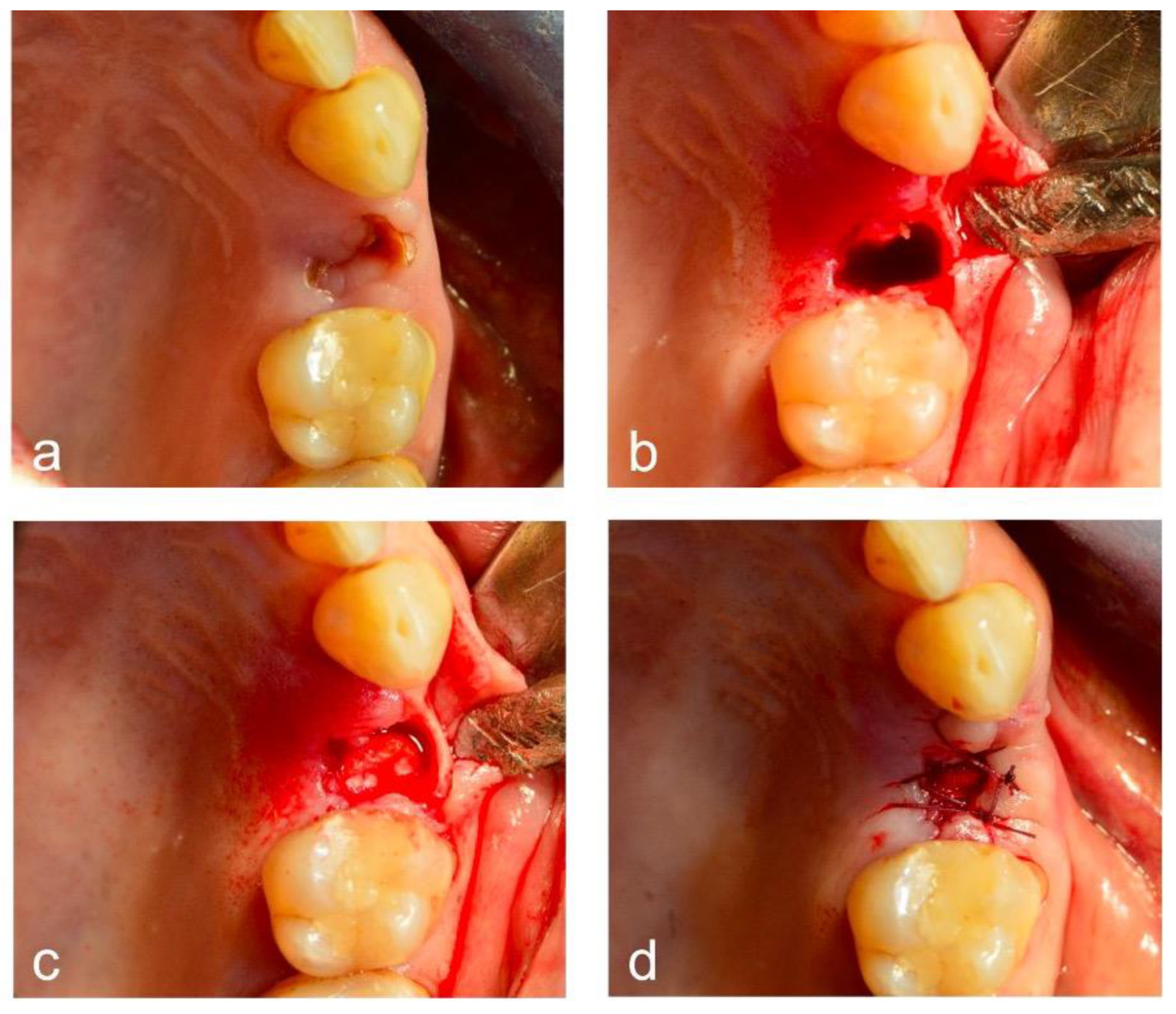
2.4. Surgical Intervention
2.5. Bleeding Episodes’ Registration
2.6. Follow-Up
2.7. Sample Size Estimation
2.8. Statistical Analysis
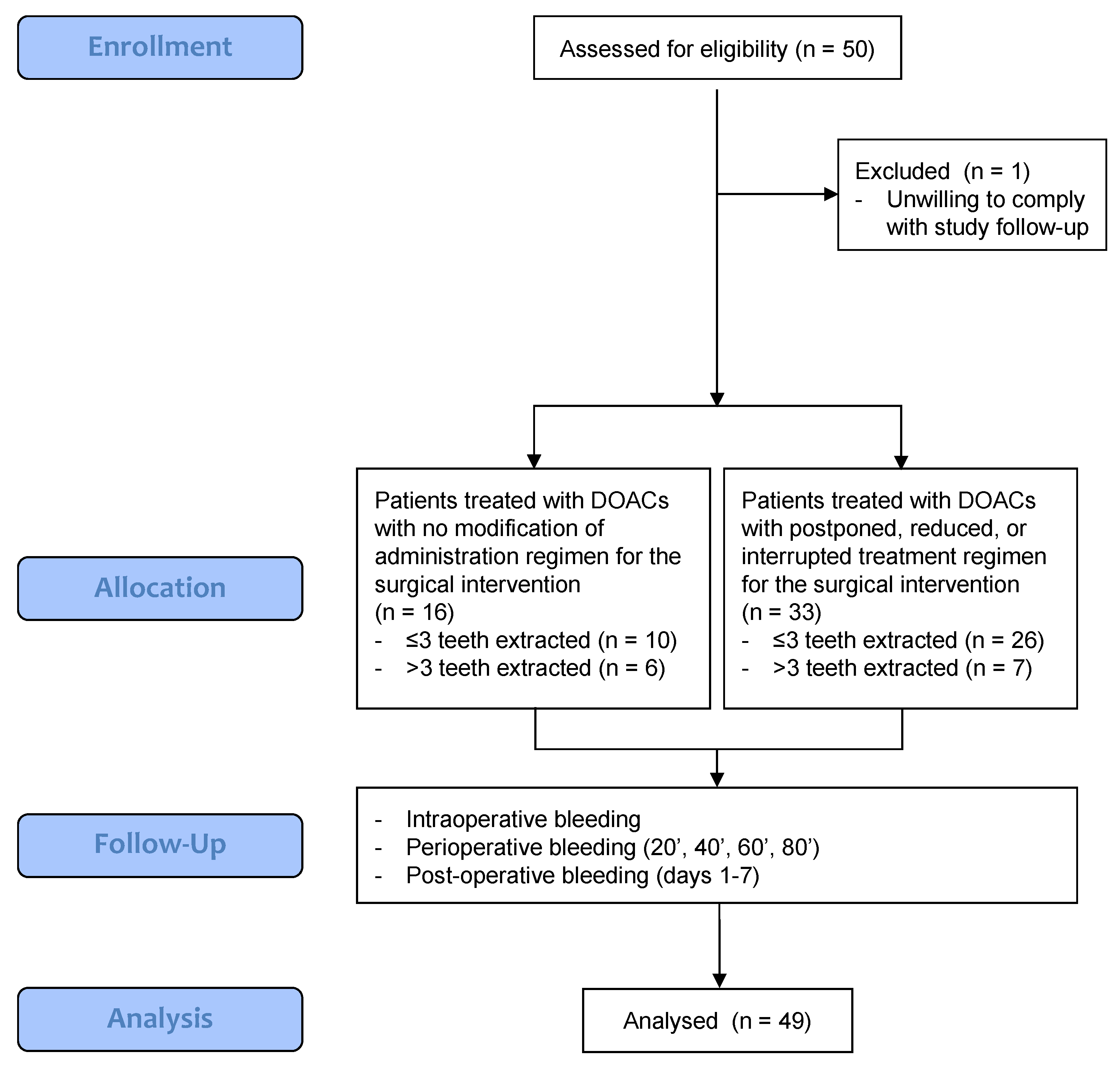
3. Results
3.1. Patient Population
3.2. Features of Tooth Extraction Procedures
3.3. Intra-Operative Bleeding
3.4. Peri-Operative Bleeding
3.5. Post-Operative Bleeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.Y.; Park, S.H.; Kim, D.M.; Ko, K.A.; Park, J.Y.; Lee, J.S.; Jung, U.W.; Cha, J.K. Risk of post-operative bleeding after dentoalveolar surgery in patients taking anticoagulants: A cohort study using the common data model. Sci. Rep. 2024, 14, 7787. [Google Scholar] [CrossRef] [PubMed]
- Darwish, G. The Effect of Direct Oral Anticoagulant Therapy (DOACs) on oral surgical procedures: A systematic review. BMC Oral Health 2023, 23, 743. [Google Scholar] [CrossRef]
- Ono, S.; Ishimaru, M.; Yokota, I.; Konishi, T.; Okada, A.; Ono, Y.; Matsui, H.; Itai, S.; Yonenaga, K.; Tonosaki, K.; et al. Risk of post-extraction bleeding with direct oral anticoagulant compared with warfarin: Retrospective cohort study using large scale claims data in Japan. Thromb. Res. 2023, 222, 24–30. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Perry, D.J.; Noakes, T.J.C.; Helliwell, P.S.; British Dental Society. Guidelines for the management of patients on oral anticoagulants requiring dental surgery. Br. Dent. J. 2007, 203, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Healey, J.S.; Brueckmann, M.; Fraessdorf, M.; Spyropoulos, A.C.; Wallentin, L.; Oldgren, J.; Reilly, P.; Ezekowitz, M.D.; Connolly, S.J.; et al. Urgent surgery or procedures in patients taking dabigatran or warfarin: Analysis of perioperative outcomes from the RE-LY trial. Thromb. Res. 2016, 139, 77–81. [Google Scholar] [CrossRef]
- Valenzuela-Mencia, J.; Serrera-Figallo, M.; Torres-Lagares, D.; Machuca-Portillo, G.; Sánchez-Fernández, E.; Valmaseda-Castellón, E.; Peñarrocha-Diago, M.; Fernández-Mosteirín, N.; Somoza-Martin, J.; Pérez-Jardón, A.; et al. Clinical practice guideline of the spanish society of oral surgery for oral surgery in patients with coagulation disorders. Med. Oral Patol. Oral Cir. Bucal 2024, 29, e58–e66. [Google Scholar] [CrossRef] [PubMed]
- Berton, F.; Costantinides, F.; Rizzo, R.; Franco, A.; Contarin, J.; Stacchi, C.; Maglione, M.; Visintini, E.; Di Lenarda, A.; Di Lenarda, R. Should we fear direct oral anticoagulants more than vitamin K antagonists in simple single tooth extraction? A prospective comparative study. Clin. Oral Investig. 2019, 23, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Woolcombe, S.A.; Ball, R.E.; Patel, J.P. Managing direct oral anticoagulants in accordance with the Scottish Dental Clinical Effectiveness Programme guidance for patients undergoing dentoalveolar surgery. Br. Dent. J. 2022, 232, 547–554. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T., Jr.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019, 16, e66–e93. [Google Scholar] [CrossRef] [PubMed]
- Nisi, M.; Carli, E.; Gennai, S.; Gulia, F.; Izzetti, R. Hemostatic Agents for the Management of Bleeding Risk Associated with Oral Anticoagulant Therapy Following Tooth Extraction: A Systematic Review. Appl. Sci. 2022, 12, 11017. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Abayon, M.; Kolokythas, A.; Harrison, S.; Elad, S. Dental management of patients on direct oral anticoagulants: Case series and literature review. Quintessence Int. 2016, 47, 687–696. [Google Scholar] [CrossRef]
- Lupi, S.M.; Rodriguez YBaena, A. Patients Taking Direct Oral Anticoagulants (DOAC) Undergoing Oral Surgery: A Review of the Literature and a Proposal of a Peri-Operative Management Protocol. Healthcare 2020, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, D.; Sugrue, A.; Amin, M.; Mehta, R.; Farwati, M.; Deshmukh, A.J.; Sridhar, H.; Ahmed, A.; Asirvatham, S.J.; Ou, N.N.; et al. Outcomes of Direct Oral Anticoagulants Co-Prescribed with Common Interacting Medications. Am. J. Cardiol. 2022, 162, 80–85. [Google Scholar] [CrossRef]
- Yagi, T.; Mannheimer, B.; Reutfors, J.; Ursing, J.; Giunta, D.H.; Kieler, H.; Linder, M. Bleeding events among patients concomitantly treated with direct oral anticoagulants and macrolide or fluoroquinolone antibiotics. Br. J. Clin. Pharmacol. 2023, 89, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Mar, P.L.; Gopinathannair, R.; Gengler, B.E.; Chung, M.K.; Perez, A.; Dukes, J.; Ezekowitz, M.D.; Lakkireddy, D.; Lip, G.Y.; Miletello, M.; et al. Drug Interactions Affecting Oral Anticoagulant Use. Circ. Arrhythm. Electrophysiol. 2022, 15, e007956. [Google Scholar] [CrossRef] [PubMed]
- Lababidi, E.; Breik, O.; Savage, J.; Engelbrecht, H.; Kumar, R.; Crossley, C.W. Assessing an oral surgery specific protocol for patients on direct oral anticoagulants: A retrospective controlled cohort study. Int. J. Oral Maxillofac. Surg. 2018, 47, 940–946. [Google Scholar] [CrossRef]
- Cocero, N.; Basso, M.; Grosso, S.; Carossa, S. Direct Oral Anticoagulants and Medical Comorbidities in Patients Needing Dental Extractions: Management of the Risk of Bleeding. J. Oral Maxillofac. Surg. 2019, 77, 463–470. [Google Scholar] [CrossRef]
- Firriolo, F.J.; Hupp, W.S. Beyond warfarin: The new generation of oral anticoagulants and their implications for the management of dental patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 431–441. [Google Scholar] [CrossRef]
- Little, J.W. New oral anticoagulants: Will they replace warfarin? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Yagyuu, T.; Kawakami, M.; Ueyama, Y.; Imada, M.; Kurihara, M.; Matsusue, Y.; Imai, Y.; Yamamoto, K.; Kirita, T. Risks of postextraction bleeding after receiving direct oral anticoagulants or warfarin: A retrospective cohort study. BMJ Open 2017, 7, e015952. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, I.; Natsuko, S.Y.; Yutaka, I.; Masayori, S.; Hiroyuki, N.; Hirohisa, I. Frequency of hemorrhage after tooth extraction in patients treated with a direct oral anticoagulant: A multicenter cross-sectional study. PLoS ONE 2022, 17, e0266011. [Google Scholar] [CrossRef]
- Patel, J.P.; Woolcombe, S.A.; Patel, R.K.; Obisesan, O.; Roberts, L.N.; Bryant, C.; Arya, R. Managing direct oral anticoagulants in patients undergoing dentoalveolar surgery. Br. Dent. J. 2017, 222, 245–249. [Google Scholar] [CrossRef]
- Cabbar, F.; Cabbar, A.T.; Coşansu, K.; Çekirdekçi, E.I. Effects of Direct Oral Anticoagulants on Quality of Life During Periprocedural Management for Dental Extractions. J. Oral Maxillofac. Surg. 2019, 77, 904–911. [Google Scholar] [CrossRef]
- Johnston, S. An evidence summary of the management of patients taking direct oral anticoagulants (DOACs) undergoing dental surgery. Int. J. Oral Maxillofac. Surg. 2016, 45, 618–630. [Google Scholar] [CrossRef]
- Van Diermen, D.E.; van der Waal, I.; Hoogstraten, J. Management recommendations for invasive dental treatment in patients using oral antithrombotic medication, including novel oral anticoagulants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 709–716. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P.; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013, 15, 625–651. [Google Scholar] [CrossRef]
- Brennan, Y.; Gu, Y.; Schifter, M.; Crowther, H.; Favaloro, E.J.; Curnow, J. Dental extractions on direct oral anticoagulants vs. warfarin: The DENTST study. Res. Pract. Thromb. Haemost. 2020, 4, 278–284. [Google Scholar] [CrossRef]
- Mauprivez, C.; Khonsari, R.H.; Razouk, O.; Goudot, P.; Lesclous, P.; Descroix, V. Management of dental extraction in patients undergoing anticoagulant oral direct treatment: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e146–e155. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.; Tükel, H.C.; Benlidayi, M.E.; Deniz, A. Is it necessary to alter anticoagulation therapy for tooth extraction in patients taking direct oral anticoagulants? Med. Oral Patol. Oral Cir. Bucal 2017, 22, e767–e773. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.G.; Miller, C.S. Direct oral anticoagulants: A retrospective study of bleeding, behavior, and documentation. Oral Dis. 2018, 24, 243–248. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Sample (n = 49) | Rivaroxaban (n = 17) | Apixaban (n = 16) | Edoxaban (n = 8) | Dabigatran (n = 8) | p Value |
|---|---|---|---|---|---|---|
| Age (years; mean [SD]) | 72.2 (8.3) | 72 (5.6) | 72.8 (9.7) | 70.8 (11) | 73.1 (8.3) | 0.807 |
| Gender (females [%]) | 21 (42.9) | 9 (53.0) | 6 (37.5) | 4 (50.0) | 2 (25.0) | 0.560 |
| BMI (kg/m2; mean [SD]) | 28.3 (5.5) | 29.2 (4.2) | 28 (5.3) | 26.4 (2.1) | 32.7 (8.6) | 0.133 |
| Non-smokers [%]) | 43 (87.8) | 12 (70.6) | 8 (50.0) | 5 (62.5) | 2 (25.0) | 0.133 |
| Systolic pressure (mean [S]D) | 133 (23.8) | 134.4 (21.3) | 125.7 (26.3) | 136.5 (12.5) | 140 (31.6) | 0.299 |
| Diastolic pressure (mean [SD]) | 79 (11.5) | 82.6 (12.5) | 76.6 (11.7) | 75.3 (8.0) | 79.3 (11.4) | 0.293 |
| Body temperature (mean [SD]) | 36.4 (0.4) | 36.1 (0.4) | 36.4 (0.3) | 36.7 (0.3) | 36.3 (0.3) | 0.026 |
| Glycemia (mean [SD]) | 121.4 (49.0) | 119.3 (30.0) | 98 (11.4) | 119.9 (28.7) | 173.9 (93.9) | 0.004 |
| Heart rate (mean [SD]) | 77.6 (14.4) | 75.8 (10.1) | 72.3 (11.1) | 83 (24.5) | 74.4 (15.0) | 0.712 |
| Variables (Mean [SD]) | Total Sample (n = 49) | Rivaroxaban (n = 17) | Apixaban (n = 16) | Edoxaban (n = 8) | Dabigatran (n = 8) | p Value |
|---|---|---|---|---|---|---|
| Red blood cells (106/mm3) | 4.33 (0.38) | 4.31 (0.37) | 4.51 (0.66) | 4.06 (0.69) | 4.43 (0.60) | 0.313 |
| Hemoglobin (g/dL) | 12.55 (2.07) | 12.29 (2.09) | 13.35 (1.99) | 12.13 (1.64) | 13.28 (1.73) | 0.431 |
| Platelets (103/mm3) | 216.47 (58.46) | 226.00 (53.53) | 233.0 (64.99) | 217.50 (110.71) | 236.13 (55.48) | 0.728 |
| PT ratio | 1.34 (0.17) | 1.37 (0.15) | 1.25 (0.22) | 1.18 (0.12) | 1.20 (0.18) | 0.014 |
| INR | 1.31 (0.15) | 1.31 (0.15) | 1.25 (0.23) | 1.25 (0.03) | 1.21 (0.09) | 0.318 |
| aPTT (sec) | 36.57 (3.75) | 37.06 (3.89) | 32.06 (3.52) | 32.14 (3.41) | 46.95 (8.75) | <0.001 |
| aPTT ratio | 1.04 (0.14) | 1.06 (0.13) | 1.02 (0.08) | 1.22 (0.01) | 1.51 (0.31) | <0.001 |
| Fibrinogen (mg/dL) | 367.07 (81.52) | 366.33 (55.80) | 351.5 (86.41) | 351.20 (63.83) | 403.29 (101.92) | 0.299 |
| Creatinine (mg/dL) | 0.98 (0.36) | 0.98 (0.35) | 0.92 (0.27) | 0.87 (0.26) | 1.07 (0.29) | 0.062 |
| Estimated glomerular filtration rate | 82.61 (10.63) | 77.23 (13.48) | 84.46 (13.43) | 84.59 (16,92) | 90.31 (21.17) | 0.646 |
| Bleeding | Total Sample (n = 49) | Rivaroxaban (n = 17) | Apixaban (n = 16) | Edoxaban (n = 8) | Dabigatran (n = 8) | p Value |
|---|---|---|---|---|---|---|
| 20′ min (n [%]) | 28 (57.1) | 5 (29.4) | 14 (87.5) | 6 (75.0) | 3 (37.5) | 0.010 |
| 40′ min (n [%]) | 19 (38.8) | 6 (35.3) | 7 (43.75) | 4 (50.0) | 2 (25.0) | 0.731 |
| 60′ min (n [%]) | 14 (28.6) | 5 (29.4) | 4 (25.0) | 3 (37.5) | 2 (25.0) | 0.928 |
| 80′ min (n [%]) | 8 (16.3) | 5 (29.4) | 3 (18.75) | 0 (0.0) | 0 (0.0) | 0.157 |
| Bleeding | DOAC Continued (n = 16) | DOAC Suspended (n = 33) | p Value |
|---|---|---|---|
| T1 (n [%]) | 8 (50.0) | 15 (45.6) | 0.357 |
| T2 (n [%]) | 5 (31.3) | 6 (18.2) | 0.304 |
| T3 (n [%]) | 1 (6.3) | 3 (9.1) | 0.733 |
| T4 (n [%]) | 1 (6.3) | 1 (3.0) | 0.587 |
| T5 (n [%]) | 0 (0.0) | 2 (6.1) | 0.315 |
| T6 (n [%]) | 0 (0.0) | 1 (3.0) | 0.482 |
| T7 (n [%]) | 0 (0.0) | 2 (6.1) | 0.315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzetti, R.; Cinquini, C.; Nisi, M.; Mattiozzi, M.; Marotta, M.; Barone, A. Direct Oral Anticoagulants and Bleeding Management Following Tooth Extractions—A Prospective Cohort Study. Dent. J. 2024, 12, 279. https://doi.org/10.3390/dj12090279
Izzetti R, Cinquini C, Nisi M, Mattiozzi M, Marotta M, Barone A. Direct Oral Anticoagulants and Bleeding Management Following Tooth Extractions—A Prospective Cohort Study. Dentistry Journal. 2024; 12(9):279. https://doi.org/10.3390/dj12090279
Chicago/Turabian StyleIzzetti, Rossana, Chiara Cinquini, Marco Nisi, Marco Mattiozzi, Monica Marotta, and Antonio Barone. 2024. "Direct Oral Anticoagulants and Bleeding Management Following Tooth Extractions—A Prospective Cohort Study" Dentistry Journal 12, no. 9: 279. https://doi.org/10.3390/dj12090279







