Debonding and Clean-Up in Orthodontics: Evaluation of Different Techniques and Micro-Morphological Aspects of the Enamel Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Sample Preparation
2.3. Duplication Protocol
2.4. Bonding of Brackets
2.5. Debonding and Clean-Up
2.5.1. SO (Sof-Lex)
2.5.2. EP (Enhance-PoGo)
2.5.3. RU (Single-Pass Rubber)
2.5.4. BLD (Blade)
2.6. Observation with an Operative Stereomicroscope
- The fidelity of the replicas, comparing natural teeth post-clean-up with the respective replicas.
- The enamel damage index (EDI), introduced in 1990 by Howell and Weekes [9], which can take four values on a numerical scale from 0 to 3:
- 0 = Smooth surface without scratches. Perikymata are visible;
- 1 = Acceptable surface with only a few superficial scratches;
- 2 = Numerous deeper scratches and grooves;
- 3 = Scratches and grooves are visible to the naked eye.
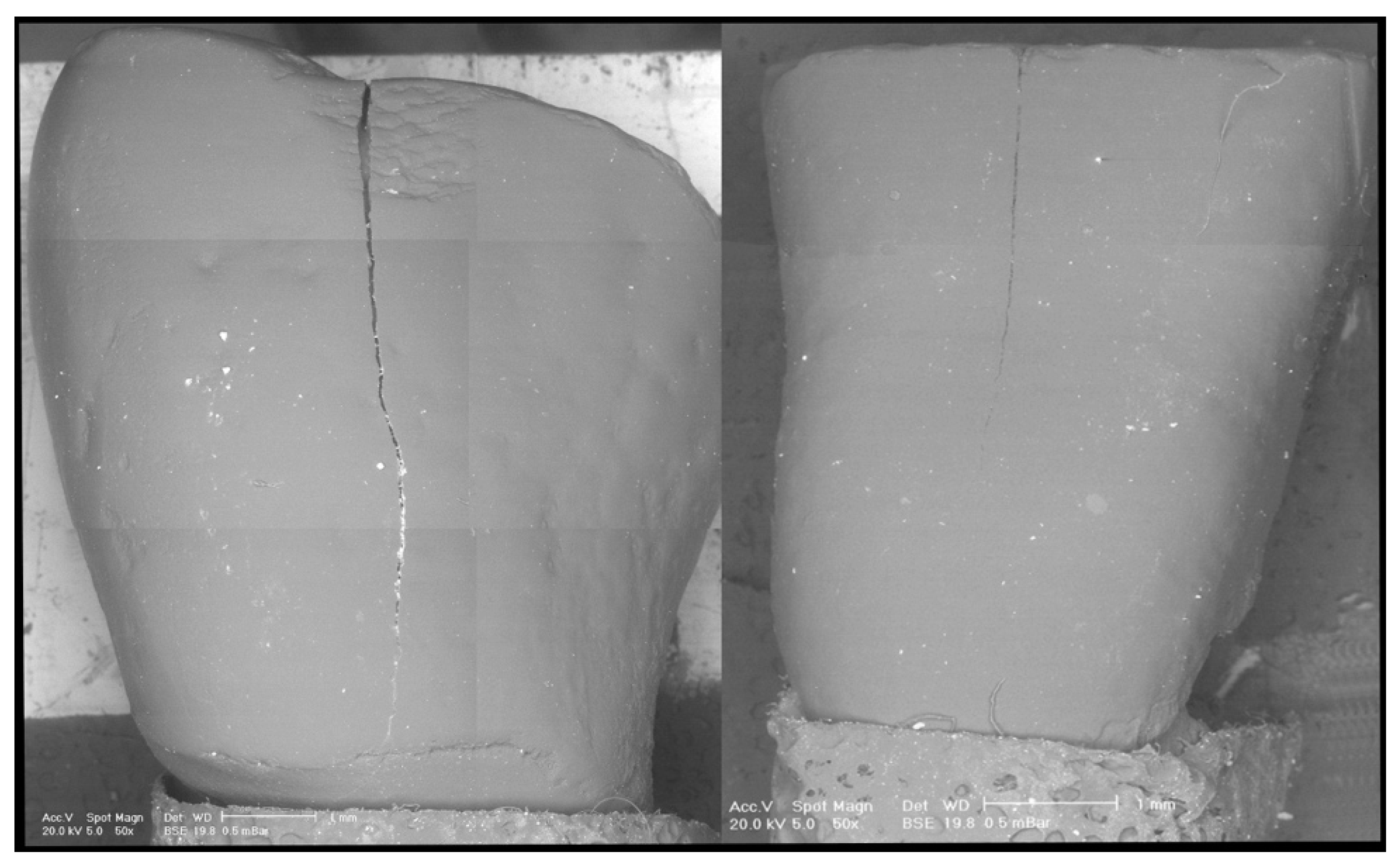
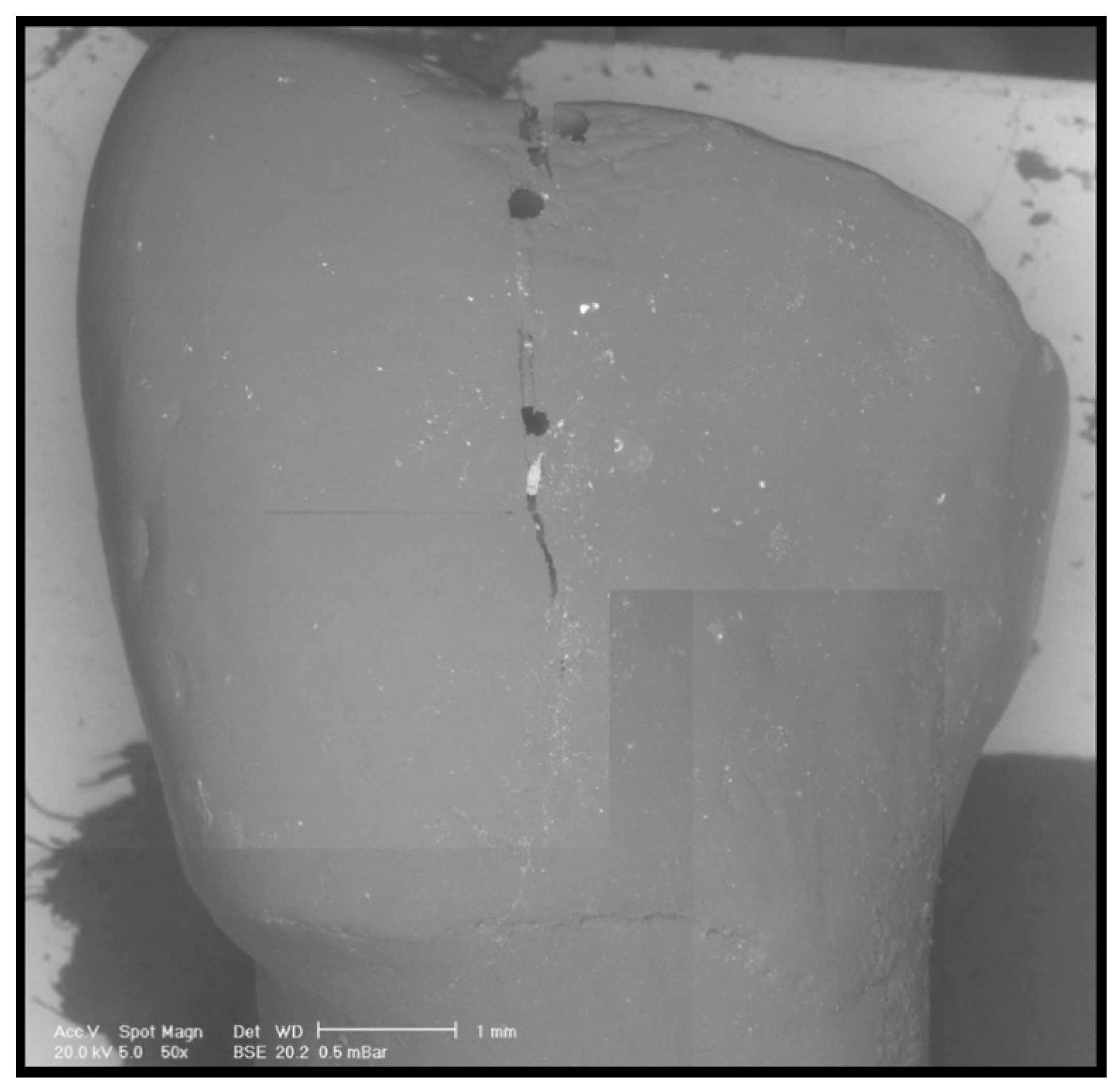
2.7. Scanning Electron Microscope (SEM) Analysis
- 0 = No adhesive on the enamel (0%);
- 1 = Percentage of residual adhesive on the enamel between 1% and 10%;
- 2 = Percentage of residual adhesive on the enamel between 11% and 20%;
- 3 = Percentage of residual adhesive on the enamel > 20%.
2.8. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Schuler, F.S.; van Waes, H. SEM-evaluation of enamel surfaces after removal of fixed orthodontic appliances. Am. J. Dent. 2003, 16, 390–394. [Google Scholar] [PubMed]
- Rocha, R.S.; Salomão, F.M.; Silveira Machado, L.; Sundfeld, R.H.; Fagundes, T.C. Efficacy of auxiliary devices for removal of fluorescent residue after bracket debonding. Angle Orthod. 2017, 87, 440–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinzi, V.; Ferro, R.; Rizzo, F.A.; Marranzini, E.M.; Federici Canova, F.; Mummolo, S.; Mattei, A.; Marzo, G. The Two by Four appliance: A nationwide cross-sectional survey. Eur. J. Paediatr. Dent. 2018, 19, 145–150. [Google Scholar] [PubMed]
- Gracco, A.; Lattuca, M.; Marchionni, S.; Siciliani, G.; Alessandri Bonetti, G. SEM-Evaluation of enamel surfaces after orthodontic debonding: A 6 and 12-month follow-up in vivo study. Scanning 2015, 37, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Koch, J.; Hagan, J.L.; Ballard, R.W.; Armbruster, P.C. Enamel surface roughness of preferred debonding and polishing protocols. J. Orthod. 2016, 43, 39–46. [Google Scholar] [CrossRef]
- Campbell, P.M. Enamel surfaces after orthodontic bracket debonding. Angle Orthod. 1995, 65, 103–110. [Google Scholar]
- Janiszewska-Olszowska, J.; Szatkiewicz, T.; Tomkowski, R.; Tandecka, K.; Grocholewicz, K. Effect of orthodontic debonding and adhesive removal on the enamel–current knowledge and future perspectives—A systematic review. Med. Sci. Monit. 2014, 20, 1991–2001. [Google Scholar]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef]
- Howell, S.; Weekes, W.T. An electron microscopic evaluation of the enamel surface subsequent to various debonding procedures. Aust. Dent. J. 1990, 35, 245–252. [Google Scholar] [CrossRef]
- Bernardi, S.; Bianchi, S.; Botticelli, G.; Rastelli, E.; Tomei, A.R.; Palmerini, M.G.; Continenza, M.A.; Macchiarelli, G. Scanning electron microscopy and microbiological approaches for the evaluation of salivary microorganisms behaviour on anatase titanium surfaces: In vitro study. Morphologie 2018, 102, 1–6. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tripodi, D.; Marzo, G.; Bernardi, S.; Continenza, M.A.; Piattelli, A.; Iaculli, F.; Mummolo, S. Microleakage of bacteria in different implant-abutment assemblies: An in vitro study. J. Appl. Biomater. Funct. Mater. 2015, 13, e174–e180. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Bianchi, S.; Tomei, A.R.; Continenza, M.A.; Macchiarelli, G. Microbiological and SEM-EDS Evaluation of Titanium Surfaces Exposed to Periodontal Gel: In Vitro Study. Materials (Basel) 2019, 12, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar]
- Bianchi, S.; Bernardi, S.; Continenza, M.A.; Vincenti, E.; Antonouli, S.; Torge, D.; Macchiarelli, G. Scanning Electron Microscopy Approach for Evaluation of Hair Dyed with Lawsonia inermis Powder: In vitro Study. Int. J. Morph. 2020, 38, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Artun, J.; Bergland, S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am. J. Orthod. 1984, 85, 333–340. [Google Scholar] [CrossRef]
- Zanarini, M.; Gracco, A.; Lattuca, M.; Marchionni, S.; Gatto, M.R.; Bonetti, G.A. Bracket base remnants after orthodontic debonding. Angle Orthod. 2013, 83, 885–891. [Google Scholar] [CrossRef]
- Kitahara-Céia, F.M.; Mucha, J.N.; Marques dos Santos, P.A. Assessment of enamel damage after removal of ceramic brackets. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 548–555. [Google Scholar] [CrossRef]
- Brosh, T.; Strouthou, S.; Sarne, O. Effects of buccal versus lingual surfaces, enamel conditioning procedures and storage duration on brackets debonding characteristics. J. Dent. 2005, 33, 99–105. [Google Scholar] [CrossRef]
- Cury-Saramago Ade, A.; Coimbra, P.R.; Izquierdo Ade, M.; Elias, C.N.; Ruellas, A.C.; Sant’Anna, E.F. Ceramic surface polishing techniques after removal of orthodontic adhesive. Angle Orthod. 2009, 79, 790–795. [Google Scholar] [CrossRef]
- Ulusoy, Ç. Comparison of finishing and polishing systems for residual resin removal after debonding. J. Appl. Oral. Sci. 2009, 17, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Baumann, D.F.; Brauchli, L.; van Waes, H. The influence of dental loupes on the quality of adhesive removal in orthodontic debonding. J. Orofac. Orthop. 2011, 72, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Scarsella, S.; Di Fabio, D.; Oliva, A.; Di Girolamo, M.; Continenza, M.A.; Cutilli, T. Giant follicular cysts extended in pterygo-maxillary fossa, antro-naso-ethmoidal and orbital space associated to exophtalmos and diplopia in young patients. Oral. Maxillofac. Surg. Cases 2018, 4, 17–22. [Google Scholar] [CrossRef]
- Del Fante, Z.; De Matteis, A.; Fazio, V.; Di Fazio, N.; Quattrocchi, A.; Romano, S.; Arcangeli, M.; Dell’Aquila, M. The importance of post mortem computed tomography (PMCT) in the reconstruction of the bullet trajectory. Clin. Ter. 2019, 170, E129–E133. [Google Scholar] [PubMed]
- Cochrane, N.J.; Lo, T.W.G.; Adams, G.G.; Schneider, P.M. Quantitative analysis of enamel on debonded orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Alessandri Bonetti, G.; Zanarini, M.; Incerti Parenti, S.; Lattuca, M.; Marchionni, S.; Gatto, M.R. Evaluation of enamel surfaces after bracket debonding: An in-vivo study with scanning electron microscopy. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Øgaard, B.; Fjeld, M. The enamel surface and bonding in orthodontics. Semin. Orthod. 2010, 16, 37–48. [Google Scholar] [CrossRef]
- Fjeld, M.; Øgaard, B. Scanning electron microscopic evaluation of enamel surfaces exposed to 3 orthodontic bonding systems. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Sigilião, L.C.; Marquezan, M.; Elias, C.N.; Ruellas, A.C.; Sant’Anna, E.F. Efficiency of different protocols for enamel clean-up after bracket debonding: An in vitro study. Dental. Press. J. Orthod. 2015, 20, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Pont, H.B.; Özcan, M.; Bagis, B.; Ren, Y. Loss of surface enamel after bracket debonding: An in-vivo and ex-vivo evaluation. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 387.e1–387.e9. [Google Scholar] [CrossRef]
- Grassia, V.; Gentile, E.; Di Stasio, D.; Jamilian, A.; Matarese, G.; D’Apuzzo, F.; Santoro, R.; Perillo, L.; Serpico, R.; Lucchese, A. In vivo confocal microscopy analysis of enamel defects after orthodontic treatments: A preliminary study. Ultrastruct. Pathol. 2016, 40, 317–323. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.A.; Arenas-Alatorre, J.; Belio-Reyes, I.A. Comparative study of dental enamel loss after debonding braces by analytical scanning electron microscopy (SEM). Microsc. Res. Tech. 2017, 80, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Silikas, N.; Lennie, A.R.; England, K.; Watts, D.C. AFM as a tool in Dental Research. Microsc. Anal. 2001, 19–21. [Google Scholar]
- D’Amario, M.; D’Attilio, M.; Baldi, M.; De Angelis, F.; Marzo, G.; Vadini, M.; Varvara, G.; D’Arcangelo, C. Histomorphologic alterations of human enamel after repeated applications of a bleaching agent. Int. J. Immunopathol. Pharmacol. 2012, 25, 1021–1027. [Google Scholar] [CrossRef]
- Vyas, N.; Sammons, R.L.; Addison, O.; Dehghani, H.; Walmsley, A.D. A quantitative method to measure biofilm removal efficiency from complex biomaterial surfaces using SEM and image analysis. Sci. Rep. 2016, 6, 32694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Bijelić, N.; Belovari, T.; Stolnik, D.; Lovrić, I.; Baus Lončar, M. Histomorphometric parameters of the growth plate and trabecular bone in wild-type and trefoil factor family 3 (Tff3)-deficient mice analyzed by free and open-source image processing software. Microsc. Microanal. 2017, 23, 818–825. [Google Scholar] [CrossRef]
- Mohan, A.; Poobal, S. Crack detection using image processing: A critical review and analysis. Alex. Eng. J. 2018, 57, 787–798. [Google Scholar] [CrossRef]



| Naked Eye | Magnification | ||||||
|---|---|---|---|---|---|---|---|
| BLD | EP | RU | BLD | EP | RU | ||
| EP | 1.00 | EP | 1.00 | ||||
| RU | 0.82 | 1.00 | RU | 0.84 | 1.00 | ||
| SO | 0.40 | 1.00 | 0.82 | SO | 0.59 | 0.35 | 0.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amario, M.; Bernardi, S.; Di Lauro, D.; Marzo, G.; Macchiarelli, G.; Capogreco, M. Debonding and Clean-Up in Orthodontics: Evaluation of Different Techniques and Micro-Morphological Aspects of the Enamel Surface. Dent. J. 2020, 8, 58. https://doi.org/10.3390/dj8020058
D’Amario M, Bernardi S, Di Lauro D, Marzo G, Macchiarelli G, Capogreco M. Debonding and Clean-Up in Orthodontics: Evaluation of Different Techniques and Micro-Morphological Aspects of the Enamel Surface. Dentistry Journal. 2020; 8(2):58. https://doi.org/10.3390/dj8020058
Chicago/Turabian StyleD’Amario, Maurizio, Sara Bernardi, Daniele Di Lauro, Giuseppe Marzo, Guido Macchiarelli, and Mario Capogreco. 2020. "Debonding and Clean-Up in Orthodontics: Evaluation of Different Techniques and Micro-Morphological Aspects of the Enamel Surface" Dentistry Journal 8, no. 2: 58. https://doi.org/10.3390/dj8020058
APA StyleD’Amario, M., Bernardi, S., Di Lauro, D., Marzo, G., Macchiarelli, G., & Capogreco, M. (2020). Debonding and Clean-Up in Orthodontics: Evaluation of Different Techniques and Micro-Morphological Aspects of the Enamel Surface. Dentistry Journal, 8(2), 58. https://doi.org/10.3390/dj8020058







