Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Sample Collection
2.3. DNA Isolation and Analysis
2.4. qPCR Screening
2.5. Scardovia Wiggsiae (SW)
2.6. Statistical Analysis
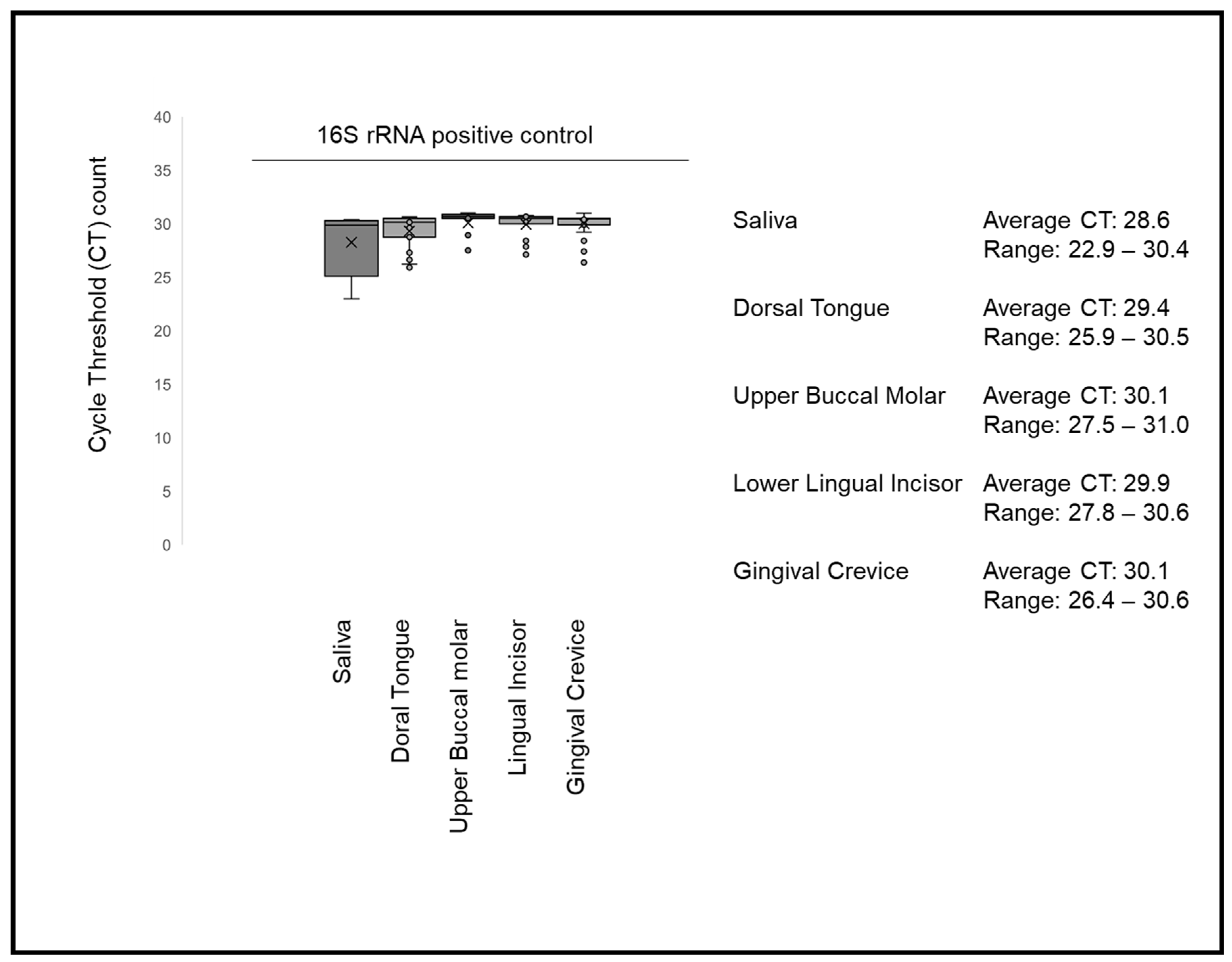
3. Results
| Key: | |
| Strength of Correlation (R) | Correlation Coefficient Range |
| Negligible/Very Weak | 0.0–0.19 or −0.19–0.0 |
| Weak | 0.20–0.39 or −0.39–−0.20 |
| Moderate | 0.40–0.59 or −0.40–−0.59 |
| Strong | 0.60–0.79 or −0.79–−0.60 |
| Very Strong | 0.80–1.0 or −1.0–−0.80 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matondkar, S.P.; Yavagal, C.; Kugaji, M.; Bhat, K.G. Quantitative assessment of Scardovia wiggsiae from dental plaque samples of children suffering from severe early childhood caries and caries free children. Anaerobe 2020, 62, 102110. [Google Scholar] [CrossRef]
- Fakhruddin, K.S.; Ngo, H.C.; Samaranayake, L.P. Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Dis. 2018, 25, 982–995. [Google Scholar] [CrossRef]
- Downes, J.; Mantzourani, M.; Beighton, D.; Hooper, S.; Wilson, M.J.; Nicholson, A.; Wade, W.G. Scardovia wiggsiae sp. nov., isolated from the human oral cavity and clinical material, and emended descriptions of the genus Scardovia and Scardovia inopinata. Int. J. Syst. Evol. Microbiol. 2011, 61, 25–29. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Mathney, J.M.J.; Kent, R.L.; Chalmers, N.I.; Hughes, C.V.; Loo, C.Y.; Pradhan, N.; Kanasi, E.; Hwang, J.; Dahlan, M.A.; et al. Cultivable Anaerobic Microbiota of Severe Early Childhood Caries. J. Clin. Microbiol. 2011, 49, 1464–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henne, K.; Gunesch, A.-P.; Walther, C.; Meyer-Lueckel, H.; Conrads, G.; Esteves-Oliveira, M. Analysis of Bacterial Activity in Sound and Cariogenic Biofilm: A Pilot in vivo Study. Caries Res. 2016, 50, 480–488. [Google Scholar] [CrossRef]
- Chandna, P.; Srivastava, N.; Sharma, A.; Sharma, V.; Gupta, N.; Adlakha, V.K. Isolation of Scardovia wiggsiae using real-time polymerase chain reaction from the saliva of children with early childhood caries. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 290. [Google Scholar] [CrossRef]
- Hurley, E.; Barrett, M.P.J.; Kinirons, M.; Whelton, H.; Ryan, C.A.; Stanton, C.; Harris, H.M.B.; O’Toole, P.W. Comparison of the salivary and dentinal microbiome of children with severe-early childhood caries to the salivary microbiome of caries-free children. BMC Oral Health 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemadi, A.S.; Huang, R.; Zhou, Y.; Zou, J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral Sci. 2017, 9, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameda, M.; Abiko, Y.; Washio, J.; Tanner, A.C.R.; Kressirer, C.A.; Mizoguchi, I.; Takahashi, N. Sugar Metabolism of Scardovia wiggsiae, a Novel Caries-Associated Bacterium. Front. Microbiol. 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Manome, A.; Abiko, Y.; Kawashima, J.; Washio, J.; Fukumoto, S.; Takahashi, N. Acidogenic Potential of Oral Bifidobacterium and Its High Fluoride Tolerance. Front. Microbiol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Kressirer, C.A.; Smith, D.J.; King, W.F.; Dobeck, J.M.; Starr, J.R.; Tanner, A.C. Scardovia wiggsiae and its potential role as a caries pathogen. J. Oral Biosci. 2017, 59, 135–141. [Google Scholar] [CrossRef]
- Bossù, M.; Selan, L.; Artini, M.; Relucenti, M.; Familiari, G.; Papa, R.; Vrenna, G.; Spigaglia, P.; Barbanti, F.; Salucci, A.; et al. Characterization of Scardovia wiggsiae Biofilm by Original Scanning Electron Microscopy Protocol. Microorganisms 2020, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Damé-Teixeira, N.; Parolo, C.C.F.; Malz, M.; Devine, D.A.; Do, T. Gene expression profile of Scardovia spp. in the metatranscriptome of root caries. Braz. Oral Res. 2020, 34, e042. [Google Scholar] [CrossRef] [PubMed]
- Baraniya, D.; Chen, T.; Nahar, A.; Alakwaa, F.; Hill, J.; Tellez, M.; Ismail, A.; Puri, S.; Al-Hebshi, N.N. Supragingival mycobiome and inter-kingdom interactions in dental caries. J. Oral Microbiol. 2020, 12, 1729305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, O.; Major, K.; Santoyo, S.; Kingsley, K.; Nguyen, L. qPCR Screening of Pediatric Saliva Samples to Evaluate Effects of Dental Sealants on Cariogenic Bacteria Streptococcus mutans and Scardovia wiggsiae. Environ. Dent. J. 2021, 2. [Google Scholar]
- Quan, K.; Kingsley, K. Effect of Dental Sealants on Oral Microbial Burden of Scardovia wiggsiae within a Pediatric Population: A Pilot Study. Microbiol. Res. J. Int. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Eriksson, L.; Holgerson, P.L.; Esberg, A.; Johansson, I. Microbial Complexes and Caries in 17-Year-Olds with and without Streptococcus mutans. J. Dent. Res. 2017, 97, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Holgerson, P.L.; Johansson, I. Saliva and tooth biofilm bacterial microbiota in adolescents in a low caries community. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Row, L.; Repp, M.R.; Kingsley, K. Screening of a Pediatric and Adult Clinic Population for Caries Pathogen Scardovia wiggsiae. J. Clin. Pediatric Dent. 2016, 40, 438–444. [Google Scholar] [CrossRef]
- McDaniel, S.; McDaniel, J.; Tam, A.; Kingsley, K.; Howard, K.M. Oral Microbial Ecology of Selenomonas noxia and Scardovia wiggsiae. Microbiol. Res. J. Int. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Sonis, A.; Holgerson, P.L.; Starr, J.; Núñez, Y.; Kressirer, C.A.; Paster, B.J.; Johansson, I. White-spot lesions and gingivitis microbiotas in orthodontic patients. J. Dent. Res. 2012, 91, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torlakovic, L.; Klepac-Ceraj, V.; Øgaard, B.; Cotton, S.L.; Paster, B.J.; Olsen, I. Microbial community succession on developing lesions on human enamel. J. Oral Microbiol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Milne, W.; Rezaei, G.; Whiteley, A.; Kingsley, K. Cariogenic pathogen Scardovia wiggsiae screening among pediatric orthodontic patients: A pilot Study. Curr. Res. Dent. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Reyes, N.; Pollock, A.; Whiteley, A.; Kingsley, K.; Howard, K.M. Prevalence of Scardovia wiggsiae among a pediatric Orthodontic patient population. EC Dental Sci. 2017, 13, 203–210. [Google Scholar]
- Whiteley, A.; Kingsley, K. Scardovia wiggsiae prevalence among adult and pediatric orthodontic and non-orthodontic patient populations. J. Med. Disc. 2017, 2, jmd17034. [Google Scholar] [CrossRef]
- Carr, G.; Alexander, A.; Nguyen, L.; Kingsley, K. Oral Site Specific Sampling Reveals Differential Location for Scardovia wiggsiae. Microbiol. Res. J. Int. 2020, 30, 47–55. [Google Scholar] [CrossRef]
- Emett, J.; David, R.; McDaniel, J.; McDaniel, S.; Kingsley, K. Comparison of DNA Extracted from Pediatric Saliva, Gingival Crevicular Fluid and Site-Specific Biofilm Samples. Methods Protoc. 2020, 3, 48. [Google Scholar] [CrossRef]
- Steigmann, L.; Maekawa, S.; Sima, C.; Travan, S.; Wang, C.-W.; Giannobile, W.V. Biosensor and Lab-on-a-chip Biomarker-identifying Technologies for Oral and Periodontal Diseases. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Vieira, A.R.; Hiller, N.L.; Powell, E.; Kim, L.H.; Spirk, T.; Modesto, A.; Kreft, R. Profiling microorganisms in whole saliva of children with and without dental caries. Clin. Exp. Dent. Res. 2019, 5, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Belstrøm, D.; Constancias, F.; Liu, Y.; Yang, L.; Drautz-Moses, D.I.; Schuster, S.C.; Kohli, G.S.; Jakobsen, T.H.; Holm, J.T.; Givskov, M. Metagenomic and metatranscriptomic analysis of saliva reveals disease-associated microbiota in patients with periodontitis and dental caries. NPJ Biofilms Microbiomes 2017, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Papagerakis, P.; Zheng, L.; Kim, D.; Said, R.; Ehlert, A.A.; Chung, K.K.M.; Papagerakis, S. Saliva and Gingival Crevicular Fluid (GCF) Collection for Biomarker Screening. Methods Mol. Biol. 2019, 1922, 549–562. [Google Scholar] [CrossRef]
- Ghallab, N.A. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: Review of the current evidence. Arch Oral Biol. 2018, 87, 115–124. [Google Scholar] [CrossRef]
- Bibi, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208. [Google Scholar] [CrossRef]
- Eggers, S.; Mc Malecki, K.; Peppard, P.; Mares, J.; Shirley, D.; Shukla, S.K.; Poulsen, K.; Gangnon, R.; Duster, M.; Kates, A.; et al. Wisconsin microbiome study, a cross-sectional investigation of dietary fibre, microbiome composition and antibiotic-resistant organisms: Rationale and methods. BMJ Open 2018, 8, e019450. [Google Scholar] [CrossRef] [PubMed]
- Chervinets, V.M.; Chervinets, Y.V.; Leont’Eva, A.V.; Kozlova, E.A.; Stulov, N.M.; Belyaev, V.S.; Grigoryants, E.O.; Mironov, A.Y. The microbiome of oral cavity patients with periodontitis, adhesive and biofilm forming properties. Klin. Lab. Diagn. 2021, 66, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wilbert, S.A.; Welch, J.L.M.; Borisy, G.G. Spatial Ecology of the Human Tongue Dorsum Microbiome. Cell Rep. 2020, 30, 4003–4015.e3. [Google Scholar] [CrossRef] [PubMed]
- Rabe, A.; Salazar, M.G.; Michalik, S.; Fuchs, S.; Welk, A.; Kocher, T.; Völker, U. Metaproteomics analysis of microbial diversity of human saliva and tongue dorsum in young healthy individuals. J. Oral Microbiol. 2019, 11, 1654786. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.; Torwane, N.A.; Tyagi, S.; Maran, S. Effectiveness of Various Tongue Cleaning Aids in the Reduction of Tongue Coating and Bacterial Load: A Comparative Clinical Study. J. Contemp. Dent. Pract. 2019, 20, 444–448. [Google Scholar] [PubMed]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.W.; Singh, N.; Ng, K.F.; Lam, D.K.; Goldberg, M.B.; Tenenbaum, H.C.; Neufeld, J.D.; Beiko, R.G.; Senadheera, D.B. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microbiomes 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Hoare, A.; Hong, B.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I. Microbial Diversity and Interactions in Subgingival Biofilm Communities. Front. Oral Biol. 2011, 15, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zuniga, C.; Zengler, K. Unraveling interactions in microbial communities-from co-cultures to microbiomes. J. Microbiol. 2015, 53, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Hoare, A.; Hong, B.-Y. Subgingival Microbiome Shifts and Community Dynamics in Periodontal Diseases. J. Calif. Dent. Assoc. 2016, 44, 27514154. [Google Scholar]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Fragkioudakis, I.; Riggio, M.P.; Apatzidou, D.A. Understanding the microbial components of periodontal diseases and periodontal treatment-induced microbiological shifts. J. Med. Microbiol. 2021, 70, 001247. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Magalhães, C.B.; Hartenbach, F.A.R.R.; Souto, R.M.D.; da Silva-Boghossian, C.M. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef]
- Tripepi, G.; Jager, K.J.; Dekker, F.W.; Zoccali, C. Selection Bias and Information Bias in Clinical Research. Nephron Clin. Pract. 2010, 115, c94–c99. [Google Scholar] [CrossRef]
- Wieringa, S.; Engebretsen, E.; Heggen, K.; Greenhalgh, T. Rethinking bias and truth in evidence-based health care. J. Eval. Clin. Pr. 2018, 24, 930–938. [Google Scholar] [CrossRef]
- Tripathi, R.; Khatri, N.; Mamde, A. Sample Size and Sampling Considerations in Published Clinical Research Articles. J. Assoc. Physicians India 2020, 68, 14–18. [Google Scholar] [PubMed]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E., Jr.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study Sample | Clinic Population | Statistical Analysis | |
|---|---|---|---|
| Sex | |||
| Females | n = 22/60 (36.7%) | 49.1% | Χ2 = 5.762, d.f. = 1 |
| Males | n = 38/60 (63.3%) | 50.9% | p = 0.0164 |
| Race/Ethnicity | |||
| White | n = 8/60 (13.3%) | 34.6% | Χ2 = 21.275, d.f. = 1 |
| Minority | n = 52/60 (86.7%) | 65.4% | p = 0.00012 |
| Hispanic | n = 40/60 (66.7%) | 58.6% | |
| Black | n = 8/60 (13.3%) | 10.2% | |
| Asian | n = 40/60 (6.7%) | 6.6% | |
| Age | |||
| Average age | 11.01 years | 10.41 years | p = 0.441 |
| Range | 5–17 years | 0–17 years |
| DNA Concentration (Average ± Standard Error or SE) | DNA Purity A260:A280 | Statistical Analysis | |
|---|---|---|---|
| Gingival Crevice | 14.69 μg/μL ± 1.24 SE | 1.701 ± 0.21 | p = 0.722 |
| Upper Buccal Molar | 14.34 μg/μL ± 1.17 SE | 1.691 ± 0.24 | p = 0.887 |
| Lower Lingual Incisor | 14.40 μg/μL ± 1.16 SE | 1.672 ± 0.27 | p = 0.772 |
| Dorsal Tongue | 14.34 μg/μL ± 1.05 SE | 1.637 ± 0.31 | p = 0.911 |
| Saliva | 13.74 μg/μL ± 1.01 SE | 1.618 ± 0.28 | p = 0.643 |
| Average | 14.30 μg/μL ± 1.12 SE | 1.664 ± 0.26 |
| Age | Sex | BMI | Dentition | Brackets | |
|---|---|---|---|---|---|
| Gingival Crevice | R = −0.1945 | R = 0.0611 | R = 0.0950 | R = 0.2444 | R = −0.1437 |
| Upper Buccal Molar | R = −0.0810 | R = −0.0024 | R = −0.0598 | R = −0.0010 | R = −0.0848 |
| Lower Lingual Incisor | R = −0.0246 | R = −0.1057 | R = −0.0278 | R = −0.1046 | R = −0.0509 |
| Dorsal Tongue | R = −0.1604 | R = 0.1211 | R = 0.0833 | R = 0.1550 | R = −0.0667 |
| Saliva | R = 0.2228 | R = 0.0399 | R = −0.1701 | R = −0.1139 | R = 0.2118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDaniel, S.; McDaniel, J.; Howard, K.M.; Kingsley, K. Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae. Dent. J. 2021, 9, 73. https://doi.org/10.3390/dj9060073
McDaniel S, McDaniel J, Howard KM, Kingsley K. Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae. Dentistry Journal. 2021; 9(6):73. https://doi.org/10.3390/dj9060073
Chicago/Turabian StyleMcDaniel, Steven, Jaydene McDaniel, Katherine M. Howard, and Karl Kingsley. 2021. "Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae" Dentistry Journal 9, no. 6: 73. https://doi.org/10.3390/dj9060073
APA StyleMcDaniel, S., McDaniel, J., Howard, K. M., & Kingsley, K. (2021). Molecular Screening and Analysis Reveal Novel Oral Site-Specific Locations for the Cariogenic Pathogen Scardovia wiggsiae. Dentistry Journal, 9(6), 73. https://doi.org/10.3390/dj9060073






