Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Rheological Behaviors
2.4. Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results and Discussion
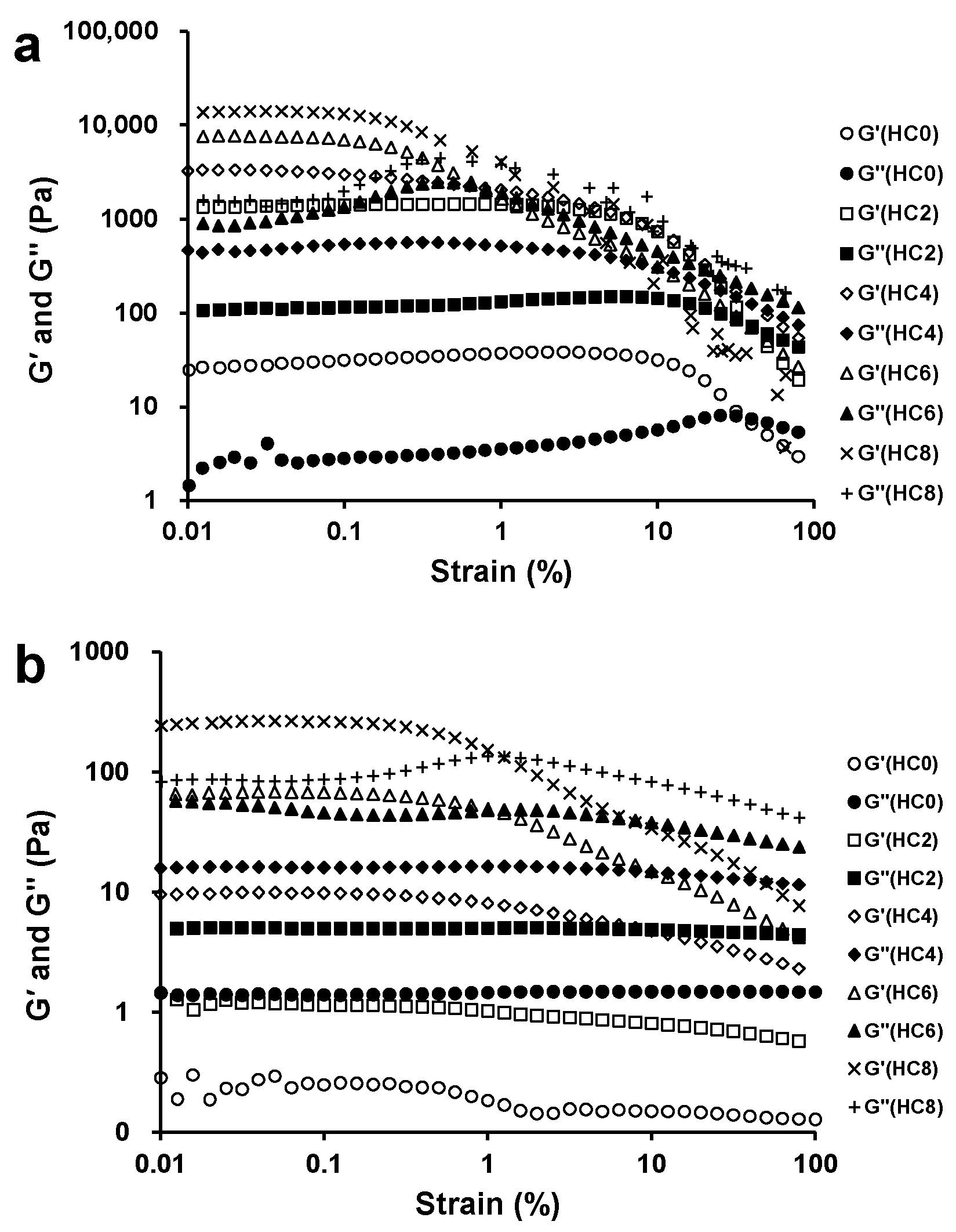
3.1. The Effect of CL on the Linear Viscoelastic Region of the HPMC System
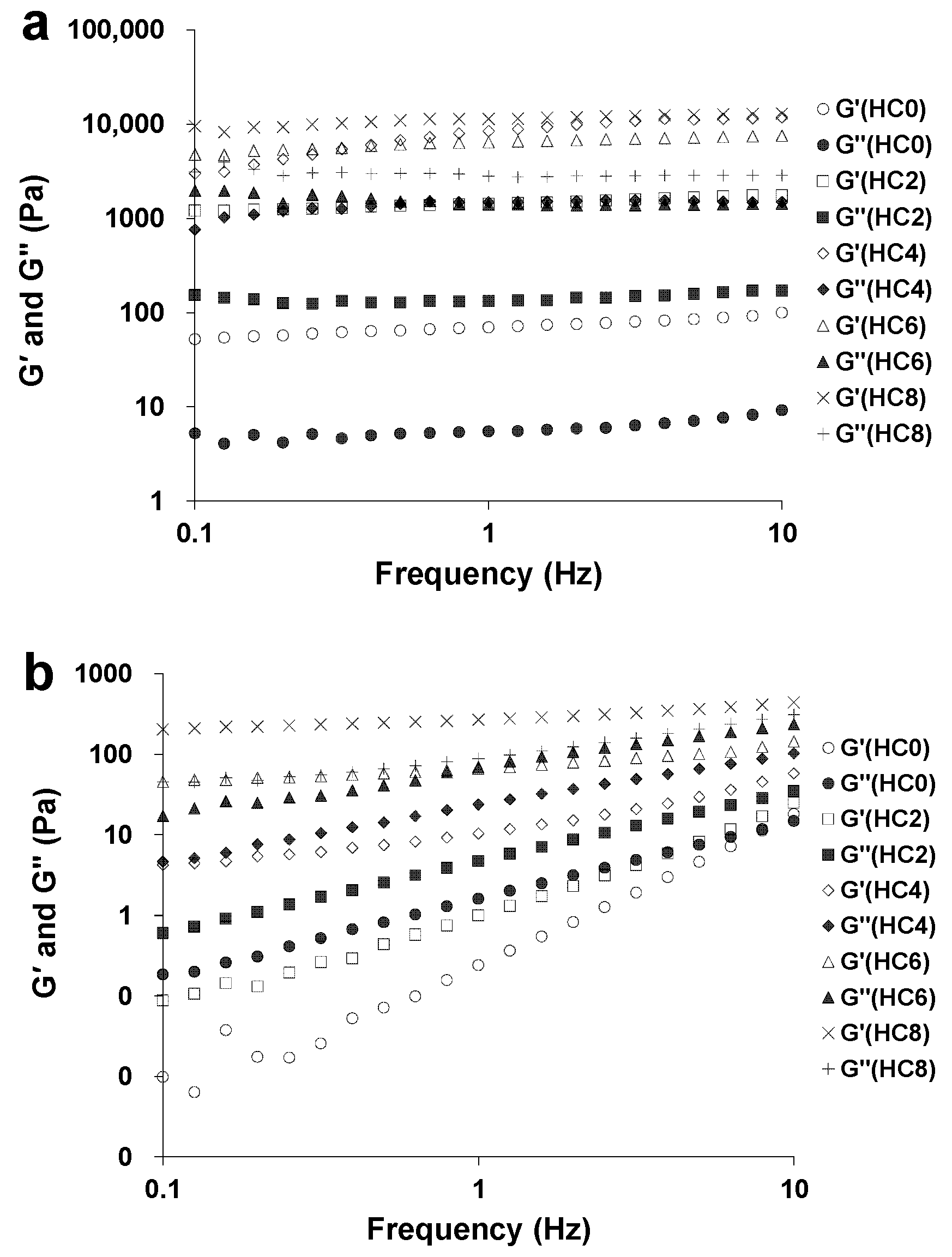
3.2. The Effect of CL on the Extent of Gel-Like Behavior of the HPMC System
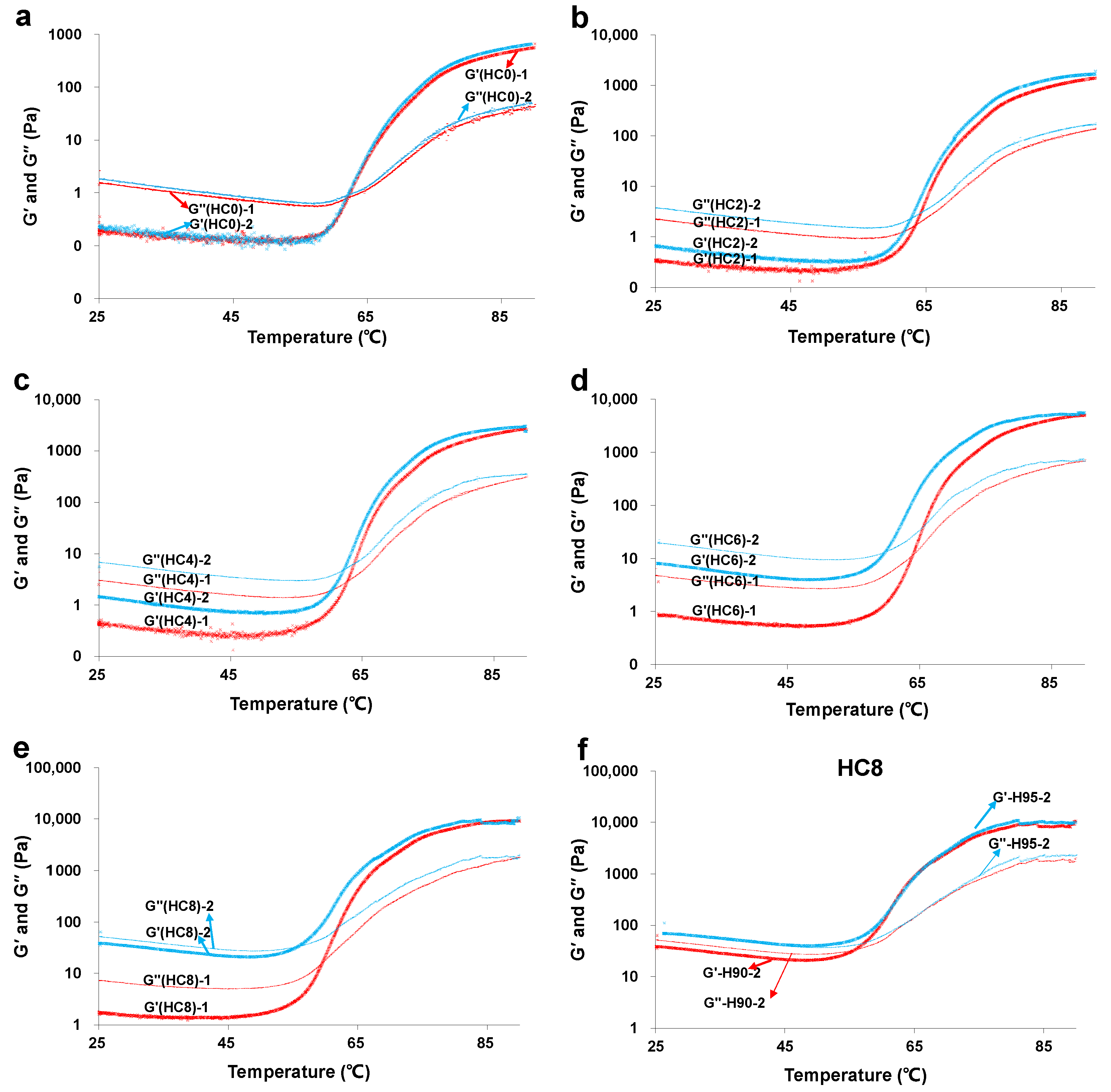
3.3. The Effect of CL on the Sol-Gel Transition of HPMC Solution
3.3.1. The Effect of CL on the Sol-Gel Transition of HPMC Solution for the First Time
3.3.2. The Effect of CL on the Repeated Sol-Gel Transition of HPMC Solution
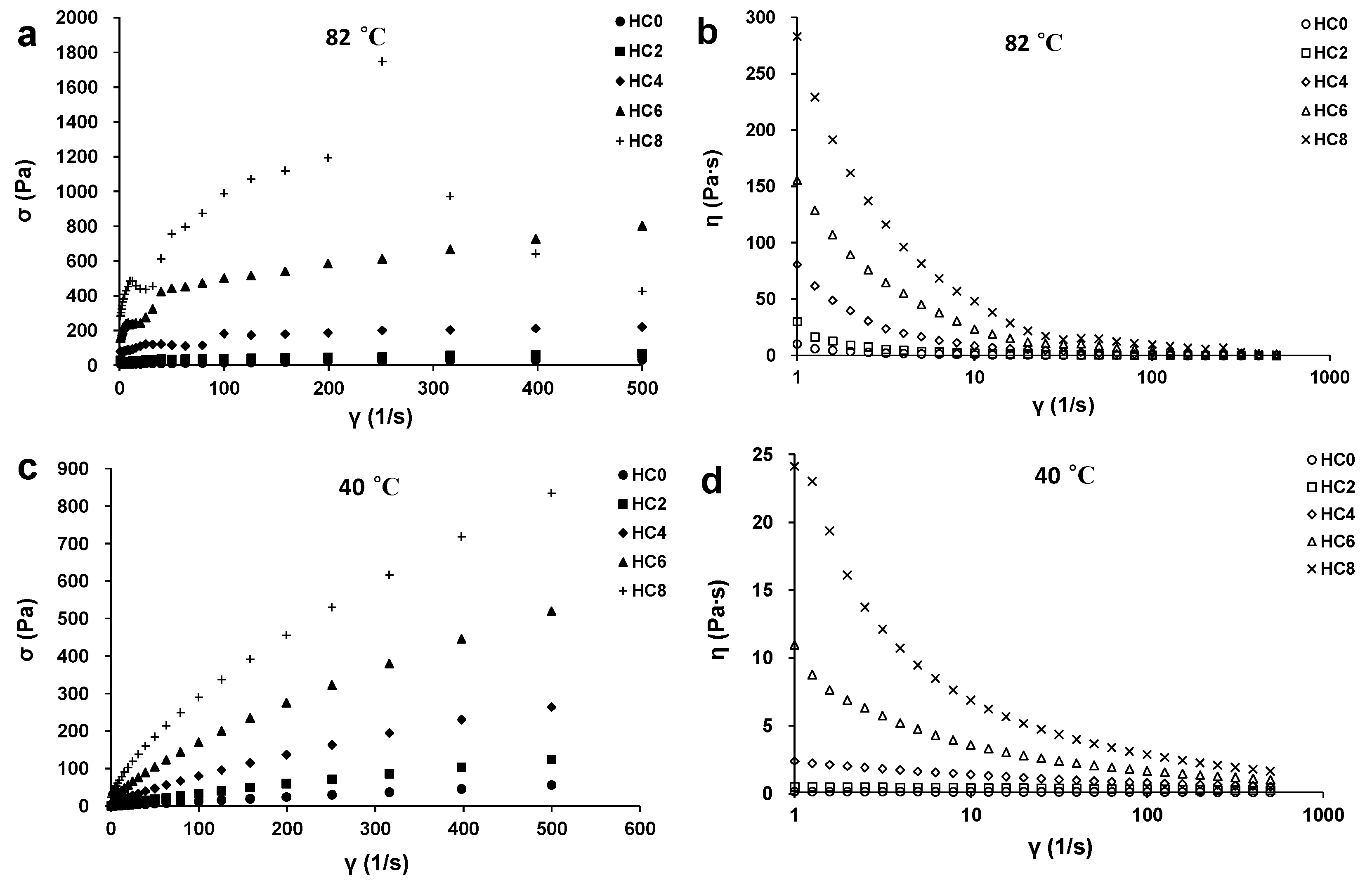
3.4. The Effect of CL on the Viscosity of the HPMC System
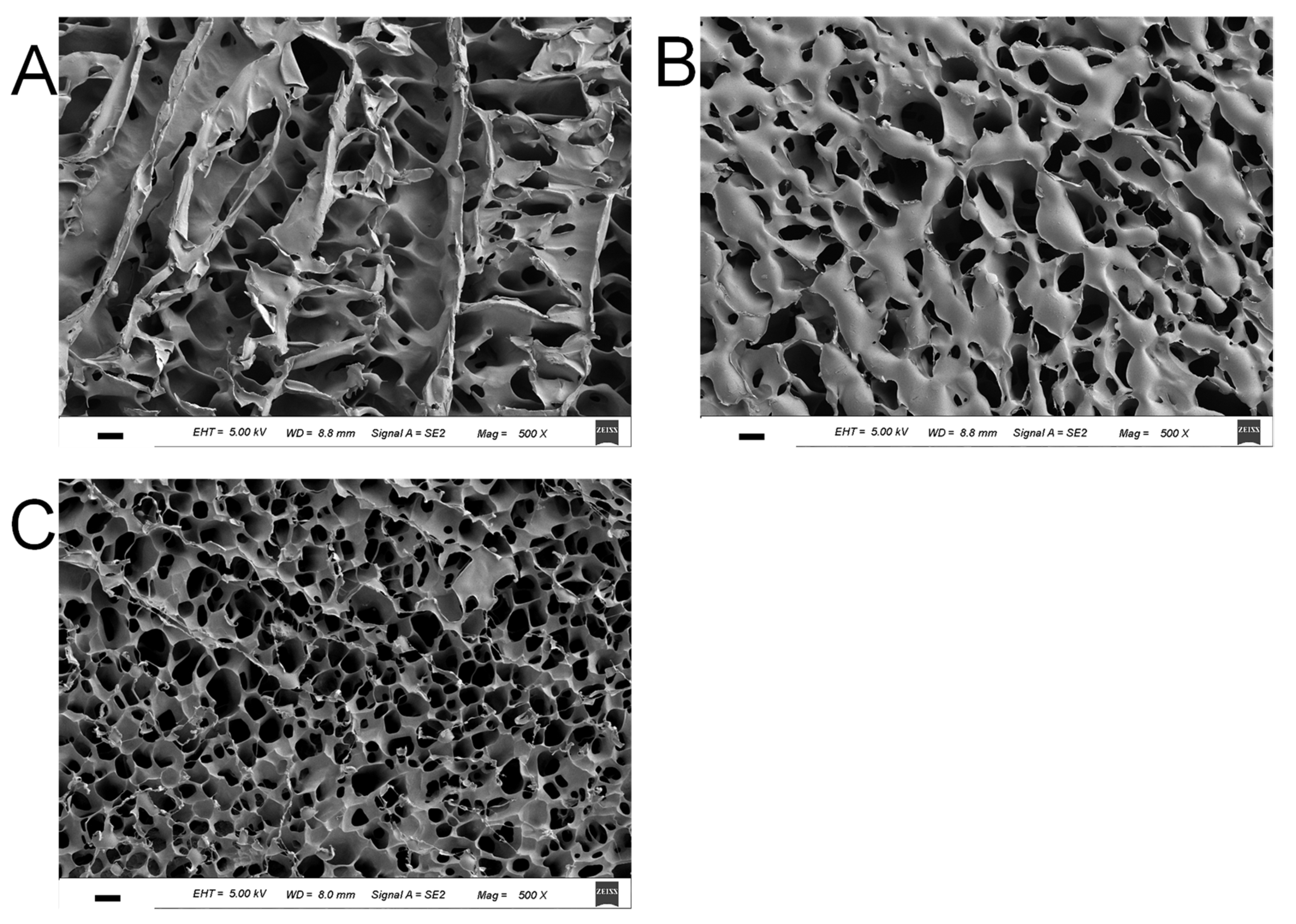
3.5. Morphology of HPMC/CL Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maki, K.C.; Davidson, M.H.; Malik, K.C.; Albrecht, H.H.; O’Mullane, J.; Daggy, B.P. Cholesterol lowering with high-viscosity hydroxypropyl methylcellulose. Am. J. Cardiol. 1999, 84, 1198–1203. [Google Scholar] [CrossRef]
- Chirico, S.; Dalmoro, A.; Lamberti, G.; Russo, G.; Titomanlio, G. Analysis and modeling of swelling and erosion behavior for pure HPMC tablet. J. Control. Release 2007, 122, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, L.; Liu, H.S.; Wang, Y.F.; Simon, G.P.; Ji, Z.L.; Qian, J. Effect of processing conditions on microstructures and properties of hydroxypropyl methylcellulose/hydroxypropyl starch blends. Food Hydrocoll. 2017, 70, 251–259. [Google Scholar] [CrossRef]
- Pourchez, J.; Peschard, A.; Grosseau, P.; Guyonnet, R.; Guilhot, B.; Vallée, F. HPMC and HEMC influence on cement hydration. Cement Concrete Res. 2006, 36, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Q.; Joshi, S.C.; Lam, Y.C.; Tam, K.C. Thermoreversible gelation of hydroxypropyl methylcellulose in simulated body fluids. Carbohyd. Polym. 2008, 72, 133–143. [Google Scholar] [CrossRef]
- Dhillon, S.; Seetharaman, K. Rheology and texture of starch gels containing iodine. J. Cereal Sci. 2011, 54, 374–379. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Um, I.C. Examination of thermo-gelation behavior of HPMC and HEMC aqueous solutions using rheology. Korea-Aust. Rheol. J. 2013, 25, 67–75. [Google Scholar] [CrossRef]
- Clasen, C.; Kulicke, W.M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919. [Google Scholar] [CrossRef]
- Lodge, T.P.; Maxwell, A.L.; Lott, J.R.; Schmidt, P.W.; McAllister, J.W.; Morozova, S.; Bates, F.S.; Li, Y.; Sammler, R.L. Gelation, phase separation, and fibril formation in aqueous hydroxypropylmethylcellulose solutions. Biomacromolecules 2018, 19, 816–824. [Google Scholar] [CrossRef]
- Talukdar, M.M.; Vinckier, I.; Moldenaers, P.; Kinget, R. Rheological characterization of xanthan gum and hydroxypropylmethyl cellulose with respect to controlled-release drug delivery. J. Pharm. Sci. 1996, 85, 537–540. [Google Scholar] [CrossRef]
- Chen, H.H.; Lin, C.H.; Kang, H.Y. Maturation effects in fish gelatin and HPMC composite gels. Food Hydrocoll. 2009, 23, 1756–1761. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.F.; Yu, L.; Liu, H.S.; Simon, G.; Zhang, N.; Chen, L. Rheological and gel properties of hydroxypropyl methylcellulose/hydroxypropyl starch blends. Colloid Polym. Sci. 2015, 293, 229–237. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Rheological properties of collagen/hydroxypropyl methylcellulose (COL/HPMC) blended solutions. J. Appl. Polym. Sci. 2014, 131, 40042. [Google Scholar] [CrossRef]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1→3)-β-D-glucans. Appl. Microbiol. Biot. 2005, 68, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Funami, M.; Yada, H.; Nakao, Y. Rheological and thermal studies on gelling characteristics of curdlan. Food Hydrocoll. 1999, 13, 317–324. [Google Scholar] [CrossRef]
- Wong, S.S.; Ngiam, Z.R.J.; Kasapis, S.; Huang, D.J. Novel sulfation of curdlan assisted by ultrasonication. Int. J. Biol. Macromol. 2010, 46, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Matsumoto, T.; Masuda, T. Dynamic viscoelasticity and small angle X-ray scattering studies on the gelation mechanism and network structure of curdlan gels. Carbohyd. Polym. 1999, 39, 53–59. [Google Scholar] [CrossRef]
- Zhang, H.; Nishinari, K.; Williams, M.A.K.; Foster, T.J.; Norton, I.T. A molecular description of the gelation mechanism of curdlan. Int. J. Biol. Macromol. 2002, 30, 7–16. [Google Scholar] [CrossRef]
- Nakao, Y.; Konno, A.; Taguchi, T.; Tawada, T.; Kasai, H.; Toda, J.; Terasaki, M. Curdlan: Properties and application to foods. J. Food Sci. 1991, 56, 769–772. [Google Scholar] [CrossRef]
- Chen, H.H.; Huang, Y.C. Rheological properties of HPMC enhanced surimi analyzed by small-and large-strain tests—Ⅱ: Effect of water content and ingredients. Food Hydrocoll. 2008, 22, 313–332. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, C.; Chen, S.; Liu, D.; Ye, X.; Hu, Y. The effect of curdlan on the rheological properties of restructured ribbonfish (Trichiurus spp.) meat gel. Food Chem. 2015, 179, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lim, S.T.; Chung, H.J. Physical modification of potato starch using mild heating and freezing with minor addition of gums. Food Hydrocoll. 2019, 94, 294–303. [Google Scholar] [CrossRef]
- Ferry, J.D.; Rice, S.A. Viscoelastic properties of polymers. Phys. Today 1980, 15, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Doi, M.; Takimoto, J.I. Molecular modelling of entanglement. Philos. Trans. R. Soc. A 2003, 361, 641–652. [Google Scholar] [CrossRef]
- Clark, A.H.; Goss-Murphy, S.B. Structure and mechanical properties of biopolymer gels. Adv. Polym. Sci. 1987, 83, 57–192. [Google Scholar]
- Lopes da Silva, J.A.; Rao, M.A. Rheological behavior of food gel system. In Rheology of Fluid and Semisolid Foods; Rao, M.A., Ed.; Aspen Publication: Gaithersburg, MD, USA, 1999; pp. 219–318. [Google Scholar]
- Musampa, R.M.; Alvesb, M.M.; Maiaa, J.M. Phase separation, rheology and microstructure of pea protein–κ-carrageenan mixtures. Food Hydrocoll. 2007, 21, 92–99. [Google Scholar] [CrossRef]
- Chen, H.H. Rheological properties of HPMC enhanced surimi analyzed by small-and large-strain tests: I. The effect of concentration and temperature on HPMC flow properties. Food Hydrocoll. 2007, 21, 1201–1208. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J.; Páez-Flor, N.M.; Velázquez-Navarro, J.F. Why monotonous and non-monotonous steady-flow curves can be obtained with the same non-Newtonian fluid? A single explanation. Rheol. Acta 2018, 57, 389–396. [Google Scholar] [CrossRef]
| HC0 | HC2 | HC4 | HC6 | HC8 | |
|---|---|---|---|---|---|
| T1 (°C) | 63.0 ± 1.2 a | 63.5 ± 0.2 a | 63.0 ± 0.5 a | 62.1 ± 0.9 a | 60.2 ± 1.0 b |
| T2 (°C) | 63.3 ± 1.1 a | 62.9 ± 0.3 a | 62.3 ± 0.3 a | 60.5 ± 1.0 b | 56.5 ± 0.5 c |
| n | K | σy | r2 | ||
|---|---|---|---|---|---|
| 82 °C | HC0 | 0.65 ± 0.14 a | 0.41 ± 0.051 b | 6.9 ± 6.6 b | 0.9819 ± 0.0094 |
| HC2 | 0.50 ± 0.15 ab | 3.0 ± 2.9 b | 16 ± 4.0 b | 0.9240 ± 0.0527 | |
| HC4 | 0.38 ± 0.073 b | 18 ± 9.7 b | 53 ± 23 a | 0.8707 ± 0.0493 | |
| HC6 | 0.36 ± 0.056 b | 86 ± 44 a | 67 ± 15 a | 0.9625 ± 0.0358 | |
| HC8 | -- | -- | -- | -- | |
| 40 °C | HC0 | 0.95 ± 0.010 a | 0.16 ± 0.017 e | -- | 0.9998 ± 0.0001 |
| HC2 | 0.89 ± 0.053 b | 0.56 ± 0.18 d | -- | 0.9991 ± 0.0005 | |
| HC4 | 0.76 ± 0.027 c | 2.4 ± 0.13 c | -- | 0.9997 ± 0.0002 | |
| HC6 | 0.65 ± 0.0080 d | 8.7 ± 0.20 b | -- | 0.9971 ± 0.0003 | |
| HC8 | 0.58 ± 0.0030 e | 20 ± 0.32 a | -- | 0.9915 ± 0.0033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yue, L.-N.; Qian, J.-Y.; Ding, X.-L. Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose. Foods 2021, 10, 34. https://doi.org/10.3390/foods10010034
Zhang L, Yue L-N, Qian J-Y, Ding X-L. Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose. Foods. 2021; 10(1):34. https://doi.org/10.3390/foods10010034
Chicago/Turabian StyleZhang, Liang, Li-Na Yue, Jian-Ya Qian, and Xiang-Li Ding. 2021. "Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose" Foods 10, no. 1: 34. https://doi.org/10.3390/foods10010034
APA StyleZhang, L., Yue, L.-N., Qian, J.-Y., & Ding, X.-L. (2021). Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose. Foods, 10(1), 34. https://doi.org/10.3390/foods10010034






