1. Introduction
Seaweed cultivation offers potential solutions to environmental challenges, such as eutrophication, by improving water quality [
1]. Seaweeds have a higher production rate than terrestrial plants, and they do not require land or fresh water [
2]. The sustainability of seaweed cultivation has increased the appeal for their production through aquaculture globally. Moreover, consumers perceive edible seaweed food products as natural and healthy [
3]. Seaweeds are rich in dietary fiber, minerals, vitamins, antioxidants, and umami flavor; they can be used in low-calorie diets and serve as functional foods [
4,
5].
There are numerous seaweed-based products in Asian countries such as China, South Korea, and Japan, with niches of products marketed in Europe and North America. The FAO reported that 290,000 wet tons of seaweed were produced in 2019 in the Americas and Europe [
6]. The principal cultivated variety (66%) was kelp, a grouping which encompasses multiple species of brown algae [
7,
8]. In the U.S., seaweed cultivation is found on the west and east coasts, with Maine and Alaska leading U.S. production (~85%) of about 600,000 wet lbs. of edible seaweed due to their extensive coastlines, as reported by the Island Institute in 2020 [
8,
9]. The increasing production provides abundant opportunities for industrial development for seaweed consumption. However, little attention has been paid to consumers’ perceptions of seaweed as a food product in the West [
10]. Additionally, the extreme seasonality and high perishability of the crop [
11,
12] may impede the availability of raw materials to produce consumer products without the use of preservation processes.
Prior studies have applied various processes, including drying, freezing, salting, and high-pressure processing, to various seaweed species to increase seaweed product availability throughout the year [
11,
13,
14]. Most of these processes reduced some bioactive compounds and changed the texture of seaweed [
15,
16]. Blanching prior to some of these preservation methods, including drying and freezing, has been suggested to retard product deterioration rates [
17]. Moreover, blanching reduces microbial counts in some vegetables [
18] and turns brown seaweed to a bright green color [
19]. Processing methods such as fermentation and salting may also add value to seaweed products in addition to providing shelf-life extension.
Fermentation is a low-cost preservation method utilized by some food processors, which increases some bioactive compounds in foods such as cabbage [
20], and gives food products unique flavor [
21]. Seaweeds can be fermented into a seaweed sauerkraut-style products to create a non-dairy alternative probiotic product for consumers [
22,
23]. Sugar kelp (
Saccharina latissima) and winged kelp (
Alaria esculenta) mixed with cabbage in various ratios were fermented with
Lactobacillus plantarum (10
6 CFU/g) and
Leuconostoc mesenteroides (10
1 CFU/g) starter cultures to produce seaweed sauerkraut with high lactic acid bacteria levels, which increased as fermentation progressed [
23]. Fermentation of sugar kelp with
L. plantarum for 48 h reduced mercury and cadmium content significantly (
p < 0.05), as compared to raw kelp [
24], which could relieve concerns about heavy metals for health-conscious consumers.
To develop appropriate food products for Western markets from the harvest of domestic seaweeds and also consider seaweed as a vegetable, it is crucial to consider cost-effective preservation methods such as blanching, freezing, and fermentation, which can extend the shelf-life of the raw materials. In the literature available to date, studies on assessment and consumer acceptance of minimally processed seaweed food products are limited. Recent work conducted in our laboratory showed that blanching of sugar kelp resulted in significant changes immediately after treatment, including differences in physicochemical properties of kelp (compared to unblanched samples), particularly color and texture, after 12 months of frozen storage (unpublished data). These significant changes in some of the kelp qualities in response to blanching and/or frozen storage may have a measurable effect on consumer acceptance and may influence commercialization of blanched and/or frozen seaweed food products. Therefore, the hypothesis of this paper was that blanching, freezing, and fermentation may increase kelp quality and consumer acceptability. The effect of these preservation processes on sugar kelp were assessed using physicochemical, sensory, and microbiological methods. To achieve this, two objectives were considered. The first objective of this study was to analyze the effect of blanching (100 °C for 1 or 3 min) on the physicochemical and microbial properties of sugar kelp and to conduct sensory evaluation of a food product (seaweed salad) developed from the blanched kelp, as compared to raw. This was done to determine the effect of minimal processing (blanching) on kelp quality and its impact on consumers’ acceptance. The second study focused on the effects of blanching and freezing on fermented kelp products to offer interesting possibilities for development of other types of kelp foods. Our prior research found no significant differences in consumer liking of sugar kelp sauerkraut-style products made with raw kelp plus 25% or 50% cabbage [
23]. Due to the similarity of fermented kelp to sauerkraut, they will be referred to as “kelp- or kelp/cabbage sauerkraut” in this paper. The consumer liking of kelp sauerkraut formulated with blanched and/or previously frozen product is unknown. Therefore, the second objective of this study was to evaluate the effects of blanching and freezing of sugar kelp on the microbial quality, physical properties, and consumer acceptability of sauerkraut containing sugar kelp. A 50% kelp/cabbage sauerkraut blend was chosen for this study and was compared to a lab-made 100% cabbage sauerkraut. Findings are of economic significance to the seaweed industry as growers and processors attempt to diversify products and increase profit.
2. Material and Methods
2.1. Sample Preparation
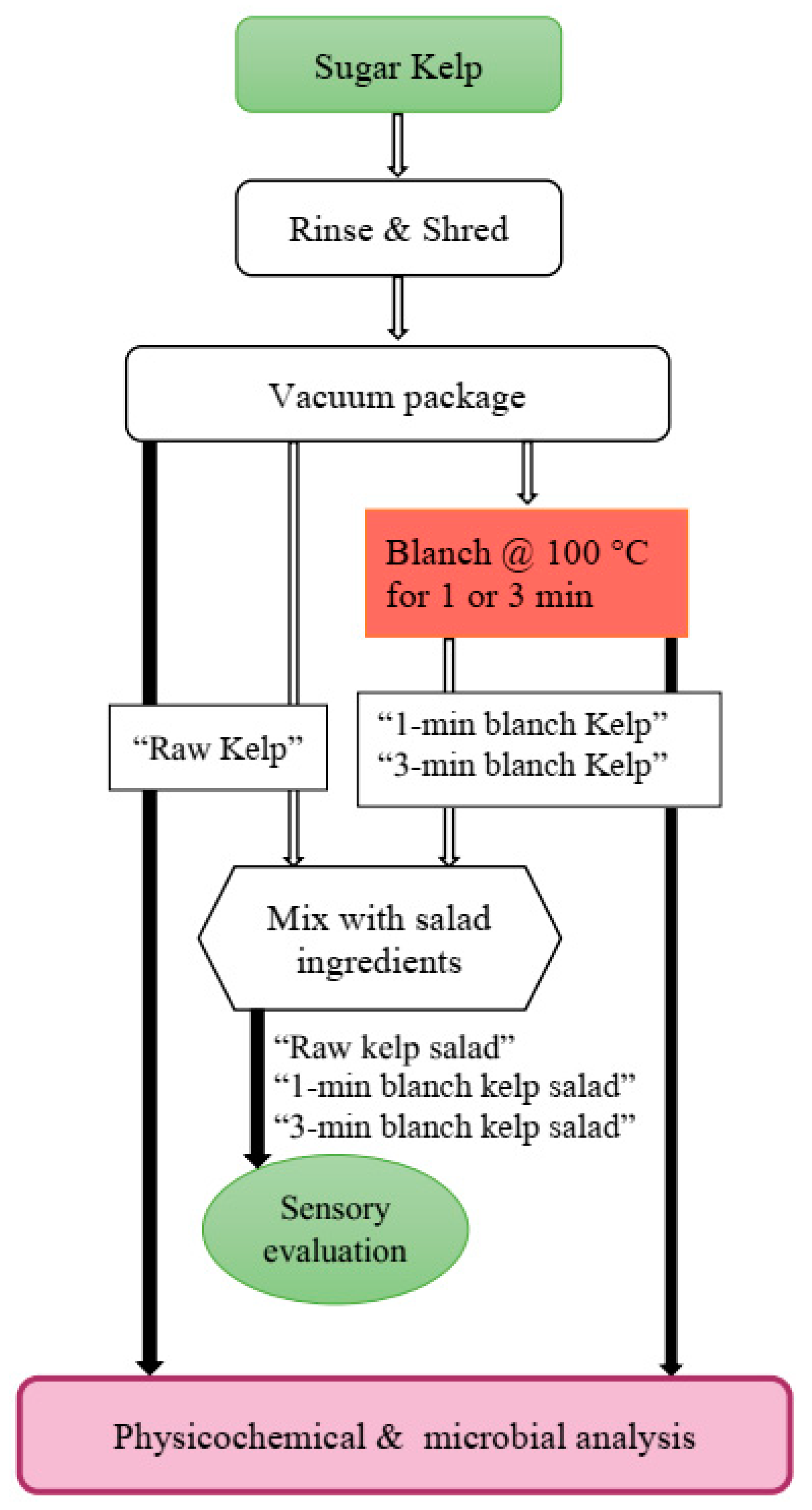
Fresh sugar kelp (Saccharina latissima) was received on two different occasions in a space of three weeks in April 2019 for the two experiments (kelp salad and sauerkraut studies). Fresh, cultivated sugar kelp received from Maine Sea Farms (South Bristol, ME) was washed with tap water to remove debris and shredded with a food processor (RobotCoupe®, CL 50 Series E, Jackson, MS, USA) fitted with a 0.32 cm slicing disc. In both experiments, about 350 g of shredded kelp were weighed into 30.48 cm × 30.48 cm plastic bags (UltraSource, Kansas, MO, USA) and vacuum packaged (KOCH Ultravac, Model UV550, Wichita, KS, USA). Vacuum-packed bags of kelp were placed in a metal strainer and submerged in boiling tap water (100 °C) of about ¾ of a 50 L steam jacketed kettle for a prescribed time according to the experimental design. After blanching, the sample bags were immediately cooled in an ice/water slurry (~1 °C) for 1 min.
2.1.1. Kelp Salad Study
Kelp was separated into three groups: a 1 min blanched, 3 min blanched, and unblanched (control) treatments. Blanching temperature, blanching time and vacuum packaging were based on the relatively higher product quality recommended by a previous study in our laboratory [
25]. Random samples were aseptically taken from the vacuumed bags after blanching and analyzed in triplicate for physicochemical and microbial quality (
Figure 1). The remaining replicates of each treatment were mixed together separately. A seaweed salad recipe from Food.com [
26] was modified for this purpose. The samples were then processed into a seaweed salad for sensory evaluation. Shredded kelp from the three previously processed treatments were mixed with shredded carrots (1.3% salad weight) and sesame seeds (10.1%), before adding 0.15% of commercial Asian balsamic vinaigrette (containing balsamic vinegar, vegetable oil (soybean and/or canola), extra virgin olive oil, salt, garlic, spice, onion, xanthan gum, red bell pepper, mustard flour) (Ken’s Lite Balsamic Vinaigrette, MA, USA). Three salad treatments (blanched for 1 min or 3 min, raw) were prepared to evaluate the effects of blanching treatment on the consumer acceptability of the kelp (
Figure 1).
2.1.2. Kelp Sauerkraut Study
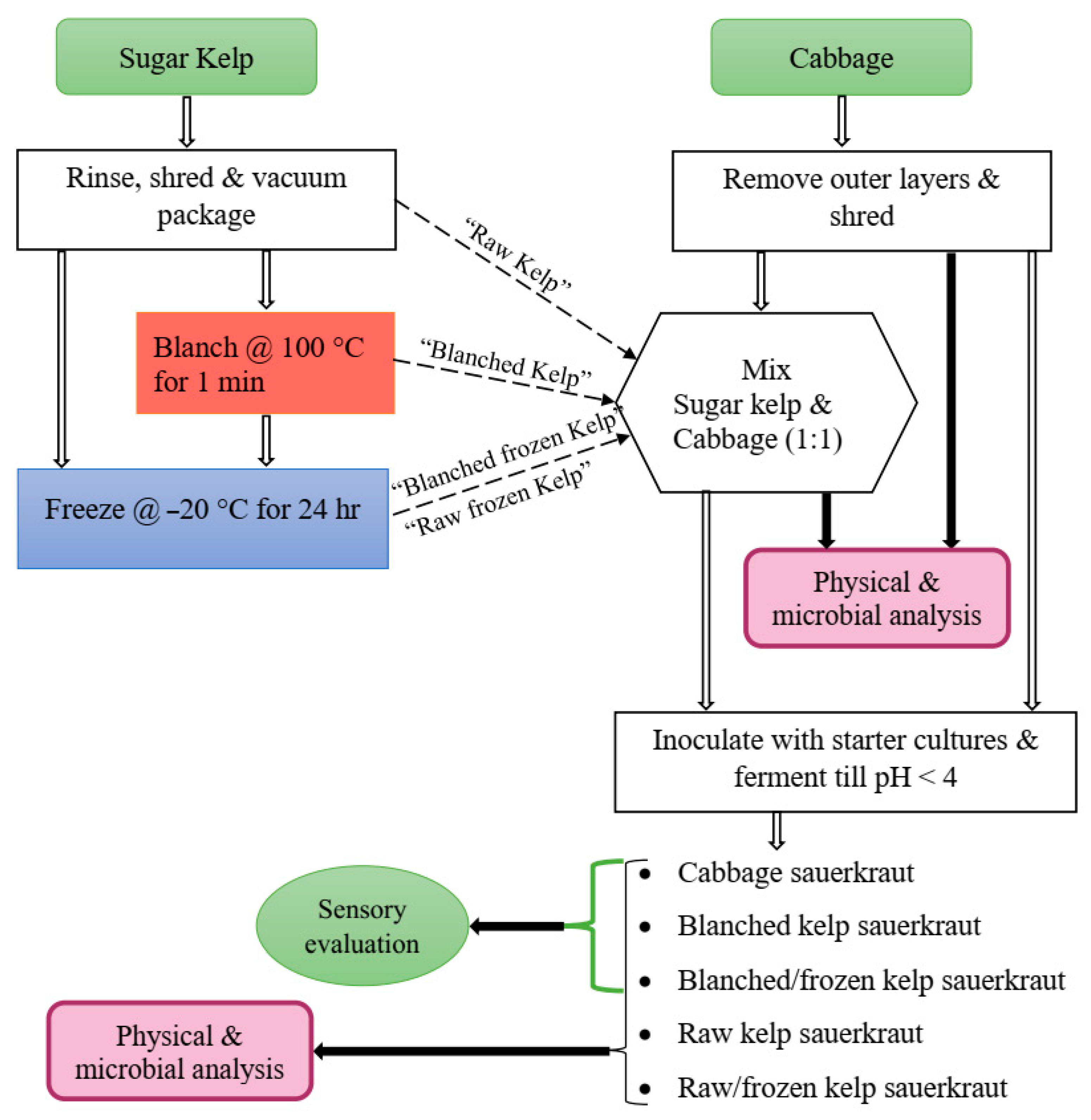
The kelp sauerkraut study was designed to test for the effect of blanching and freezing on physicochemical and microbial properties of sugar kelp, which was developed into a value-added food product (kelp sauerkraut). The shredded kelp was divided into four treatments: raw, raw/frozen (−20 °C, 24 h), blanched (100 °C, 1 min), or blanched/frozen. Specifically, one of the blanched treatments (blanched/frozen) was immediately blast frozen after blanching, together with one of the raw kelp treatments (raw/frozen) at −30 °C (Southeast Cooler, Lithia Springs, GA, USA) for an hour, and then stored at −20 °C for 24 h before further processing. White cabbage (
Brassica oleracea) was purchased from a local grocer. The outer leaves of cabbage were discarded, and the rest were washed and shredded with the same food processor used for shredding kelp. The four kelp treatments were combined with shredded cabbage (50% ratio) and manually mixed with kosher salt (2% of kelp/cabbage mix weight, Morton coarse Kosher salt, Chicago, IL, USA) for 5 min to produce a brine solution (
Figure 2). The last treatment was 100% cabbage with 2% kosher salt, which served as a control. Each of the five treatments was packed into 3.785 L glass fermentation jars (Kombucha Brooklyn, Kingston, NY, USA) with a plastic lid and airlock. Treatments were subsequently inoculated aseptically in triplicate with starter cultures (see below) to ferment at ambient temperature (~22 °C) until a pH < 4.0 was achieved (an average of six days for all cabbage sauerkraut and nine days for kelp-containing sauerkrauts), at which time sauerkrauts were placed in storage at 4 °C for further analysis and sensory evaluation.
2.1.3. Starter Culture Preparation
Lactobacillus plantarum (ATCC 8014) and L. mesenteroides subsp. cremoris were obtained from Microbiologics (St. Cloud, MN, USA) and DuPont (Danisco, Paris, France), respectively. Cultures were stored at −80 °C before use. The cultures were streaked separately onto Lactobacilli MRS agar (Alpha Biosciences, Baltimore, MD, USA) and placed into a 30 °C incubator for 48 h. One single colony of each culture was aseptically transferred into 9 mL of room temperature Lactobacilli MRS broth (Alpha Biosciences, Baltimore, MD, USA) and incubated at 30 °C for 24 h to achieve a population of ~9 log CFU/g for both cultures, verified by direct plating, which was used to inoculate the five treatments to achieve a target concentration of 101 CFU/g for L. mesenteroides and 106 CFU/g for L. plantarum.
2.2. Physicochemical Analyses
2.2.1. Colorimetric Analyses
Color change in sample treatments was measured with a colorimeter (LabScan XE, Hunter Labs, USA) fitted with a 5.1 cm diameter aperture, which was standardized with white and black tiles. Sample shreds were placed to cover the bottom of a transparent cup and Hunter L*, a*, b* values were determined. Ten readings were recorded for each treatment replicate. Color change (ΔE) after processing was calculated in comparison to raw values using the following formula:
where L* denotes lightness using a scale from black (0) to white (100), a* denotes the red (+a) to green (−a) color axis, and b* denotes the yellow (+b) to blue (−b) color axis. For the kelp salad study, the subscript 1 represents color values for raw samples before blanching and 2 represents color values after blanching.
2.2.2. Instrumental Texture
Texture analysis for all treatments was conducted using the Kramer shear method. Briefly, 10–15 g of shredded sample were loaded into a mini Kramer shear cell (TA-XTi2, Texture Technologies Inc., Scarsdale, NY, USA) with five flat blades set to travel 5 cm in a downward direction at 2 mm/s. Force (N) required to shear the sample was recorded as the firmness of the shredded kelp. Ten subsamples from each treatment replicate were analyzed, and values were averaged.
2.2.3. Moisture Content
Moisture content (%) was determined using a convection oven (VWR International, Radnor, PA). Each treatment replicate was evaluated in duplicate, and values were averaged in percentage on a wet weight basis (wwb). Briefly, homogenized kelp samples (5 ± 0.002 g) in a pre-weighed aluminum pan were dried at 105 °C for 6 h (AOAC, Method 950.46) [
27]. Pans containing the dried samples were re-weighed and the percent moisture was calculated.
2.2.4. Total Phenolic Content (TPC) and Antioxidant Analysis
Blanched and raw samples used for salad were freeze-dried (VirTis Ultra, Warminster, PA, USA), ground, and extracted for analysis as previously described by Rajauria [
28] with slight modifications. Freeze-dried samples (2 g) were mixed with 20 mL of 60% methanol (
v/
v) and shaken on a lab plate shaker at 210 rpm for 24 h at room temperature. The mixture was centrifuged at 2100×
g for 10 min. All supernatants from the extraction and pellet wash (2 times) were collected and then brought to a final volume of 50 mL with deionized water. The extracts were stored at −20 °C prior to conducting total phenolic content (TPC) and ferric reducing antioxidant power (FRAP) assays.
Total phenolics were determined in duplicate using the Folin–Ciocalteau reagent. Absorbance was measured at 725 nm against a 42% methanol blank. Total phenol content was expressed as mg of gallic acid equivalent (GAE) per g of freeze-dried sample based on a gallic acid reference curve (0–200 ug/mL) [
28].
The assay for ferric reducing antioxidant power (FRAP) procedure was conducted according to the method described by Rajauria [
28]. Fe
3+ in the FRAP reagent, which included 2,4,6-tripyridy-s-triazine (TPTZ), was reduced in the presence of the sample extracts, and a colored TPTZ–Fe
2+ complex was formed. After 4 min, sample absorbance was measured at 595 nm against a deionized water sample blank. A standard curve was derived from the absorbances of 50–750 μM ferrous sulfate (FeSO
4·7H
2O) in deionized water. All samples were analyzed in duplicate and results were expressed as μmol ferrous sulfate equivalents (FSE) per gram of freeze-dried sample.
2.3. Determination of Microbiological Quality
In the kelp salad study, microbial safety analysis was performed on the raw control and blanched kelp treatments before incorporating them into salads. In the second study, samples were tested before and after fermentation of the five treatments. The presence of
Vibrio spp.,
Listeria monocytogenes,
Salmonella spp., and
Staphylococcus aureus was assessed as described by FDA’s Bacteriological Analytical Manual [
29]. Briefly, 25 g of each of the samples were placed aseptically into 225 mL of alkaline peptone water (28 °C) for
Vibrio, Listeria enrichment broth (28 °C) for
Listeria, lactose broth (35 °C) for
Salmonella and tryptic soy broth with 10% NaCl and 1% sodium pyruvate (35 °C) for
S. aureus in a stomacher bag and homogenized for two minutes using a BAGMixer 400 (Model P, Spiral Biotech, Advanced Instruments, Norwood, MA, USA). Afterward, the stomacher bag was incubated for 24 h and samples were plated (0.1 mL) on Thiosulfate–citrate–bile salts–sucrose agar (28 °C,
Vibrio), Modified oxford agar (28 °C,
Listeria), Xylose lysine deoxycholate agar (35 °C,
Salmonella) and Baird–Parker (35 °C,
S. aureus) in duplicate and incubated for 48 h for each of the treatment replicates. The presence of colony growth with expected morphology denoted the presumptive presence of pathogens.
To assess microbial quality, duplicate samples (10 g) of all treatment replicates in both experiments were mixed with 0.1% peptone and agitated for 2 min. After agitation, the samples were serially diluted in 0.1% peptone and spread plated onto tryptic soy agar (TSA) (Alpha Biosciences, Baltimore, MD, USA) and acidified potato dextrose agar (APDA, Alpha Biosciences, Baltimore, MD, USA) for aerobic plate counts (APC) and fungi, respectively. Plates were incubated at 37 °C for 48 h (TSA), and at room temperature for 5 days (APDA). Microbial populations were determined in log CFU/g for APC and fungi.
2.4. Sensory Evaluation
This research was approved by the University of Maine Institutional Review Board for the protection of human subjects. All research participants provided their informed consent. In the kelp salad study, sensory evaluation was conducted to determine the effects of two blanching times on consumer acceptance of salad made from blanched or raw kelp One hundred and two sensory panelists (at least 18 years old) in the greater Orono, ME area interested in seaweed and not allergic to seaweed or the other salad ingredients were recruited via email and flyer notices to assess the acceptability of sugar kelp salad. Each of the three salads was kept at 5–10 °C in a covered aluminum dish before being served. Panelists were simultaneously presented with three 30 g samples of three kelp salads for evaluation (
Figure 3a).
In the kelp sauerkraut study, 30 g of sauerkraut prepared as described previously was served for each of the three treatments: blanched kelp sauerkraut, blanched/frozen kelp sauerkraut, and the raw cabbage sauerkraut control. Eighty sensory panelists (older than 18 years) interested in consuming seaweed and sauerkraut were recruited via email and flyer notices to assess the acceptability of kelp and/or cabbage sauerkraut. Each treatment was kept at 5–10 °C in a covered aluminum dish prior to being served (
Figure 3b).
For both studies, panelists were seated in individual booths with a combination of fluorescent and incandescent lighting at the Sensory Evaluation Center at the University of Maine. The three products were labeled with 3-digit random codes and were served in a ceramic ramekin with small cups of ~4 °C Poland spring water alongside. Sample order was randomized in each study to reduce the effects of flavor carry-over and order bias. Panelists were instructed to evaluate the samples, take a sip of water before testing each sample, and rate the acceptance of specific sensory attributes of the samples. A 9-point hedonic scale (from 1 = “Dislike Extremely” to 9 = “Like Extremely,” with 5 = “Neither Like nor Dislike”) was used to assess the acceptability of appearance, color, flavor, texture, and overall liking of samples [
30] and a 5-point Just-About-Right (JAR) scale (1 = Not Firm/Tender, 2 = Somewhat Firm/Tender, 3 = Just About Right, 4 = Somewhat Too Firm/Tender, and 5 = Much Too Firm/Tender) was used to examine specific texture attributes (firmness and tenderness) for salad only [
31]. Penalty analysis was performed for scores that were not JAR. Participants were asked to answer a set of questions relating to demographic characteristics, seaweed consumption habits, and attitudes towards consuming seaweed in both studies prior to consuming samples. Panelists were also asked if they would like to consume raw seaweed in the kelp salad study prior to consuming samples. Panelist were asked to select one descriptor that best described each salad treatment from a short list (chewy, firm, tender, juicy, mushy, soft, tough) based on previous research [
32,
33]. Additionally, panelists choose which forms they consume seaweed (as part of other foods like sushi, salad, soup, frozen smoothie cubes or in other form). In the kelp sauerkraut study, participants were additionally asked to check all that apply (CATA) for words that best described each sauerkraut sample after consumption. Panelists were asked to provide comments about the three treatments at the end of both studies. The test randomizations, experimental designs, and analyses were executed using SIMS 2000 (Sensory Computer Systems, Berkeley Heights, NJ, USA) software.
2.5. Statistical Analysis
Data from physicochemical, microbial, and sensory tests were analyzed using SPSS 20 (IBM, Armonk, NY, USA) at a significance level of p ≤ 0.05. One-way analysis of variance (ANOVA) was used to assess all one-level (treatment) effects. Multiway ANOVA was used to assess salad type and consumption frequency. Separation of treatment means was accomplished using Tukey’s honest significant difference (HSD) post hoc test. Pearson’s correlation was performed to evaluate correlations among variables. An independent t-test was used to compare the changes in color between the two blanched treatments in study one, and a pairwise t-test was used to compare kelp/cabbage qualities in treatments before and after fermentation in study two. A Cochran–Mantel–Haenszel test was used to determine whether JAR score distributions were different among the three products for firmness and tenderness attributes.
5. Conclusions
With the increase in production of seaweed in the West, data gathered from this research show that kelp could be utilized and consumed as vegetables by consumers. The study revealed that preservation processes had some positive impact on kelp quality and consumer acceptability. Blanching increased greenness but decreased firmness of kelp. Results from sensory acceptability tests indicate that consumers may like blanched kelp food products more than raw, possibly due to the color change and reduced firmness. Therefore, we can recommend minimal processes such as blanching and freezing of seaweed for extension of the short shelf life of fresh kelp. Use of such processes will extend marketable life of kelp and may allow preservation for use in formulated foods independent of harvest season. However, cost of water and energy should be considered. Moreover, the absence of pathogens after fermentation from the kelp sauerkraut study confirm that fermented foods are typically safe however, proper hygiene and sanitation practices should not be compromised to prevent possible cross-contamination from the environment during and after kelp sauerkraut production. Moreover, freezing can increase kelp retail availability throughout the year and also mask some aroma notes of kelp such as pungency and fishiness when used to develop products. Future studies are warranted to evaluate the impact of an extended frozen storage on value added kelp products, since this study focused on the immediate effect of freezing on kelp sauerkraut. Additionally, blanching, freezing and fermenting kelp into sauerkraut can increase the commercial availability of seaweed products and promote the development of diverse seaweed products that could be easily made at home or conveniently sold in the marketplace year-round. These findings have important implications for the growing U.S. seaweed industry for many economical and nutritional reasons.