Effect of Physical and Enzymatic Pre-Treatment on the Nutritional and Functional Properties of Fermented Beverages Enriched with Cricket Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasound Pre-Treatment
2.3. γ-Irradiation Treatment
2.4. Enzymatic Hydrolysis and Combined Treatments
2.5. Beverage Preparation
2.6. Protein Solubility
2.7. Measurement of Sulfhydryl (SH) Bonds
2.8. Surface Hydrophobicity
2.9. Molecular Characterization by Fourier Transform Infrared (FTIR) Spectroscopy
2.10. In Vitro Digestibility
2.11. Peptide Profile (SEC-HPLC)
2.12. Statistical Analysis
3. Results and Discussion
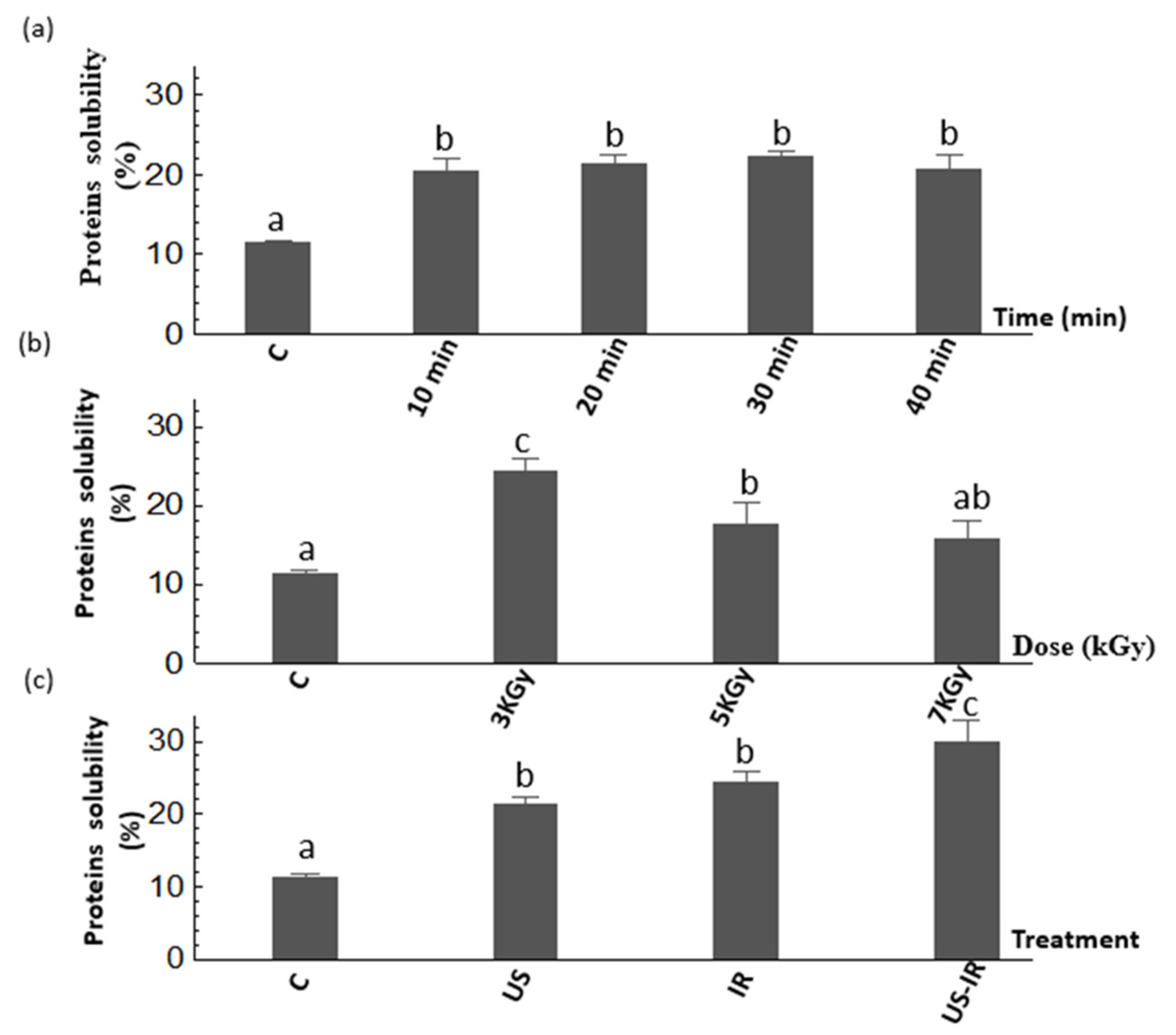
3.1. Protein Solubility
3.2. Sulfhydryl Groups and Surface Hydrophobicity
3.3. ATR-FTIR Analysis
3.4. In Vitro Digestibility
3.5. Molecular Weight (Mw) Distribution by SEC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Didari, T. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. WJG 2015, 21, 3072. [Google Scholar] [CrossRef]
- Michail, S.; Kenche, H. Gut Microbiota is Not Modified by Randomized, Double-Blind, Placebo-Controlled Trial of VSL#3 in Diarrhea-Predominant Irritable Bowel Syndrome. Probiotics Antimicro. Prot. 2011, 3, 1–7. [Google Scholar]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Desrouillères, K.; Millette, M.; Bagheri, L.; Maherani, B.; Jamshidian, M.; Lacroix, M. The synergistic effect of cell wall extracted from probiotic biomass containing Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 on the anticancer activity of cranberry juice—HPLC fractions. J. Food Biochem. 2020, 44, e13195. [Google Scholar] [CrossRef]
- Desrouillères, K.; Millette, M.; Jamshidian, M.; Maherani, B.; Fortin, O.; Lacroix, M. Cancer preventive effect of a specific probiotic fermented milk components and cell walls extracted from a biomass containing L. acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 on male F344 rats treated with 1,2-dimethylhydrazine. J. Funct. Foods 2016, 26, 373–384. [Google Scholar]
- Song, X.; Pérez-Cueto, F.J.A.; Bølling-Laugesen, S.M.; van der Zanden, L.D.T.; Giacalone, D. Older consumers’ attitudes towards food carriers for protein-enrichment. Appetite 2019, 135, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Ortinau, L.C.; Hoertel, H.A.; Leidy, H.J. Low, moderate, or high protein yogurt snacks on appetite control and subsequent eating in healthy women. Appetite 2013, 60, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Felix da Silva, D.; Junior, N.N.T.; Gomes, R.G.; dos Santos Pozza, M.S.; Britten, M.; Matumoto-Pintro, P.T. Physical, microbiological and rheological properties of probiotic yogurt supplemented with grape extract. J. Food Sci. Technol. 2017, 54, 1608–1615. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.Z.; Wang, J.S.; Zhao, M.M.; Jiang, Y.M.; Chun, C. Effect of Casein Hydrolysates on Yogurt Fermentation and Texture Properties during Storage. Food Technol. Biotechnol. 2006, 44, 429–434. [Google Scholar]
- Durst, P.B. FAO. Forest insects as food: Humans bite back. In Proceedings of the a Workshop on Asia-Pacific Resources and Their Potential for Development, Chiang Mai, Thailand, 19–21 February 2008; Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; p. 231. [Google Scholar]
- Van Huis, A. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 187. [Google Scholar]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects: Nutrient Content of Feeder Insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Boveraa, F.; Parisib, G. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Anim. Feed. Sci. Technol. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed. Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In Vitro Crude Protein Digestibility of Tenebrio Molitor and Hermetia Illucens Insect Meals and its Correlation with Chemical Composition Traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.M.; Abdalla, I.G.; Salih, A.M.; Hassan, A.B. Effect of gamma radiation on storability and functional properties of sorghum grains ( Sorghum bicolor L.). Food Sci. Nutr. 2018, 6, 1933–1939. [Google Scholar] [CrossRef]
- Ebrahimi, S.R.; Nikkhah, A.; Sadeghi, A.A.; Raisali, G. Chemical composition, secondary compounds, ruminal degradation and in vitro crude protein digestibility of gamma irradiated canola seed. Anim. Feed. Sci. Tech. 2009, 151, 184–193. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Chen, F. Effect of gamma irradiation on the physicochemical properties and nutrient contents of peanut. LWT 2018, 96, 535–542. [Google Scholar] [CrossRef]
- Gölge, E.; Ova, G. The effects of food irradiation on quality of pine nut kernels. Radiat. Phys. Chem. 2008, 77, 365–369. [Google Scholar] [CrossRef]
- Rooney, P.; Eagle, M.; Hogg, P.; Lomas, R.; Kearney, J. Sterilisation of skin allograft with gamma irradiation. Burns 2008, 34, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- López-Ferrer, D.; Heibeck, T.H.; Petritis, K.; Hixson, K.K.; Qian, W.; Monroe, M.E.; Mayampurath, A.; Moore, J.R.; Belov, M.E.; Camp, D.G.; et al. Rapid Sample Processing for LC-MS-Based Quantitative Proteomics Using High Intensity Focused Ultrasound. J. Proteome Res 2008, 7, 3860–3867. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Cai, M.; Cheng, J.H.; Sun, D.W. Effects of electric fields and electromagnetic wave on food protein structure and functionality: A review. Trends Food Sci. Tech. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Chen, W.; Wang, Z.; He, R.; Ma, H. Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason. Sonochemistry 2018, 42, 250–259. [Google Scholar] [CrossRef]
- Tang, C.H.; Wang, X.S.; Yang, X.Q. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009, 114, 1484–1490. [Google Scholar] [CrossRef]
- Ali, M.A.M.; El Tinay, A.H.; Abdalla, A.H. Effect of fermentation on the in vitro protein digestibility of pearl millet. Food Chem. 2003, 80, 51–54. [Google Scholar] [CrossRef]
- Beausoleil, M.; Fortier, N.; Guénette, S.; L’Ecuyer, A.; Savoie, M.; Franco, M.; Lachaîne, J.; Weiss, K. Effect of a Fermented Milk Combining Lactobacillus Acidophilus CL1285 and Lactobacillus Casei in the Prevention of Antibiotic-Associated Diarrhea: A Randomized, Double-Blind, Placebo-Controlled Trial. Can. J. Gastroenterol. 2007, 21, 732–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [Green Version]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef] [Green Version]
- Prajapat, A.L.; Subhedar, P.B.; Gogate, P.R. Ultrasound assisted enzymatic depolymerization of aqueous guar gum solution. Ultrason. Sonochem. 2016, 29, 84–92. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Li, P.; Cai, P.; Zhang, M.; Sun, Z.; Sun, C.; Geng, Z.; Xu, W.; Xu, X.; et al. Effects of ultrasound assisted extraction on the physiochemical, structural and functional characteristics of duck liver protein isolate. Process. Biochem. 2017, 52, 174–182. [Google Scholar] [CrossRef]
- Sousa, P.; Borges, S.; Pintado, M. Enzymatic hydrolysis of insect Alphitobius diaperinus towards the development of bioactive peptide hydrolysates. Food Funct. 2020, 11, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Li, M.Y.; Tian, G.; Zhang, T.H.; Ren, H.; Quek, S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019, 299, 125103. [Google Scholar] [CrossRef]
- Lacroix, M.; Amiot, J.; Brisson, G.J. Hydrolysis and Ultrafiltration Treatment to Improve the Nutritive Value of Rapeseed Proteins. J. Food Sci. 1983, 48, 1644–1645. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- AOAC: Method 991. Official Methods of Analysis of the Association of Analytical Chemists International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Shimada, K.; Cheftel, J.C. Texture characteristics, protein solubility, and sulfhydryl group/disulfide bond contents of heat-induced gels of whey protein isolate. J. Agric. Food Chem. 1988, 36, 1018–1025. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. et Biophys. Acta (BBA)—Protein Struct. 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Application of computerized infrared and Raman spectroscopy to conformation studies of casein and other food proteins. J. Ind. Microbiol. 1988, 3, 73–88. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Yang, X.Q.; Gao, W.R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Kjartansson, G.T.; Zivanovic, S.; Kristbergsson, K.; Weiss, J. Sonication-Assisted Extraction of Chitin from Shells of Fresh Water Prawns (Macrobrachium rosenbergii). J. Agric. Food Chem. 2006, 54, 3317–3323. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Amiri, A.; Sharifian, P.; Soltanizadeh, N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018, 111, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Morel, M.H.; Dehlon, P.; Autran, J.C.; Leygue, J.P.; Bar-L’Helgouac’h, C. Effects of Temperature, Sonication Time, and Power Settings on Size Distribution and Extractability of Total Wheat Flour Proteins as Determined by Size-Exclusion High-Performance Liquid Chromatography. Cereal Chem. J. 2000, 77, 685–691. [Google Scholar] [CrossRef]
- Hassan, A.B.; Mahmoud, N.S.; Elmamoun, K.; Adiamo, O.Q.; Mohamed Ahmed, I.A. Effects of gamma irradiation on the protein characteristics and functional properties of sesame (Sesamum indicum L.) seeds. Radiat. Phys. Chem. 2018, 144, 85–91. [Google Scholar] [CrossRef]
- Dogbevi, M.K.; Vachon, C.; Lacroix, M. Effect of gamma irradiation on the microbiological quality and on the functional properties of proteins in dry red kidney beans (Phaseolus vulgaris). Radiat. Phys. Chem. 2000, 4, 265–268. [Google Scholar] [CrossRef]
- Shih, F.F.; Kalmar, A.D. SDS-catalyzed deamidation of oilseed proteins. J. Agric. Food Chem. 1987, 35, 672–675. [Google Scholar] [CrossRef]
- Dogbevi, M.K.; Vachon, C.; Lacroix, M. Physicochemical Properties of Dry Red Kidney Bean Proteins and Natural Microflora as Affected by Gamma Irradiation. J. Food Sci. 1999, 64, 540–542. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; Rashed, M.M.; Mahmoud, E.A.; El-Beltagi, H.S. Effect of Gamma Radiation on Protein Profile, Protein Fraction and Solubility’s of Three Oil Seeds: Soybean, Peanut and Sesame. Not. Bot. Hort. Agrobot. Cluj 2011, 39, 90. [Google Scholar] [CrossRef] [Green Version]
- Maity, J.P.; Chakraborty, S.; Kar, S.; Panja, S.; Jean, J.S.; Samal, A.C.; Chakraborty, A.; Santra, S.C. Effects of gamma irradiation on edible seed protein, amino acids and genomic DNA during sterilization. Food Chem. 2009, 114, 1237–1244. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali solution extraction of rice residue protein isolates: Influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Barraza, O.A.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Cinco-Moroyoqui, F.J.; Rodríguez Figueroa, J.C.; Marquez-Ríos, E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sonochem. 2017, 38, 829–834. [Google Scholar] [CrossRef]
- Fombang, E.N.; Taylor, J.R.N.; Mbofung, C.M.F.; Minnaar, A. Use of γ-irradiation to alleviate the poor protein digestibility of sorghum porridge. Food Chem. 2005, 91, 695–703. [Google Scholar] [CrossRef]
- Hooshmand, H.; Klopfenstein, C.F. Effects of gamma irradiation on mycotoxin disappearance and amino acid contents of corn, wheat, and soybeans with different moisture contents. Plant Food Hum. Nutr. 1995, 47, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.A.; Delgado-Macuil, R.J.; Ruiz-Espinosa, H.; Rojas-López, M.; Amador-Espejo, G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: Evaluation of selected functional properties. Ultrason. Sonochem. 2021, 70, 105340. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, W. FTIR Analysis of Protein Structure. 8. Available online: https://www.chem.uwec.edu/chem455_S05/Pages/Manuals/FTIR_of_proteins.pdf (accessed on 31 August 2021).
- Garidel, P.; Schott, H. Fourier-Transform Midinfrared Spectroscopy for Analysis and Screening of Liquid Protein Formulations. Bioprocess Int. 2006, 4, 48–55. [Google Scholar]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Yang, X.; Zhao, C.; Guo, M. Ultrasound-induced changes in structural and physicochemical properties of β-lactoglobulin. Food Sci. Nutr. 2018, 6, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, C.; Guo, M. Changes in structure and antioxidant activity of β-lactoglobulin by ultrasound and enzymatic treatment. Ultrason. Sonochem. 2018, 43, 227–236. [Google Scholar] [CrossRef]
- Aouzelleg, A.; Bull, L.A.; Price, N.C.; Kelly, S.M. Molecular studies of pressure/temperature-induced structural changes in bovine β-lactoglobulin: Pressure/temperature effects on β-lactoglobulin. J. Sci. Food Agric. 2004, 84, 398–404. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. Effect of gamma irradiation on structural, molecular, thermal and rheological properties of sunflower protein isolate. Food Hydrocoll. 2017, 72, 312–322. [Google Scholar] [CrossRef]
- Lee, Y.W.; Song, K.B. Effect of γ-Irradiation on the Molecular Properties of Myoglobin. BMB Rep. 2002, 35, 590–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Tien, C.; Letendre, M.; Ispas-Szabo, P.; Mateescu, M.A.; Delmas-Patterson, G.; Yu, H.L.; Lacroix, M. Development of Biodegradable Films from Whey Proteins by Cross-Linking and Entrapment in Cellulose. J. Agric. Food Chem. 2000, 48, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- Karra, S.; Sebii, H.; Bouaziz, M.A.; Blecker, C.; Danthine, S.; Attia, H.; Besbes, S. Effect of sonication pretreatment on physicochemical, surface, thermal, and functional properties of fibro-proteic extracts from male date palm flowers. J. Food Process. Preserv. 2020, 44, e14963. [Google Scholar] [CrossRef]
- Tian, R.; Feng, J.; Huang, G.; Tian, B.; Zhang, Y.; Jianga, L.; Suia, X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020, 68, 105202. [Google Scholar] [CrossRef]
- Dogan, A.; Siyakus, G.; Severcan, F. FTIR spectroscopic characterization of irradiated hazelnut (Corylus avellana L.). Food Chem. 2007, 100, 1106–1114. [Google Scholar] [CrossRef]
- Gaber, M.H. Effect of γ-irradiation on the molecular properties of bovine serum albumin. J. Biosci. Bioeng. 2005, 100, 203–206. [Google Scholar] [CrossRef]
- Gildberg, A.; Stenberg, E. A new process for advanced utilisation of shrimp waste. Process. Biochem. 2001, 36, 809–812. [Google Scholar] [CrossRef]
- Yi, L.; Van Boekel, M.A.J.S.; Boeren, S.; Lakemond, C.M.M. Protein identification and in vitro digestion of fractions from Tenebrio molitor. Eur. Food Res. Technol. 2016, 242, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Yang, H.J.; Kim, J.; Kim, J. Effects of temperature on ultrasound-assisted tryptic protein digestion. Anal. Biochem. 2011, 414, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Li, C.; Yang, F.; Zhao, S.; Liang, Y.; Jin, G. Effects of gamma ray irradiation-induced protein hydrolysis and oxidation on tenderness change of fresh pork during storage. Meat Sci. 2020, 163, 108058. [Google Scholar] [CrossRef]
- Zuleta, A.; Dyner, L.; Sambucetti, M.E.; de Francisco, A. Effect of Gamma Irradiation on the Functional and Nutritive Properties of Rice Flours from Different Cultivars. Cereal Chem. J. 2006, 83, 76–79. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Sun, D.; Li, Y.; Chen, Z. Effect of enzymolysis-assisted electron beam irradiation on structural characteristics and antioxidant activity of rice protein. J. Cereal Sci. 2019, 89, 102789. [Google Scholar] [CrossRef]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.; Boirie, Y.; van Loon, L.J. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W.; Guo, X. Comparative Study About Some Physical Properties, In vitro Digestibility and Immunoreactivity of Soybean Protein Isolate for Infant Formula. Plant Foods Hum. Nutr. 2013, 68, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ogodo, A.C.; Ugbogu, O.C.; Onyeagba, R.A.; Okereke, H.C. In-Vitro starch and protein digestibility and proximate composition of soybean flour fermented with lactic acid bacteria (LAB) consortia. Agric. Nat. Resour. 2018, 52, 503–509. [Google Scholar] [CrossRef]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of natural and Lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Biosci. 2013, 2, 46–52. [Google Scholar] [CrossRef]

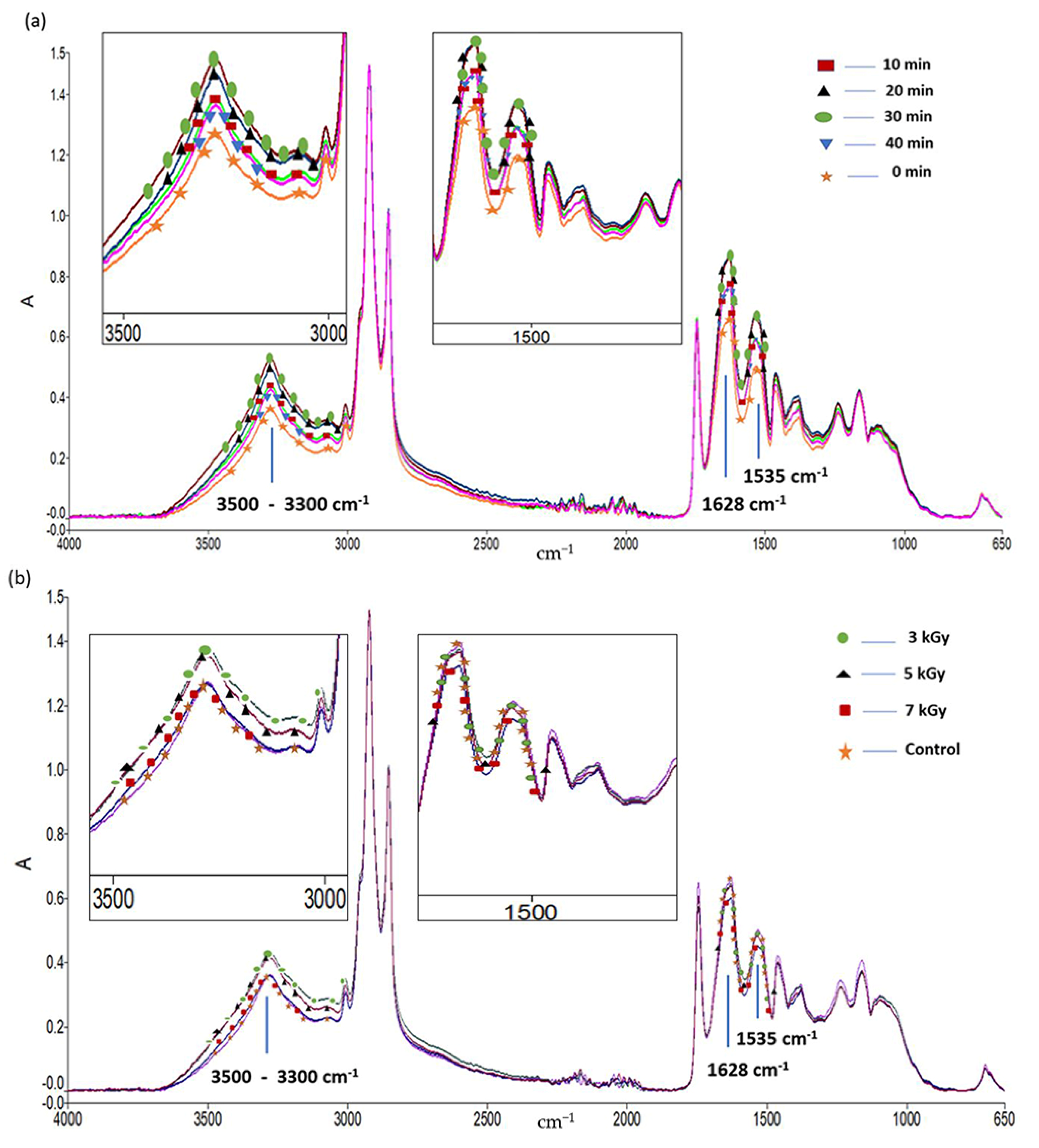
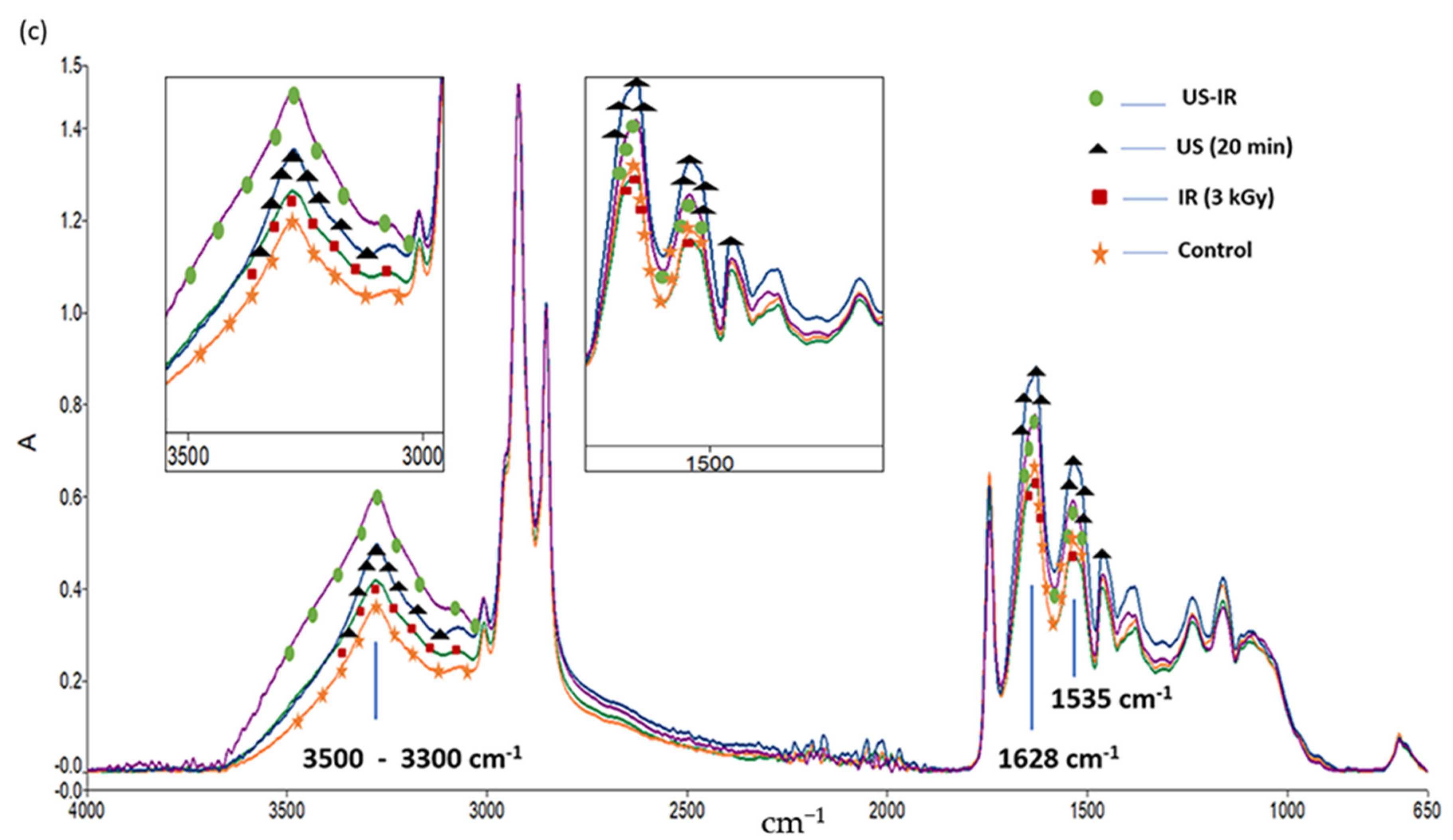

| Treatment Effect (Ratio Treatment/Control) | α-Helix 1653 cm−1 | β-Sheets 1623 cm−1 | β-Sheets 1637 cm−1 | Random Coils 1645 cm−1 | β-Turns 1663 cm−1 | β-Turns 1694 cm−1 |
|---|---|---|---|---|---|---|
| US effect | ||||||
| 10 min | 1.32 | 1.08 | 0.79 | 1.19 | 1.38 | 1.00 |
| 20 min | 1.17 | 1.29 | 1.20 | 1.74 | 3.11 | 1.21 |
| 30 min | 0.88 | 1.18 | 1.01 | 1.83 | 1.55 | 1.40 |
| 40 min | 0.96 | 1.18 | 1.19 | 1.36 | 1.77 | 1.22 |
| IR effect | ||||||
| 3 kGy | 0.20 | 0.74 | 0.70 | 1.68 | 1.59 | 0.74 |
| 5 kGy | 0.96 | 0.70 | 0.48 | 1.35 | 1.35 | 0.42 |
| 7 kGy | 0.18 | 0.67 | 0.55 | 1.66 | 1.08 | 0.50 |
| US-IR effect | ||||||
| US-IR | 1.02 | 1.07 | 1.00 | 3.47 | 1.70 | 0.55 |
| Percentage of MW Distribution, (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | MW (Da) | NF 1 (0 min) | NFP 2 (120 min) | NFPT 3 (240 min) | ΔNF7 | F 4 (0 min) | FP 5 (120 min) | FPT 6 (240 min) | ΔF 7 |
| Control | >3000 | 6.7 ± 0.3 | 3.9 ± 0.1 | 2.2 ± 0.0 | −67.1 | 6.4 ± 0.0 | 4.6 ± 0.2 | 2.3 ± 0.1 | −62.5 |
| 3000–260 | 60.0 ± 0.5 | 61.6 ± 0.5 | 63.6 ± 0.8 | 6.0 | 59.1 ± 1.1 | 60.0 ± 0.4 | 63.4 ± 0.6 | 7.6 | |
| <260 | 33.3 ± 0.9 | 34.5 ± 0.4 | 34.2 ± 0.7 | 2.7 | 34.5 ± 1.0 | 35.4 ± 0.1 | 34.3 ± 0.6 | −0.6 | |
| Cr | >3000 | 5.0 ± 0.61 | 3.8 ± 0.41 | 3.2 ± 0.35 | −36.0 | 4.9 ± 0.7 | 3.8 ± 0.4 | 3.0 ± 0.4 | −38.7 |
| 3000–260 | 37.7 ± 2.9 | 38.8 ± 0.2 | 43.3 ± 0.1 | 17.9 | 41.4 ± 0.8 | 43.3 ± 0.6 | 45.5 ± 0.5 | 10 | |
| <260 | 57.3 ± 3.2 | 57.4 ± 0.5 | 53.5 ± 0.3 | −6.6 | 53.7 ± 0.0 | 52.9 ± 0.2 | 51.5 ± 0.0 | −4.1 | |
| US-IR | >3000 | 4.5 ± 0.1 | 3.0 ± 0.1 | 3.3 ± 0.4 | −26.6 | 4.5 ± 0.1 | 3.7 ± 0.2 | 3.5 ± 0.1 | −22.2 |
| 3000–260 | 37.1 ± 0.7 | 39.3 ± 0.3 | 49.1 ± 0.3 | 32.3 | 44.7 ± 1.61 | 43.1 ± 1.6 | 46.0 ± 0.4 | 2.9 | |
| <260 | 58.4 ± 0.6 | 57.7 ± 0.2 | 47.6 ± 0.4 | −18.4 | 50.8 ± 1.4 | 53.2 ± 1.5 | 50.5 ± 0.5 | −0.6 | |
| US-E | >3000 | 1.8 ± 0.0 | 1.7 ± 0.1 | 0.9 ± 0.2 | −50.0 | 2.2 ± 0.7 | 1.3 ± 0.0 | 0.9 ± 0.7 | −59.0 |
| 3000–260 | 38.7 ± 0.3 | 39.4 ± 0.0 | 41.7 ± 0.4 | 7.7 | 39.1 ± 0.4 | 40.5 ± 0.3 | 41.4 ± 1.2 | 5.9 | |
| <260 | 59.5 ± 0.3 | 58.9 ± 0.1 | 57.4 ± 0.4 | −3.5 | 58.7 ± 0.3 | 58.2 ± 0.2 | 57.7 ± 0.1 | −1.7 | |
| US-EWC | >3000 | 1.3 ± 0.6 | 0.8 ± 0.0 | 0.5 ± 0.0 | −61.5 | 1.8 ± 0.3 | 0.7 ± 0.0 | 0.5 ± 1.0 | −72.2 |
| 3000–260 | 29.4 ± 0.3 | 30.0 ± 0.0 | 30.5 ± 0.3 | 3.7 | 30.1 ± 0.3 | 31.1 ± 0.0 | 34.7 ± 2.5 | 15.3 | |
| <260 | 69.3 ± 0.4 | 69.1 ± 0.5 | 69.0 ± 0.4 | −0.5 | 68.2 ± 0.4 | 68.2 ± 0.2 | 64.8 ± 2.8 | −4.9 | |
| US-IRE | >3000 | 1.3 ± 0.2 | 0.8 ± 0.5 | 0.7 ± 0.6 | −85.7 | 1.3 ± 0.1 | 0.7 ± 0.4 | 0.3 ± 1.0 | −76.9 |
| 3000–260 | 18.9 ± 0.3 | 19.3 ± 0.4 | 18.5 ± 0.1 | −2.1 | 20.4 ± 0.4 | 19.4 ± 0.0 | 19.0 ± 2.5 | −6.8 | |
| <260 | 79.8 ± 0.3 | 79.8 ± 1.0 | 80.7 ± 0.7 | 1.1 | 78.3 ± 0.4 | 79.8 ± 0.3 | 80.7 ± 2.8 | 3.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dridi, C.; Millette, M.; Aguilar, B.; Manus, J.; Salmieri, S.; Lacroix, M. Effect of Physical and Enzymatic Pre-Treatment on the Nutritional and Functional Properties of Fermented Beverages Enriched with Cricket Proteins. Foods 2021, 10, 2259. https://doi.org/10.3390/foods10102259
Dridi C, Millette M, Aguilar B, Manus J, Salmieri S, Lacroix M. Effect of Physical and Enzymatic Pre-Treatment on the Nutritional and Functional Properties of Fermented Beverages Enriched with Cricket Proteins. Foods. 2021; 10(10):2259. https://doi.org/10.3390/foods10102259
Chicago/Turabian StyleDridi, Chaima, Mathieu Millette, Blanca Aguilar, Johanne Manus, Stephane Salmieri, and Monique Lacroix. 2021. "Effect of Physical and Enzymatic Pre-Treatment on the Nutritional and Functional Properties of Fermented Beverages Enriched with Cricket Proteins" Foods 10, no. 10: 2259. https://doi.org/10.3390/foods10102259






