Interaction of Carbon Dots from Grilled Spanish Mackerel with Human Serum Albumin, ?-Globulin and Fibrinogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
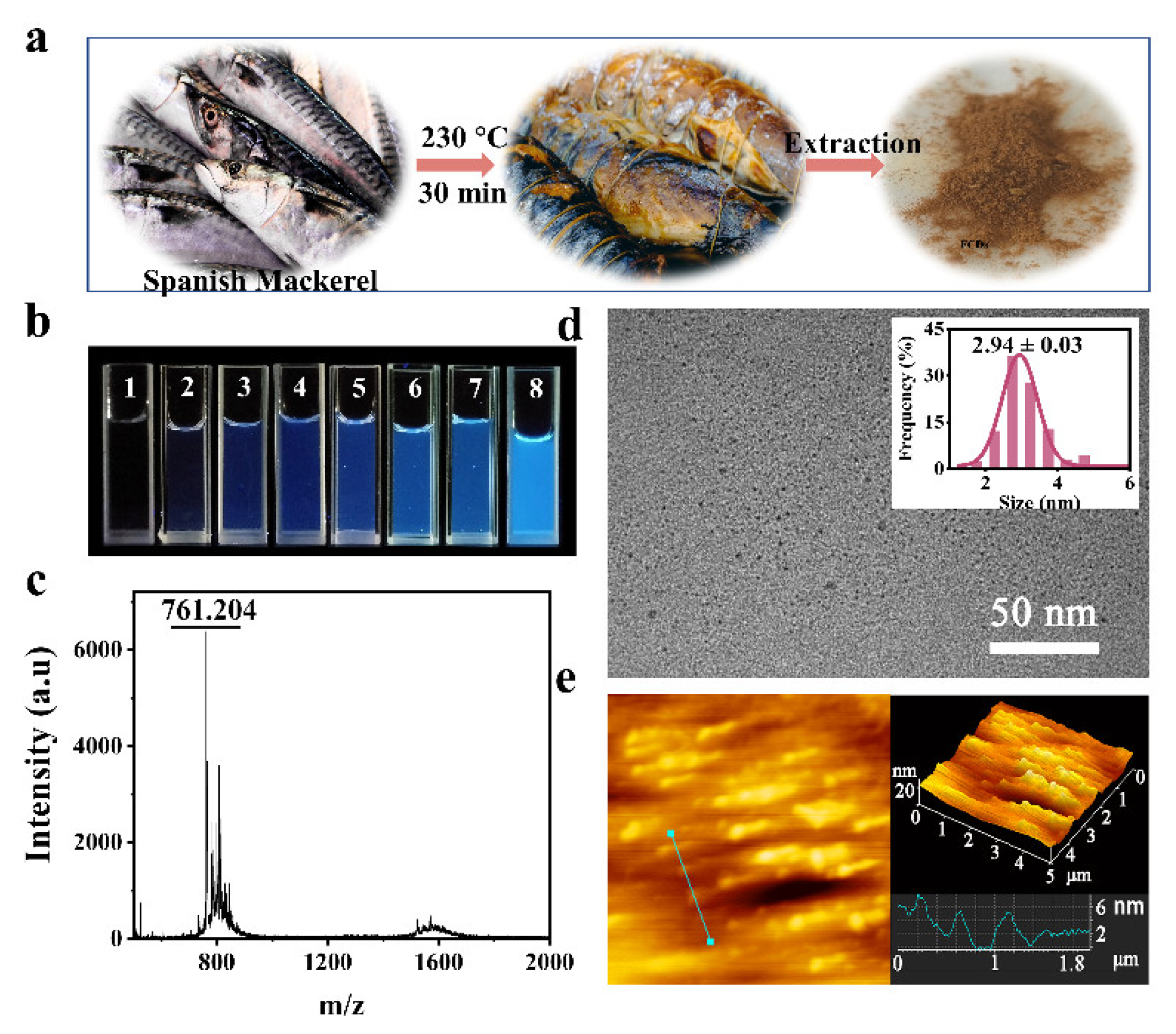
2.3. Preparation of FCDs
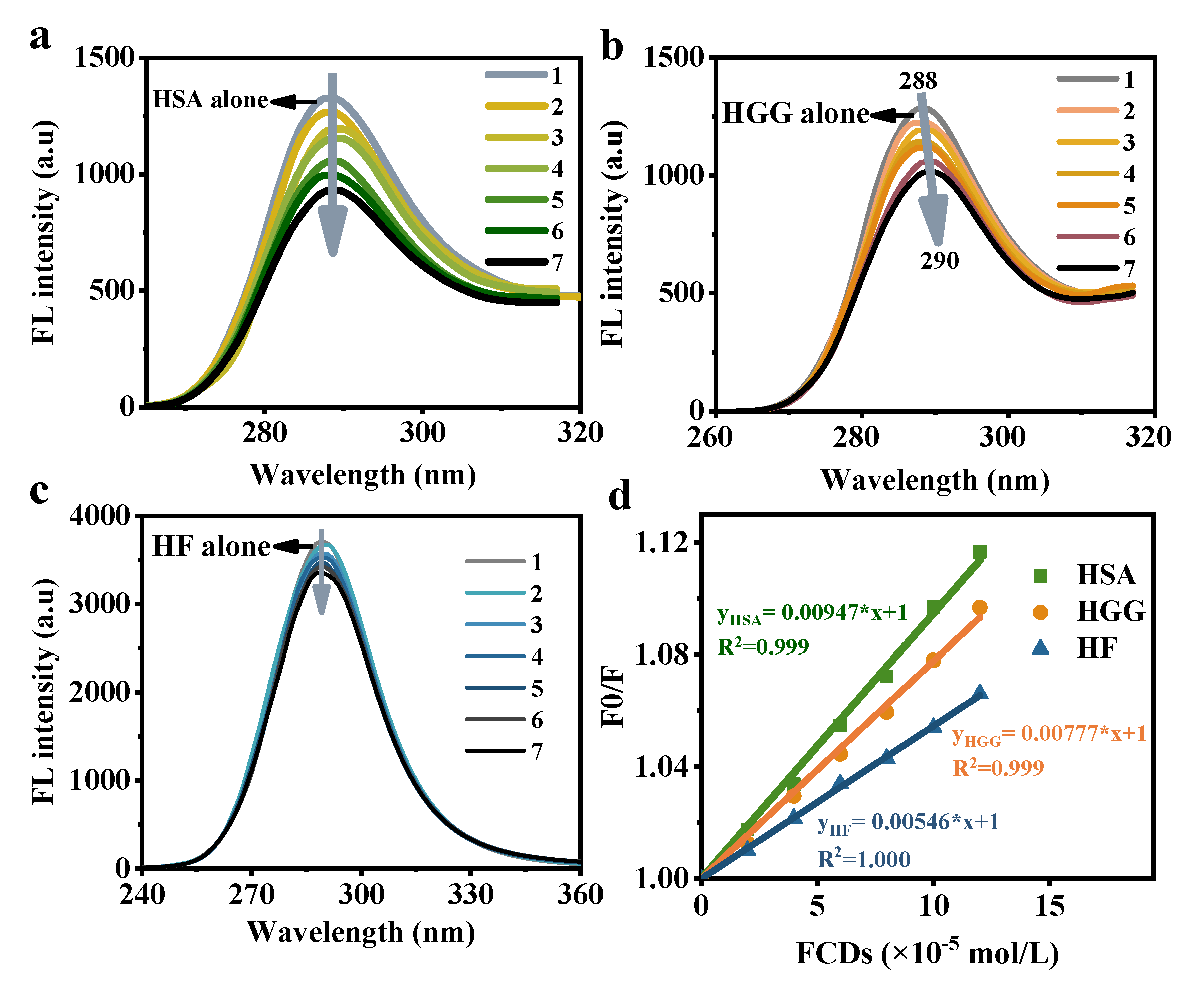
2.4. Fluorescence Analysis
2.5. Adsorption Measurement
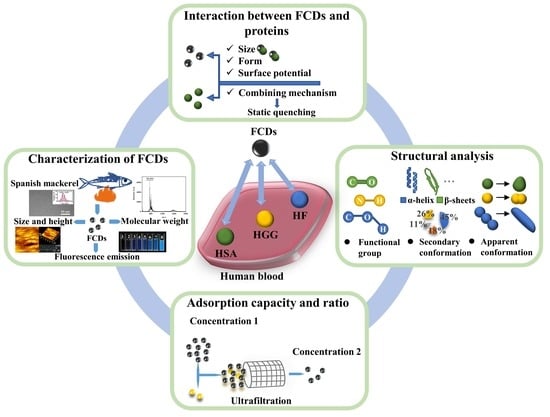
3. Results and Discussion
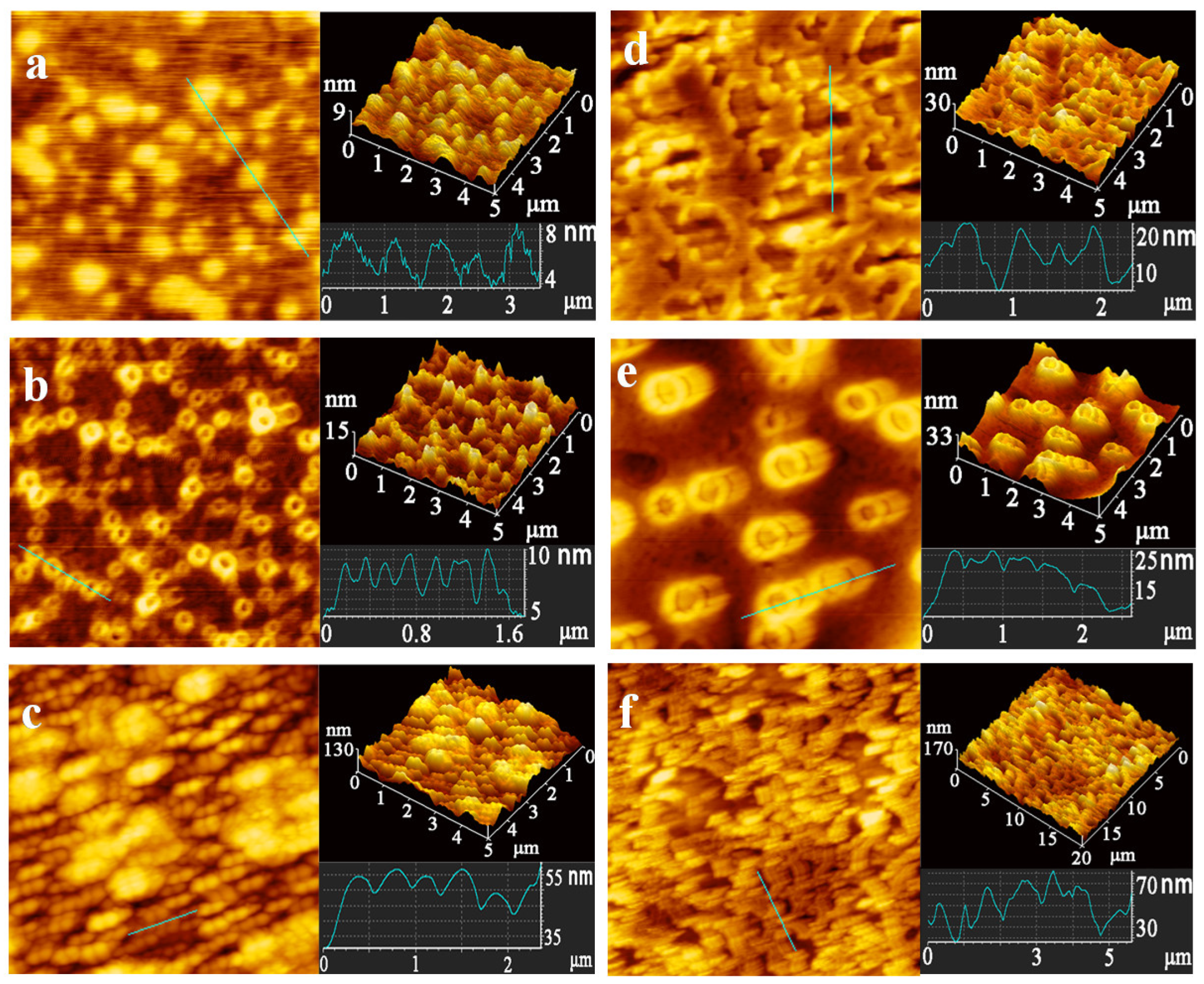
3.1. Characterization of FCDs
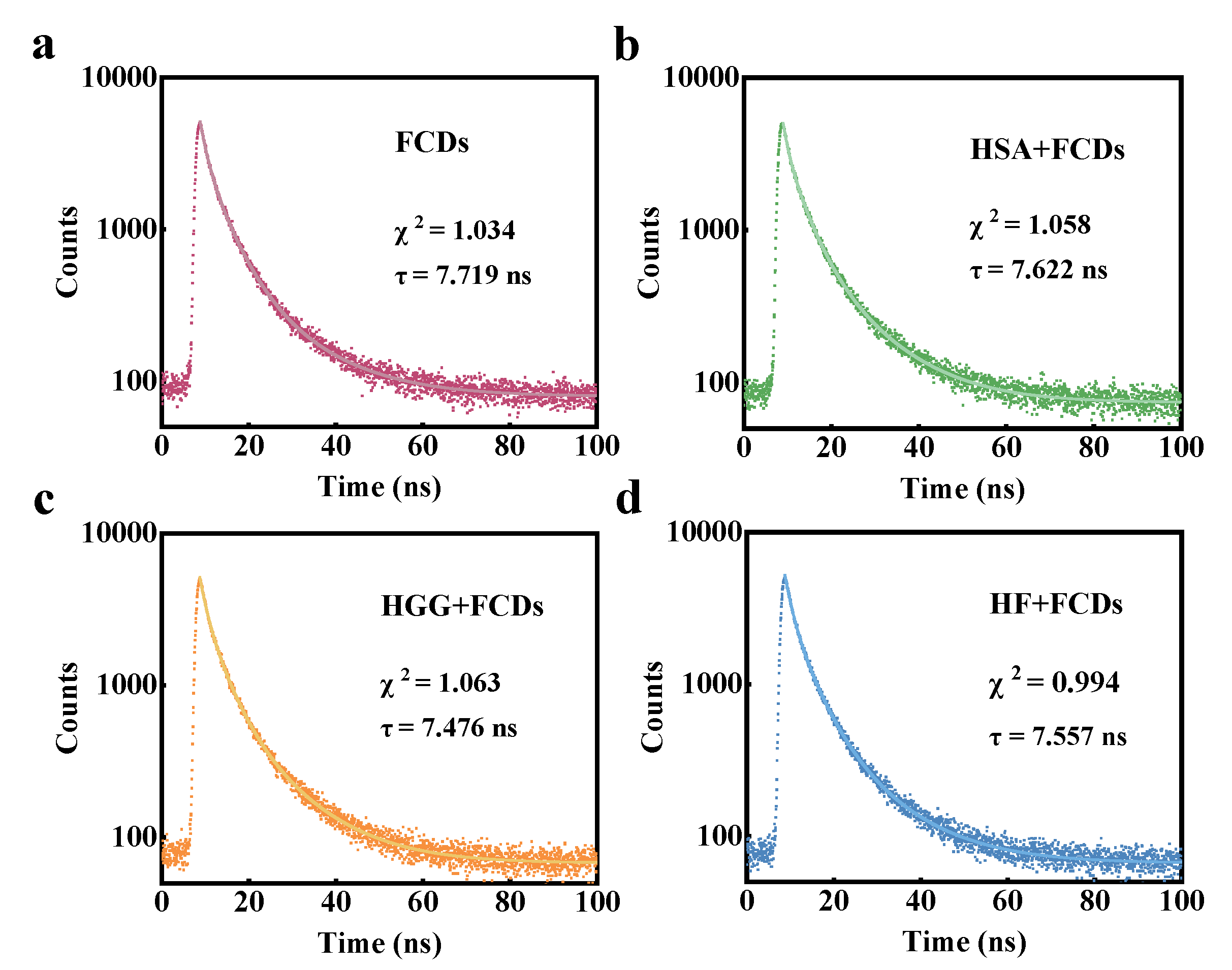
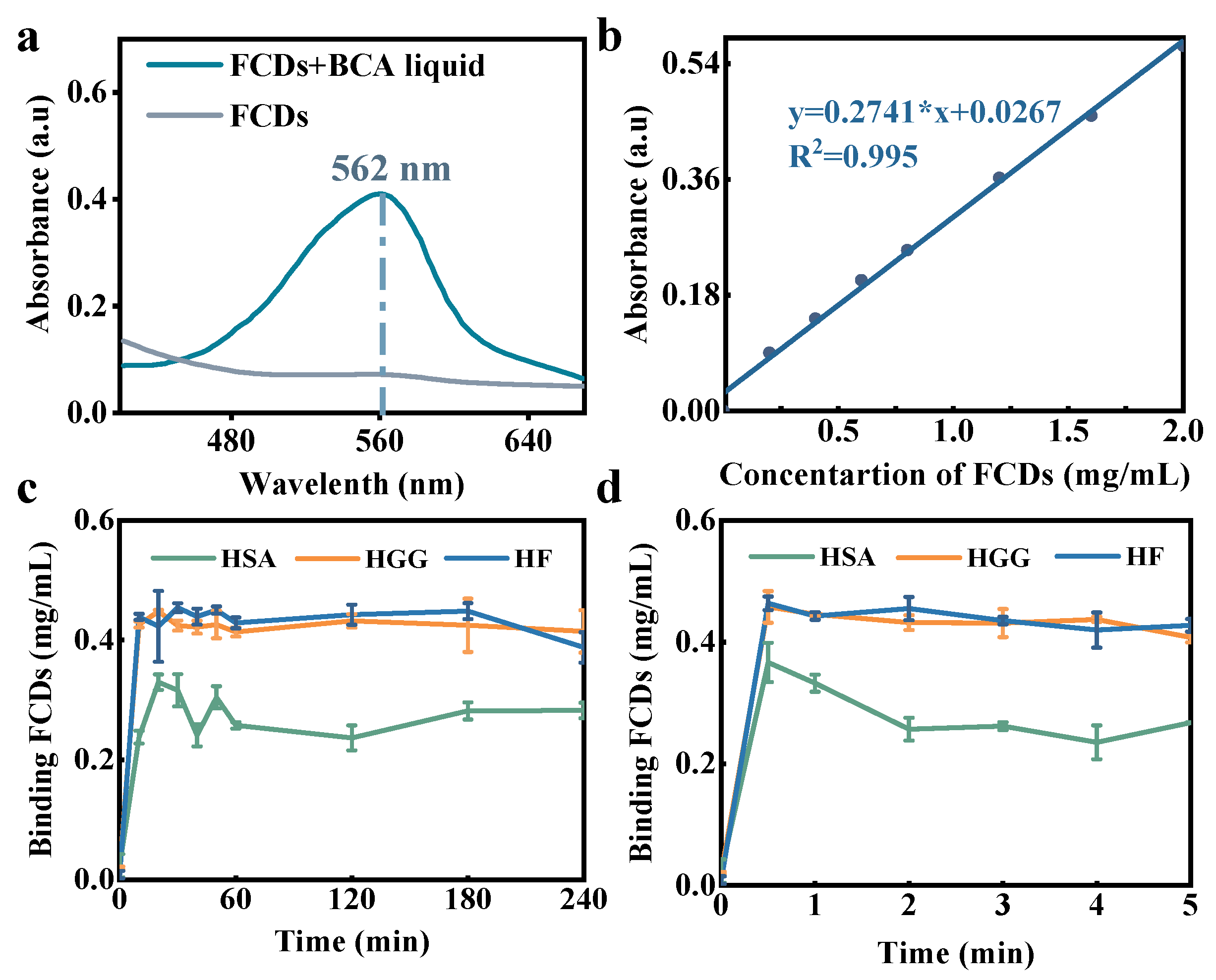
3.2. Interaction between FCDs and Proteins
3.3. Structural Analysis
3.4. Adsorption Capacity and Ratio
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Su, W.; Tan, M. Endogenous fluorescence carbon dots derived from food items. Innovation 2020, 1, 100009. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001, 90, 1927–1936. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Y.; Wang, H.; Liu, S.; Song, L.; Li, S.; Tan, M. Carbon quantum dots from roasted Atlantic salmon (Salmo salar L.): Formation, biodistribution and cytotoxicity. Food Chem. 2019, 293, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Liu, K.; Qiao, F.; Song, Y.; Tan, M. Biocompatible fluorescent carbon dots derived from roast duck for in vitro cellular and in vivo C. elegans bio-imaging. Methods 2019, 168, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, H.; Zhang, L.; Lai, B.; Liu, K.; Tan, M. Protein corona formation of human serum albumin with carbon quantum dots from roast salmon. Food Funct. 2020, 11, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Song, Y.; Tan, M. Toxicity alleviation of carbon dots from roast beef after forming protein coronas with human serum albumin. J. Agric. Food Chem. 2020, 68, 9789–9795. [Google Scholar] [CrossRef]
- Nanjappa, V.; Thomas, J.K.; Marimuthu, A.; Muthusamy, B.; Radhakrishnan, A.; Sharma, R.; Khan, A.A.; Balakrishnan, L.; Sahasrabuddhe, N.A.; Kumar, S.; et al. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 2013, 42, D959–D965. [Google Scholar] [CrossRef] [Green Version]
- Nair, M. The Circulatory System. Fundamentals of Anatomy and Physiology for Student Nurses, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2016. [Google Scholar]
- Wimuktiwan, P.; Shiowatana, J.; Siripinyanond, A. Investigation of silver nanoparticles and plasma protein association using flow field-flow fractionation coupled with inductively coupled plasma mass spectrometry (FlFFF-ICP-MS). J. Anal. At. Spectrom. 2015, 30, 245–253. [Google Scholar] [CrossRef]
- Busher, J.T. Serum albumin and globulin. In Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworth: Boston, MA, USA, 1990; Volume 3, pp. 497–499. [Google Scholar]
- Melillo, A. Applications of serum protein electrophoresis in exotic pet medicine. Vet. Clin. N. Am. Exot. Anim. Pract. 2013, 16, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Imbach, P. Immune thrombocytopenic purpura and intravenous immunoglobulin. Cancer 1991, 68, 1422–1425. [Google Scholar] [CrossRef]
- Samuelsson, A.; Towers, T.L.; Ravetch, J.V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001, 291, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Herrick, S.; Blanc-Brude, O.; Gray, A.; Laurent, G. Fibrinogen. Int. J. Biochem. Cell Biol. 1999, 31, 741–746. [Google Scholar] [CrossRef]
- Zhdanova, N.; Shirshin, E.A.; Maksimov, E.; Panchishin, I.M.; Saletsky, A.M.; Fadeev, V.V. Tyrosine fluorescence probing of the surfactant-induced conformational changes of albumin. Photochem. Photobiol. Sci. 2015, 14, 897–908. [Google Scholar] [CrossRef]
- Cong, S.; Bi, J.; Song, X.; Yu, C.; Tan, M. Ultrasmall fluorescent nanoparticles derived from roast duck: Their physicochemical characteristics and interaction with human serum albumin. Food Funct. 2018, 9, 2490–2495. [Google Scholar] [CrossRef]
- Liu, R.; Liu, K.; Tan, M. Nanocorona formation between foodborne nanoparticles extracted from roast squid and human serum albumin. J. Agric. Food Chem. 2019, 67, 10470–10480. [Google Scholar] [CrossRef]
- Carter, D.C.; He, X.M.; Munson, S.; Twigg, P.D.; Gernert, K.M.; Broom, M.B.; Miller, T.Y. Three-dimensional structure of human serum albumin. Science 1989, 244, 1195–1198. [Google Scholar] [CrossRef] [Green Version]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. Des. Sel. 1999, 12, 439–446. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumakura, M.; Yoshida, M.; Asano, M. Adsorption of γ-globulin on polymer surfaces having various chemical and physical structures. J. Appl. Polym. Sci. 1990, 41, 177–184. [Google Scholar] [CrossRef]
- Finlayson, J.S.; Mosesson, M.W. Heterogeneity of human fibrinogen. Biochemistry 1963, 2, 42–46. [Google Scholar] [CrossRef]
- Mosesson, M.; Finlayson, J.; Umfleet, R. Human fibrinogen heterogeneities: III. Identification of γ chain variants. J. Biol. Chem. 1972, 247, 5223–5227. [Google Scholar] [CrossRef]
- Mosesson, M. Fibrinogen structure and fibrin clot assembly. Semin. Thromb. Hemost. 1998, 24, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, H.A. The thrombin–fibrinogen interaction. Biophys. Chem. 2004, 112, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005, 70, 247–299. [Google Scholar]
- Krakow, W.; Endres, G.; Siegbl, B.; Schekaga, H. An electron microscopic investigation of the polymerization of bovine fibrin monomer. J. Mol. Biol. 1972, 71, 95–103. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Il’Ichev, Y.V.; Perry, A.J.L.; Simon, J.D. Interaction of ochratoxin a with human serum albumin. Preferential binding of the dianion and Ph effects. J. Phys. Chem. B 2001, 106, 452–459. [Google Scholar] [CrossRef]
- Li, X.; Wang, S. Study on the interaction of (+)-catechin with human serum albumin using isothermal titration calorimetry and spectroscopic techniques. New J. Chem. 2015, 39, 386–395. [Google Scholar] [CrossRef]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Parham, H.; Saeed, S. Resonance Rayleigh scattering method for determination of ethion using silver nanoparticles as probe. Talanta 2015, 131, 570–576. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Masters, B.R. Principles of fluorescence spectroscopy, third edition. J. Biomed. Opt. 2008, 13, 029901. [Google Scholar] [CrossRef]
- Peng, X.; Wang, X.; Qi, W.; Su, R.; He, Z. Affinity of rosmarinic acid to human serum albumin and its effect on protein con-formation stability. Food Chem. 2016, 192, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, R.; Zhang, H. Potential enzyme toxicity of oxytetracycline to catalase. Sci. Total Environ. 2010, 408, 5399–5404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Yang, J.; Dai, L.; Ji, N.; Xiong, L.; Sun, Q. Interactions of surface-functionalized starch nanoparticles with pepsin and trypsin in simulated gastrointestinal fluids. J. Agric. Food Chem. 2020, 68, 10174–10183. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M. Interactions of hemin with bovine serum albumin and human hemoglobin: A fluorescence quenching study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 23–32. [Google Scholar] [CrossRef]
- Yan, F.; Zou, Y.; Wang, M.; Mu, X.; Yang, N.; Chen, L. Highly photoluminescent carbon dots-based fluorescent chemosensors for sensitive and selective detection of mercury ions and application of imaging in living cells. Sens. Actuators B Chem. 2014, 192, 488–495. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, L.; Pan, J. Probing the binding of the flavonoid diosmetin to human serum albumin by multispectroscopic techniques. J. Agric. Food Chem. 2012, 60, 2721–2729. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, F.; Lu, D.; Liu, W.; Zhou, Q.; Jiang, G. Influence of the surface functional group density on the car-bon-nanotube-induced α-chymotrypsin structure and activity alterations. ACS Appl. Mater. Interf. 2015, 7, 18880–18890. [Google Scholar] [CrossRef]
- Arakawa, H.; Umemura, K.; Ikai, A. Protein images obtained by STM, AFM and TEM. Nature 1992, 358, 171–173. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Gryzunov, I.A.; Dobretsov, G.E.; Komarova, M.N. Size of a human serum albumin molecule in solution. Biofizika 2001, 46, 423–427. [Google Scholar] [PubMed]
- Arwin, H. Optical properties of thin layers of bovine serum albumin, γ-globulin, and hemoglobin. Appl. Spectrosc. 1986, 40, 313–318. [Google Scholar] [CrossRef]
- Bae, Y.M.; Oh, B.-K.; Lee, W.; Lee, W.H.; Choi, J.-W. Study on orientation of immunoglobulin G on protein G layer. Biosens. Bioelectron. 2005, 21, 103–110. [Google Scholar] [CrossRef]
- Fuss, C.; Palmaz, J.C.; Sprague, E.A. Fibrinogen: Structure, function, and surface interactions. J. Vasc. Interv. Radiol. 2001, 12, 677–682. [Google Scholar] [CrossRef]
- Marchin, K.L.; Berrie, C.L. Conformational changes in the plasma protein fibrinogen upon adsorption to graphite and mica investigated by atomic force microscopy. Langmuir 2003, 19, 9883–9888. [Google Scholar] [CrossRef]
- Evans-Nguyen, K.M.; Fuierer, R.R.; Fitchett, B.D.; Tolles, L.R.; Conboy, J.C.; Schoenfisch, M.H. Changes in adsorbed fibrinogen upon conversion to fibrin. Langmuir 2006, 22, 5115–5121. [Google Scholar] [CrossRef]
- Kollman, J.M.; Pandi, L.; Sawaya, M.R.; Riley, M.; Doolittle, R.F. Crystal structure of human fibrinogen. Biochemistry 2009, 48, 3877–3886. [Google Scholar] [CrossRef]
- Fowler, W.E.; Erickson, H.P. Trinodular structure of fibrinogen: Confirmation by both shadowing and negative stain electron microscopy. J. Mol. Biol. 1979, 134, 241–249. [Google Scholar] [CrossRef]
- Zhang, H.; Burnum-Johnson, K.; Luna, M.L.; Petritis, B.O.; Kim, J.-S.; Qian, W.-J.; Moore, R.J.; Heredia-Langner, A.; Webb-Robertson, B.-J.; Thrall, B.D.; et al. Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size. Proteomics 2011, 11, 4569–4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orco, D.; Lundqvist, M.; Oslakovic, C.; Cedervall, T.; Linse, S. Modeling the time evolution of the nanoparticle-protein corona in a body fluid. PLoS ONE 2010, 5, e10949. [Google Scholar] [CrossRef] [PubMed] [Green Version]




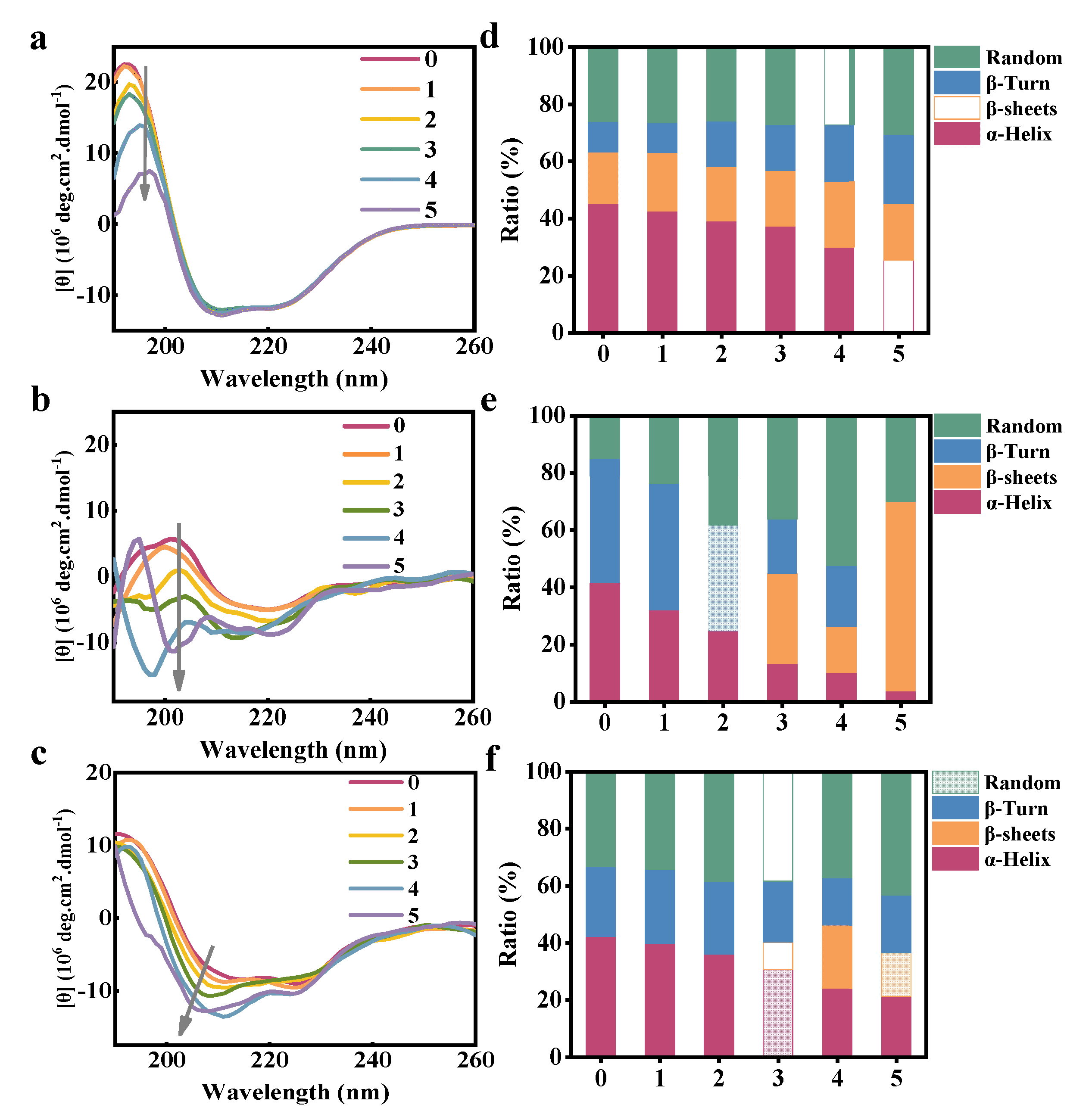
| Ratio (%) | FCDs (mmol/L) | 0.00 | 0.02 | 0.04 | 0.06 | 0.08 | 0.10 |
|---|---|---|---|---|---|---|---|
| HSA | α-Helix | 45.0 | 42.6 | 39.1 | 37.3 | 29.9 | 25.3 |
| β-sheet | 18.2 | 20.4 | 19.0 | 19.4 | 23.0 | 19.8 | |
| β-Turn | 10.8 | 10.7 | 16.0 | 16.2 | 19.8 | 24.2 | |
| Random | 26.0 | 26.2 | 26.0 | 27.1 | 27.2 | 30.7 | |
| HGG | α-Helix | 41.3 | 32.0 | 24.6 | 13.1 | 10.1 | 3.7 |
| β-sheet | 0.0 | 0.0 | 0.0 | 31.6 | 16.2 | 66.3 | |
| β-Turn | 43.6 | 44.3 | 37.0 | 19.1 | 21.2 | 0.0 | |
| Random | 15.1 | 23.8 | 38.3 | 36.2 | 52.5 | 30.0 | |
| HF | α-Helix | 42.2 | 39.7 | 36.0 | 30.6 | 23.9 | 21.1 |
| β-sheet | 0.0 | 0.0 | 0.0 | 9.6 | 22.3 | 15.4 | |
| β-Turn | 24.4 | 26.0 | 25.4 | 21.4 | 16.6 | 20.2 | |
| Random | 33.4 | 34.3 | 38.6 | 38.3 | 37.2 | 43.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, G.; Song, Y.; Liu, K.; Tan, M. Interaction of Carbon Dots from Grilled Spanish Mackerel with Human Serum Albumin, ?-Globulin and Fibrinogen. Foods 2021, 10, 2336. https://doi.org/10.3390/foods10102336
Cui G, Song Y, Liu K, Tan M. Interaction of Carbon Dots from Grilled Spanish Mackerel with Human Serum Albumin, ?-Globulin and Fibrinogen. Foods. 2021; 10(10):2336. https://doi.org/10.3390/foods10102336
Chicago/Turabian StyleCui, Guoxin, Yukun Song, Kangjing Liu, and Mingqian Tan. 2021. "Interaction of Carbon Dots from Grilled Spanish Mackerel with Human Serum Albumin, ?-Globulin and Fibrinogen" Foods 10, no. 10: 2336. https://doi.org/10.3390/foods10102336
APA StyleCui, G., Song, Y., Liu, K., & Tan, M. (2021). Interaction of Carbon Dots from Grilled Spanish Mackerel with Human Serum Albumin, ?-Globulin and Fibrinogen. Foods, 10(10), 2336. https://doi.org/10.3390/foods10102336