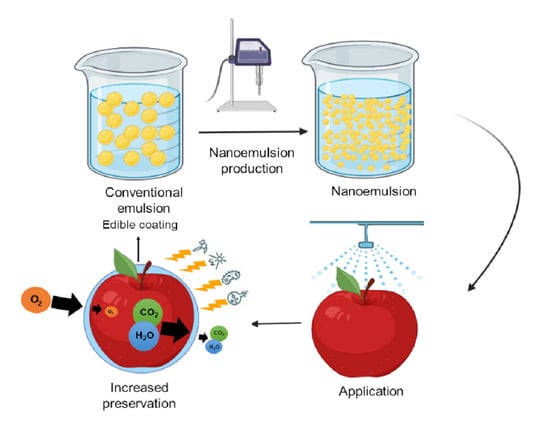
Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation
Abstract
1. Introduction
2. Edible Coatings—An Overview
3. Methods to Apply Edible Coatings
4. Nanomaterials in Edible Coatings
5. Fundamentals of Nanoemulsions
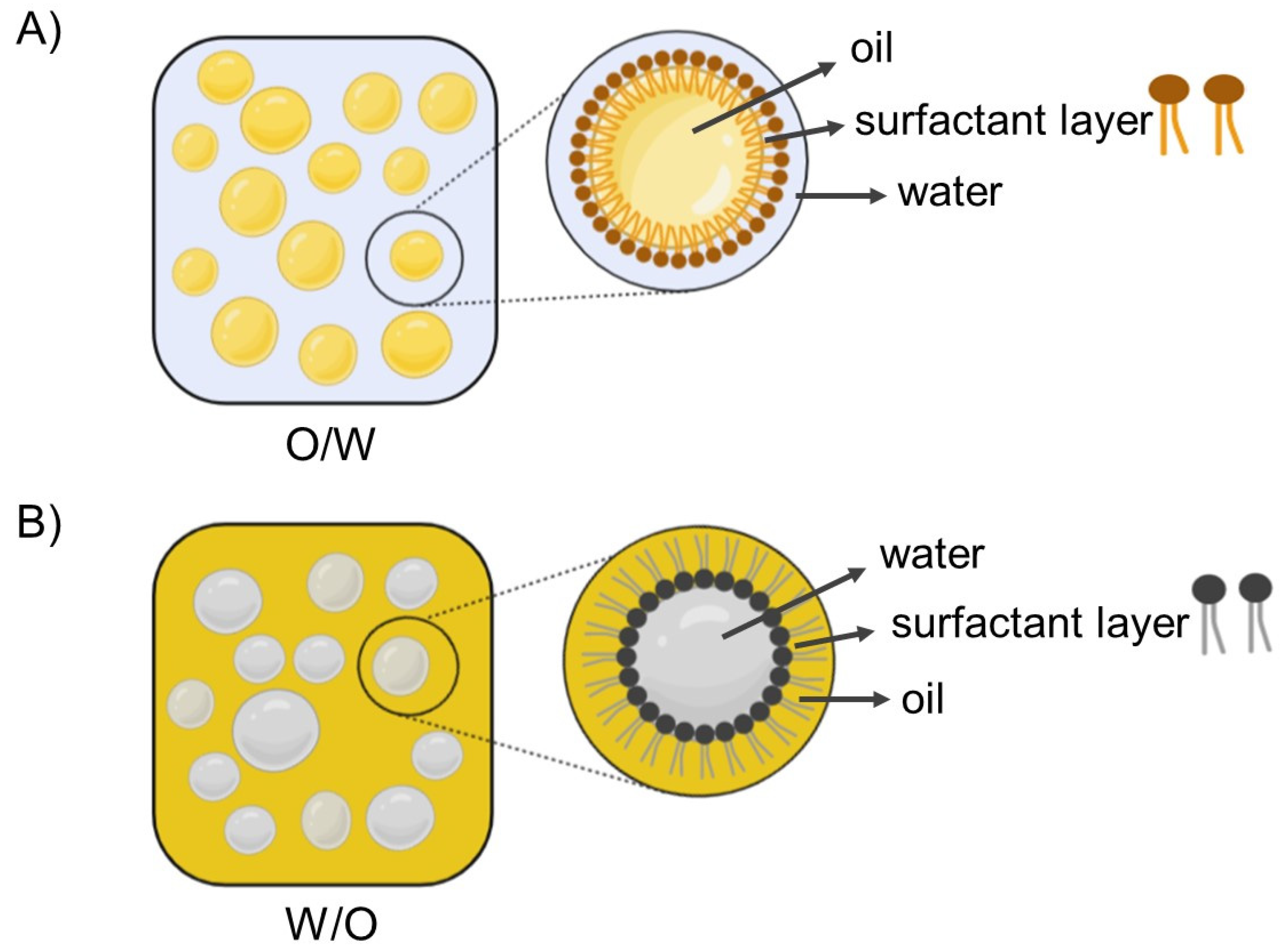
5.1. Nanoemulsions and Production Methods
5.2. Surfactants
6. Plant-Based Nanoemulsions as Edible Coatings on Fruits and Vegetables Postharvest
6.1. Coatings Based on Essential Oil Nanoemulsions
6.2. Coatings Based on Plant-Based Wax Nanoemulsions
7. Trends in Materials Based on Nanoemulsions with Potential for Application in the Preservation of Fruits and Vegetables
8. Potential Toxicity, Limitations, and Regulatory Aspects of Nanoemulsions
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Dhandevi, P.; Jeewon, R. Fruit and vegetable intake: Benefits and progress of nutrition education interventions-narrative review article. Iran. J. Public Health 2015, 44, 1309–1321. [Google Scholar]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Mattoo, A.K.; Handa, A.K. Ripening and senescence of fleshy fruits. In Postharvest Biology and Nanotechnology; Paliyath, G., Subramanian, J., Lim, L.-T., Subramanian, K.S., Handa, A.K., Mattoo, A.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 15–51. [Google Scholar]
- Nunes, C.N.; Emond, J.-P. Relationship between weight loss and visual quality of fruits and vegetables. Proc. Fla. State Hort. Soc. 2007, 120, 235–245. [Google Scholar]
- Baldwin, E.A. Edible coatings for fresh fruits and vegetables: Past, present, and future. Edible Coat. Film. Improv. Food Qual. 1994, 1, 25. [Google Scholar]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanoemulsions as edible coatings. Curr. Opin. Food Sci. 2017, 15, 43–49. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Hagenmaier, R.H. Alternatives to shellac coatings provide comparable gloss, internal gas modification, and quality for ‘Delicious’ apple fruit. HortScience 2002, 37, 559–563. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.-L.; Perez-Gago, M.-B.; Goodner, K.; Plotto, A. A new composite coating containing HPMC, beeswax, and Shellac for ‘Valencia’ oranges and ‘Marisol’ tangerines. Proc. Fla. State Hort. Soc. 2007, 120, 228–234. [Google Scholar]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; del Río, M.Á.; Palou, L. Effect of antifungal hydroxypropyl methylcellulose (HPMC)–lipid edible composite coatings on postharvest decay development and quality attributes of cold-stored ‘Valencia’ oranges. Postharvest Biol. Technol. 2009, 54, 72–79. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf-life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Hasan, S.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced edible coatings to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible nanoemulsions as carriers of active ingredients: A review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef]
- Jin, W.; Xu, W.; Liang, H.; Li, Y.; Liu, S.; Li, B. Nanoemulsions for food: Properties, production, characterization, and applications. In Emulsions; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–36. [Google Scholar]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible coating of fruits and vegetables: A review. Int. J. Sci. Res. Mod. Educ. 2016, I, 188–204. [Google Scholar]
- Ghoora, M.D.; Srividya, N. Effect of packaging and coating technique on postharvest quality and shelf life of Raphanus sativus L. and Hibiscus sabdariffa L. microgreens. Foods 2020, 9, 653. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Ference, C.; Plotto, A.; Bai, J.; Wood, D.; Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Nano-and Micro-Carnauba Wax Emulsions versus Shellac Protective Coatings on Postharvest Citrus Quality. J. Am. Soc. Hortic. Sci. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Eddin, A.S.; Ibrahim, S.A.; Tahergorabi, R. Egg quality and safety with an overview of edible coating application for egg preservation. Food Chem. 2019, 296, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Gozalbo, A.M.; Sun, X.; Plotto, A.; Bai, J.; de Assis, O.; Ferreira, M.; Baldwin, E. Effect of mono and bilayer of carnauba wax based nano-emulsion and HPMC coatings on popst-harvest quality of ‘redtainung’ papaya. In Proceedings of the Embrapa Instrumentação-Artigo em anais de congresso (ALICE), São Carlos, Brazil, 3–5 December 2019. [Google Scholar]
- De Oliveira Filho, J.G.; Bezerra, C.C.d.O.N.; Albiero, B.R.; Oldoni, F.C.A.; Miranda, M.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. New approach in the development of edible films: The use of carnauba wax micro-or nanoemulsions in arrowroot starch-based films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- Bodbodak, S.; Moshfeghifar, M. Advances in modified atmosphere packaging of fruits and vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 127–183. [Google Scholar]
- Nor, S.M.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Porat, R.; Fallik, E. Production of off-flavours in fruit and vegetables under fermentative conditions. In Fruit and Vegetable Flavour; Brückner, B., Wyllie, S.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 150–164. [Google Scholar]
- Baldwin, E.A.; Nisperos-Carriedo, M.; Shaw, P.E.; Burns, J.K. Effect of coatings and prolonged storage conditions on fresh orange flavor volatiles, degrees brix, and ascorbic acid levels. J. Agric. Food Chem. 1995, 43, 1321–1331. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Baldwin, E.; Ritenour, M.; Plotto, A.; Bai, J. Evaluation of natural colorants and their application on citrus fruit as alternatives to Citrus Red No. 2. HortScience 2015, 50, 1353–1357. [Google Scholar] [CrossRef]
- Marin, A.; Sun, X.; Miranda, M.; Ference, C.; Baldwin, E.A.; Bai, J.; Ritenour, M.A.; Zhang, J.; Plotto, A. Optimizing Essential Oil Applications to Prevent Postharvest Decay in Strawberries. Proc. Fla. State Hort. Soc 2019, 132, 185–188. [Google Scholar]
- Basaglia, R.R.; Pizato, S.; Santiago, N.G.; de Almeida, M.M.M.; Pinedo, R.A.; Cortez-Vega, W.R. Effect of edible chitosan and cinnamon essential oil coatings on the shelf life of minimally processed pineapple (Smooth cayenne). Food Biosci. 2021, 41, 100966. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; da Cruz Silva, G.; de Aguiar, A.C.; Cipriano, L.; de Azeredo, H.M.C.; Junior, S.B.; Ferreira, M.D. Chemical composition and antifungal activity of essential oils and their combinations against Botrytis cinerea in strawberries. J. Food Meas. Charact. 2021, 15, 1815–1825. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- de Jesús Salas-Méndez, E.; Vicente, A.; Pinheiro, A.C.; Ballesteros, L.F.; Silva, P.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.d.L.V.; Flores-López, M.L.; Villarreal-Quintanilla, J.Á. Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2019, 150, 19–27. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum postharvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Ghidelli, C.; Pérez-Gago, M. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.; Baldwin, E.A.; Bai, J.; Wood, D.; Ference, C.; Sun, X.; Brecht, J.K.; Plotto, A. Edible coatings as carriers of antibrowning compounds to maintain appealing appearance of fresh-cut mango. HortTechnology 2021, 31, 27–35. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Schmid, M.; Müller, K. Effect of dipping and vacuum impregnation coating techniques with alginate based coating on physical quality parameters of cantaloupe melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.; Sasikala, P.; Rajamani, L.; Kavitha, V.; Yazhini, G.; Banu, M.S. Edible films from polysaccharides. Food Sci. Qual. Manag. 2012, 3, 9. [Google Scholar]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of coating application methods on the postharvest quality of cassava. Int. J. Food Sci. 2019, 2019, 2148914. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Liu, Y.; Song, Y.; Wu, T.; Zhang, M. Using soy protein SiOx nanocomposite film coating to extend the shelf life of apple fruit. Int. J. Food Sci. Technol. 2017, 52, 2018–2030. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2020, 101081. [Google Scholar]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; McClements, D.J. Nanoemulsions: An emerging platform for increasing the efficacy of nutraceuticals in foods. Colloids Surf. B Biointerfaces 2020, 194, 111202. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Queste, S.; Salager, J.; Strey, R.; Aubry, J. The EACN scale for oil classification revisited thanks to fish diagrams. J. Colloid Interface Sci. 2007, 312, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, E.; Claesson, P.M. Surface forces and emulsion stability: Chapter 7. In Food Emulsions; Friberg, S.E., Larsson, K., Sjöblom, J., Eds.; CRC Press: New York, NY, USA, 2004; pp. 254–297. [Google Scholar]
- Yao, K.; McClements, D.J.; Xiang, J.; Zhang, Z.; Cao, Y.; Xiao, H.; Liu, X. Improvement of carotenoid bioaccessibility from spinach by co-ingesting with excipient nanoemulsions: Impact of the oil phase composition. Food Funct. 2019, 10, 5302–5311. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D.J. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, D.R. Nanoemulsions based edible coatings with potential food applications. Int. J. Biobased Plast. 2021, 3, 112–125. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanostructured emulsions and nanolaminates for delivery of active ingredients: Improving food safety and functionality. Trends Food Sci. Technol. 2017, 60, 12–22. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.; Jos, A.; Cameán, A. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel edible coating containing essential oil nanoemulsions to prolong the shelf life of vegetable products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157: H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Gundewadi, G.; Rudra, S.G.; Sarkar, D.J.; Singh, D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packag. Shelf Life 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Kim, I.H.; Lee, H.; Kim, J.E.; Song, K.B.; Lee, Y.S.; Chung, D.S.; Min, S.C. Plum coatings of lemongrass oil-incorporating carnauba wax-based nanoemulsion. J. Food Sci. 2013, 78, E1551–E1559. [Google Scholar] [CrossRef]
- Kim, I.-H.; Oh, Y.A.; Lee, H.; Song, K.B.; Min, S.C. Grape berry coatings of lemongrass oil-incorporating nanoemulsion. LWT—Food Sci. Technol. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Pastrana-Castro, L.; Barbosa-Pereira, L.; Rua-Rodríguez, M.L.; Saucedo, S.; Ventura-Sobrevilla, J.M.; Salinas-Jasso, T.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Nanocoating with extract of tarbush to retard Fuji apples senescence. Postharvest Biol. Technol. 2017, 134, 67–75. [Google Scholar] [CrossRef]
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria× ananassa) under commercial storage environment. Food Bioprocess Technol. 2018, 11, 1566–1574. [Google Scholar] [CrossRef]
- Deng, Z.; Jung, J.; Simonsen, J.; Wang, Y.; Zhao, Y. Cellulose nanocrystal reinforced chitosan coatings for improving the storability of postharvest pears under both ambient and cold storages. J. Food Sci. 2017, 82, 453–462. [Google Scholar] [CrossRef]
- Kumar, R. Health effects of morpholine based coating for fruits and vegetables. Int. J. Med. Res. Health Sci. 2016, 5, 32–38. [Google Scholar]
- Kumar, R.; Kapur, S. Morpholine: A glazing agent for fruits and vegetables coating/waxing. Int. J. Sci. Technol. Eng. 2016, 2, 694–697. [Google Scholar]
- Ohnishi, T. Studies on mutagenicity of the food additive morpholine (fatty acid salt). Nippon Eiseigaku Zasshi Jpn. J. Hyg. 1984, 39, 729–748. [Google Scholar] [CrossRef][Green Version]
- Mirvish, S.; Salmasi, S.; Cohen, S.; Patil, K.; Mahboubi, E. Liver and forestomach tumors and other forestomach lesions in rats treated with morpholine and sodium nitrite, with and without sodium ascorbate. J. Natl. Cancer Inst. 1983, 71, 81–85. [Google Scholar]
- Health Canada. ARCHIVED—A Summary of the Health Hazard Assessment of Morpholine in Wax Coatings of Apples. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/information-product/summary-health-hazard-assessment-morpholine-coatings-apples.html (accessed on 29 September 2021).
- Hagenmaier, R.D.; Baker, R.A. Edible coatings from morpholine-free wax microemulsions. J. Agric. Food Chem. 1997, 45, 349–352. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.; Mditshwa, A.; Tesfay, S. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.; Albiero, B.; Cipriano, L.; Bezerra, C.d.O.; Oldoni, F.; Egea, M.; de Azeredo, H.; Ferreira, M. Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511. [Google Scholar] [CrossRef]
- Qi, W.; Li, T.; Zhang, Z.; Wu, T. Preparation and characterization of oleogel-in-water pickering emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2021, 110, 106206. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C. Properties of starch nanoparticle obtained by ultrasonication and high pressure homogenization for developing carotenoids-enriched powder and Pickering nanoemulsion. Innov. Food Sci. Emerg. Technol. 2021, 74, 102822. [Google Scholar] [CrossRef]
- Nandy, M.; Lahiri, B.; Philip, J. Inter-droplet force between magnetically polarizable Pickering oil-in-water nanoemulsions stabilized with γ-Al2O3 nanoparticles: Role of electrostatic and electric dipolar interactions. J. Colloid Interface Sci. 2021, 607, 1671–1686. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, Y.; Niu, Y.; Kou, X. Cyclodextrin supermolecules as excellent stabilizers for Pickering nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124367. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Liu, P.; Ma, J.; Zhang, W.; Liu, C.; Simion, D. Novel fabrication of stable Pickering emulsion and latex by hollow silica nanoparticles. J. Colloid Interface Sci. 2019, 553, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- López-Monterrubio, D.; Lobato-Calleros, C.; Vernon-Carter, E.; Alvarez-Ramirez, J. Influence of β-carotene concentration on the physicochemical properties, degradation and antioxidant activity of nanoemulsions stabilized by whey protein hydrolyzate-pectin soluble complexes. LWT 2021, 143, 111148. [Google Scholar] [CrossRef]
- Wani, T.; Masoodi, F.; Jafari, S.M.; McClements, D. Safety of nanoemulsions and their regulatory status. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 613–628. [Google Scholar]
- McClements, D.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, R.; Goel, S.; Uppal, S.; Bhatia, A.; Mehta, S. Physiochemical and cytotoxicity study of TPGS stabilized nanoemulsion designed by ultrasonication method. Ultrason. Sonochem. 2017, 34, 173–182. [Google Scholar] [CrossRef]
- Marchese, E.; D’onofrio, N.; Balestrieri, M.; Castaldo, D.; Ferrari, G.; Donsì, F. Bergamot essential oil nanoemulsions: Antimicrobial and cytotoxic activity. Z. Nat. C 2020, 75, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hort, M.; Alves, B.d.S.; Ramires Junior, O.; Falkembach, M.; Araújo, G.d.M.; Fernandes, C.; Tavella, R.; Bidone, J.; Dora, C.; da Silva Júnior, F. In vivo toxicity evaluation of nanoemulsions for drug delivery. Drug Chem. Toxicol. 2021, 44, 585–594. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Moniz, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.; Mech, A.; Pesudo, L. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef] [PubMed]
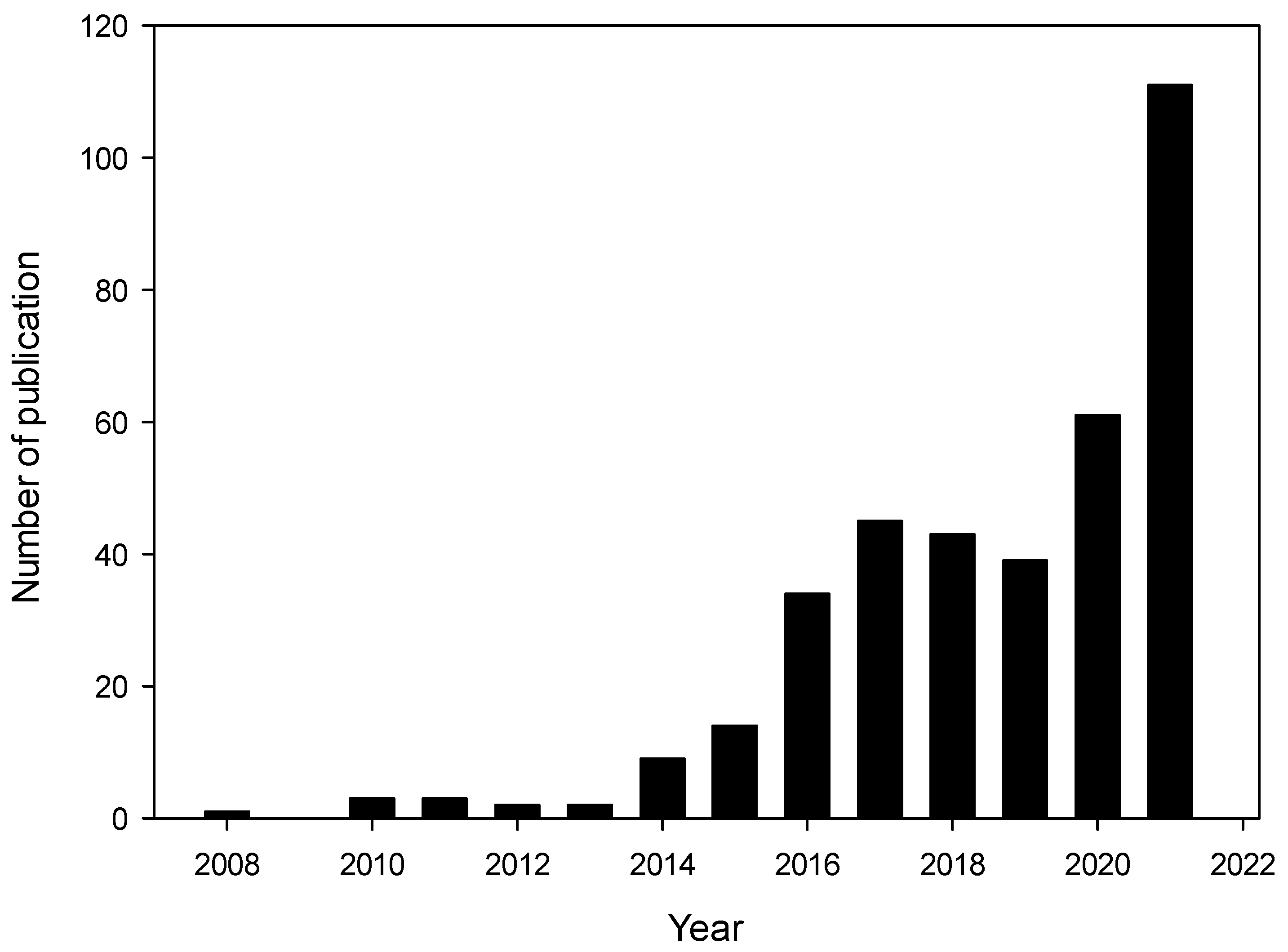


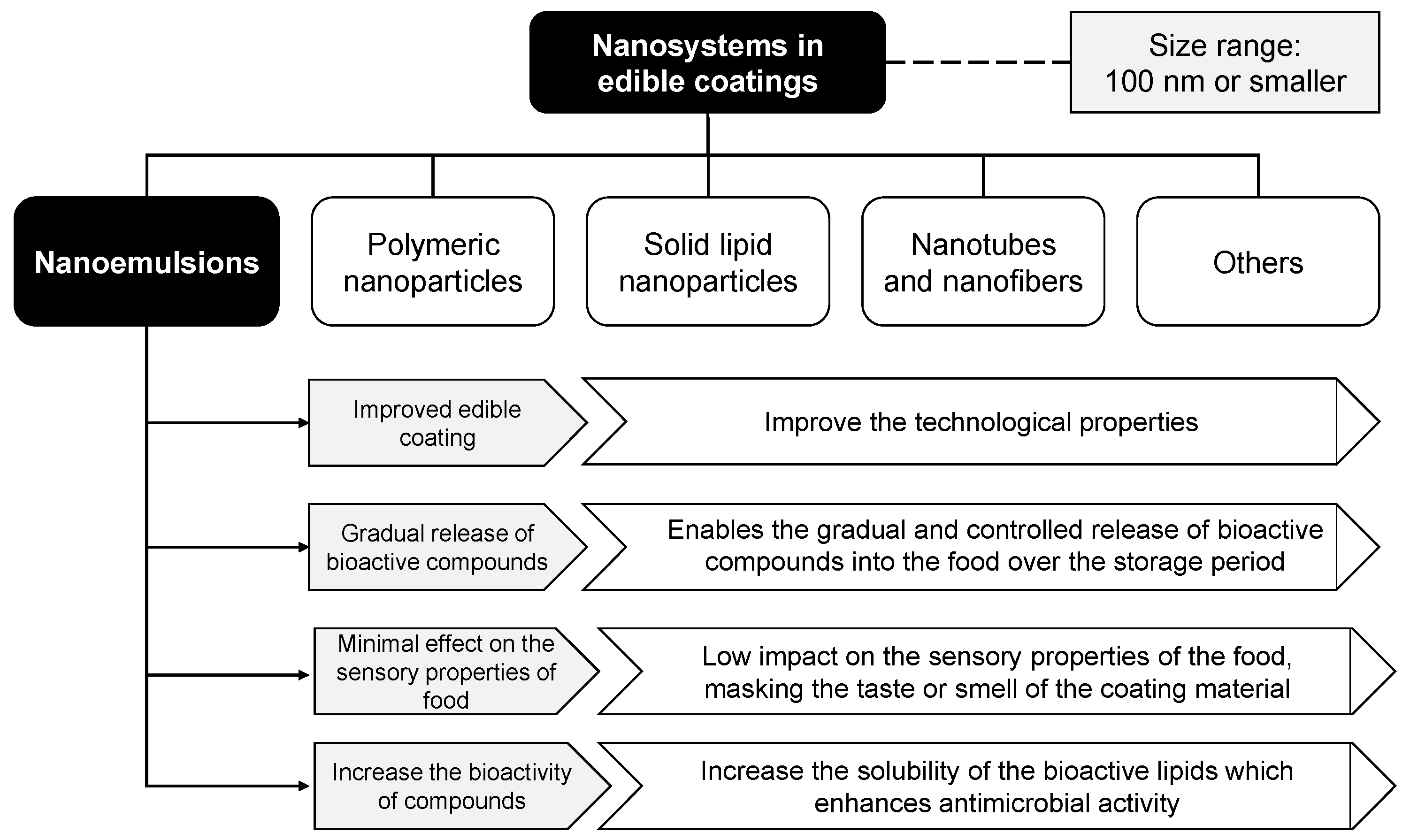

| Material | Main Matrices | Positive Points | Negative Points | References |
|---|---|---|---|---|
| Polysaccharide | Starch, chitosan, alginate, cellulose, and its derivatives, and pectin | Good gas and mechanical barrier properties | Poor moisture barrier due to hydrophilic nature | [21,22] |
| Lipid | Animal, vegetable waxes and resins, vegetable oil, and fatty acids | Good moisture barrier properties with a shiny appearance | Poor mechanical and gas barrier properties | [18,23,24] |
| Protein | Gelatin, casein, whey protein, zein, soy protein, myofibrillar protein, and quinoa protein | Good gas barrier properties without anaerobic conditions | Brittle and susceptible to cracking | [25] |
| Composite | Combination of polysaccharide and/or protein with lipids | Good moisture and gas barrier properties | Formation of non-homogeneous emulsion | [26,27,28,29] |
| Matrix | Bioactive Substance or Lipid Compound | Production Technique | Functionality | Fruit or Vegetable | Reference |
|---|---|---|---|---|---|
| Modified chitosan | Lemon, mandarin, oregano, or clove essential oils | High-pressure homogenization (HPH) | Increase the antimicrobial activity of the essential oil and improve the homogeneity and stability of the emulsion | Arugula leaf (Eruca sativa) | [72] |
| Chitosan | Carvacrol, bergamot, mandarin, and lemon essential oils | High-pressure homogenization | Increase the antimicrobial activity of essential oils | Green beans (Phaseolus vulgaris) | [73] |
| Sodium alginate | Basil essential oil | Ultrasound | Increase the antimicrobial activity of essential oil | Okra (Abelmoschus esculentus) | [74] |
| Pullulan | Cinnamon essential oil | Ultrasound | Improve the distribution of oil in the matrix and increase its antimicrobial activity | Strawberry (Fragaria × ananassa) | [75] |
| Carnauba wax | Lemongrass essential oil | Dynamic high pressure | Increase the antimicrobial activity of the essential oil and improve the homogeneity and stability of the emulsion | Plums (Prunus salicina) | [76] |
| Carnauba wax | Lemongrass essential oil | High shear probe and high-pressure dynamic processing (DHP) | Increase the antimicrobial activity of essential oil | Grape berry (Vitis labruscana Bailey) | [77] |
| Candelilla wax | Extract of tarbush | High-speed stirrer | Improved the wettability of the nanocoating on the Fuji apple surface | Fuji apple (Malus domestica ‘Fuji) | [78] |
| Quinoa protein/chitosan | Thymol | 1200 rpm agitation | Increase the antimicrobial activity of the active compound and improve dispersion in the matrix | Strawberry (Fragaria × ananassa) | [79] |
| Sodium alginate | Lemongrass essential oil | Microfluidization | Improve the stability of the emulsion and increase the antimicrobial activity of the essential oil | Fresh-cut Fuji apples (Malus domestica ‘Fuji) | [59] |
| Hydroxypropyl methylcellulose | Carnauba wax nano-emulsion | High-pressure homogenization (HPH) and mechanical stirring | Reduce gas permeability and moisture loss | ‘Redtainung’ Papaya (Carica papaya) | [28] |
| Sodium alginate | Citral | Ultrasound | Improve the dispersion of the active compound in the matrix and increase its antimicrobial activity | Fresh cut pineapples (Ananas comosus) | [60] |
| Carnauba wax | Oleic acid and Carnauba wax | High-pressure homogenization (HPH) | Improve optical properties, and emulsion stability | ‘Nova’ mandarins (Citrus reticulata) and ‘Unique’ tangors (C. reticulata C. sinensis) | [18] |
| Chitosan | Cellulose nanocrystal and oleic acid | Ultra turrax homogenizer | Increase coating stability at high humidity, adhesion on fruit surface and delayed ripening of pears | Bartlett pears (Pyrus communis) | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Filho, J.G.; Miranda, M.; Ferreira, M.D.; Plotto, A. Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods 2021, 10, 2438. https://doi.org/10.3390/foods10102438
de Oliveira Filho JG, Miranda M, Ferreira MD, Plotto A. Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods. 2021; 10(10):2438. https://doi.org/10.3390/foods10102438
Chicago/Turabian Stylede Oliveira Filho, Josemar Gonçalves, Marcela Miranda, Marcos David Ferreira, and Anne Plotto. 2021. "Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation" Foods 10, no. 10: 2438. https://doi.org/10.3390/foods10102438
APA Stylede Oliveira Filho, J. G., Miranda, M., Ferreira, M. D., & Plotto, A. (2021). Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods, 10(10), 2438. https://doi.org/10.3390/foods10102438