Rheological and Pipe Flow Properties of Chocolate Masses at Different Temperatures
Abstract
:1. Introduction
- Chocolate masses have a plastic property with shear thinning behaviour (pseudoplastic with a yield stress).
- With an increasing temperature, the plastic viscosity or the consistency (depends on used rheological model) and thixotropy of the chocolate masses decreases.
2. Materials and Methods
2.1. Samples of Chocolate Masses
2.2. Rheological Measurements
2.3. Hysteresis Loop Test
2.4. Mathematical Modelling
2.5. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties
3.2. Hysteresis Area
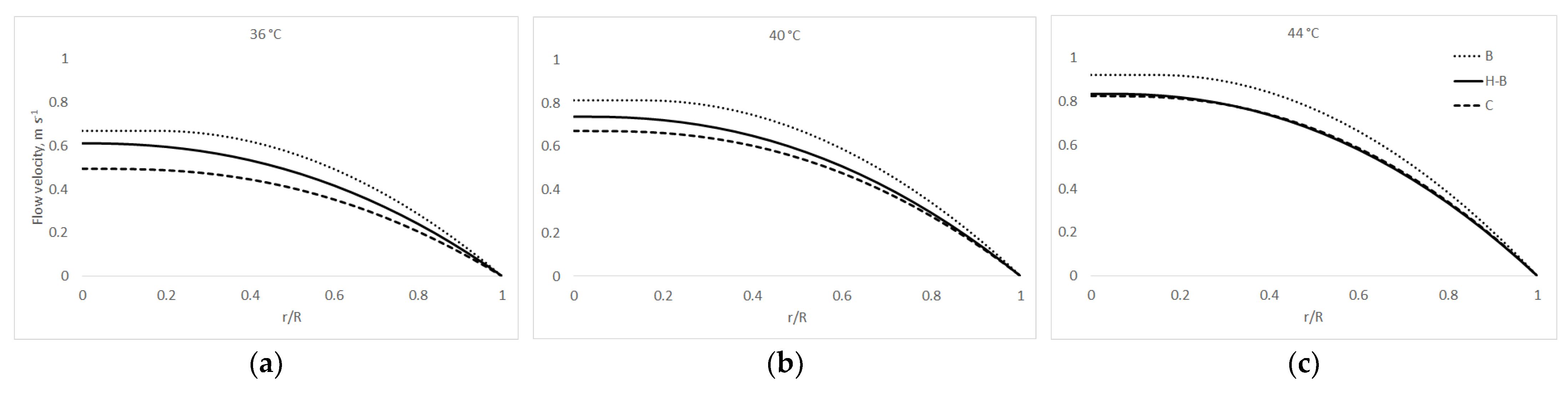
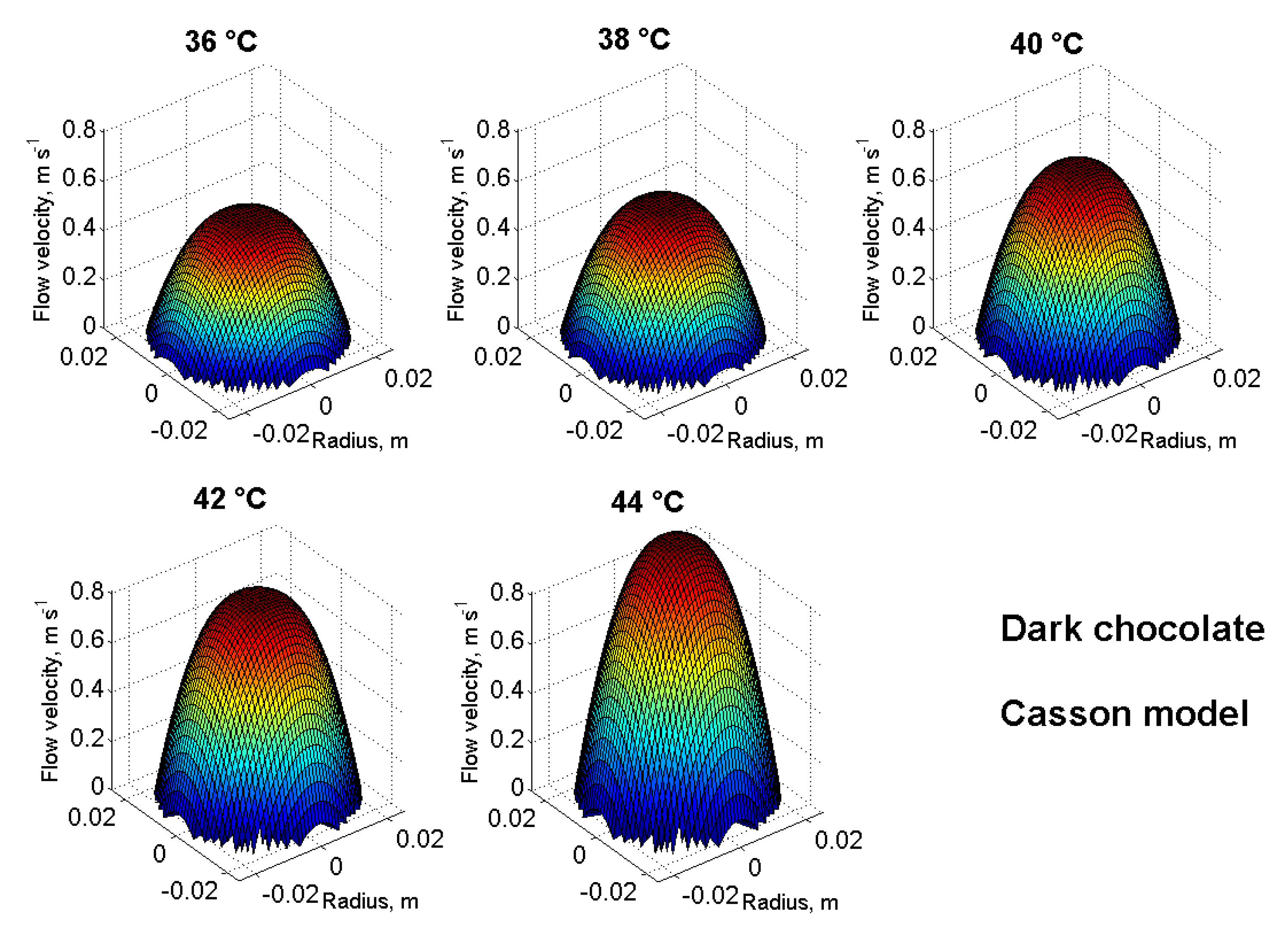
3.3. Pipe Flow Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvasekaran, P.; Chidambaram, R. Advances in formulation for the production of low-fat, fat-free, low-sugar, and sugar-free chocolates: An overview of the past decade. Trends Food Sci. Technol. 2021, 113, 315–334. [Google Scholar] [CrossRef]
- Directive (EC) No 36/2000 of the European parliament and of the council of 23 June 2000 relating to cocoa and chocolate products intended for human consumption. OJ L 197 2000, 3, 19.
- Afoakwa, E.O. Chocolate Science and Technology, 1st ed.; Willey Blackwell: York, UK, 2010; 275p. [Google Scholar]
- Glicerina, V.; Balestra, F.; Dalla Rosa, M.; Romani, S. Microstructural and rheological characteristics of dark, milk and white chocolate. J. Food Eng. 2016, 169, 165–171. [Google Scholar] [CrossRef]
- Martinez-Inchausti, A. Legal aspects of chocolate manufacture. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2009; pp. 576–594. [Google Scholar] [CrossRef]
- Dumarche, A.; Troplin, P.; Bernaert, H.; Lechevalier, P.; Beerens, H.; Landuyt, A. Process for Producing Cocoa-Derived Material. European Patent EP2237677B1, 13 June 2012. [Google Scholar]
- Tuenter, E.; Sakavitsi, M.E.; Rivera-Mondragon, A.; Hermans, N.; Foubert, K.; Halabalaki, M.; Pieters, L. Ruby chocolate: A study of its phytochemical composition and quantitative comparison with dark, milk and white chocolate. Food Chem. 2021, 343, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.C.; Valencia, W.G.; Sierra, J.A.; Penagos, L. Enhanced pink-red hues in processed powders from unfermented cacao beans. LWT 2021, 138, 110671. [Google Scholar] [CrossRef]
- Lillah, A.; Asghar, I.; Pasha, G.; Murtaza, M.A. Improving heat stability along with quality of compound dark chocolate by adding optimized cocoa butter substitute (hydrogenated palm kernel stearin) emulsion. LWT-Food Sci. Technol. 2017, 80, 531–536. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Budryn, G.; Oracz, J.; Antolak, H.; Kręgiel, D.; Kaczmarska, M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018, 113, 234–244. [Google Scholar] [CrossRef]
- Juszczak, L.; Witczak, M.; Fortuna, T.; Banys, A. Rheological properties of commercial mustards. J. Food Eng. 2004, 63, 209–271. [Google Scholar] [CrossRef]
- Beckett, S.T. The Science of Chocolate, 1st ed.; Royal Society of Chemistry Paperbacks: London, UK, 2000; 175p. [Google Scholar]
- Balmforth, N.J.; Frigaard, I.A.; Ovarlez, G. Yielding to Stress: Recent Developments in Viscoplastic Fluid Mechanics. Annu. Rev. Fluid Mech. 2014, 46, 121–146. [Google Scholar] [CrossRef]
- Tran, P.D.; Van Durme, J.; Van de Walle, D.; de Winne, A.; Delbaere, C.; de Clercq, N.; Dewettinck, K. Quality attributes of dark chocolate produced from vietnamese cocoa liquors. J. Food Qual. 2016, 39, 311–322. [Google Scholar] [CrossRef]
- Hinneh, M.; Van de Walle, D.; Haeck, J.; Abotsi, E.E.; De Winne, A.; Saputro, A.D.; Dewettinck, K. Applicability of the melanger for chocolate refining and stephan mixer for conching as small-scale alternative chocolate production techniques. J. Food Eng. 2019, 253, 59–71. [Google Scholar] [CrossRef]
- Přidal, A.; Trávníček, P.; Kudělka, J.; Nedomová, Š.; Ondrušíková, S.; Trost, D.; Kumbár, V. A rheological analysis of biomaterial behaviour as a tool to detect the dilution of heather honey. Materials 2021, 14, 2472. [Google Scholar] [CrossRef]
- Bergemann, N.; Heil, M.; Smith, B.; Juel, A. From elastic deformation to flow in tempered chocolate. J. Rheol. 2018, 62, 1187–1195. [Google Scholar] [CrossRef]
- Kumbár, V.; Nedomová, Š.; Trnka, J.; Buchar, J.; Pytel, R. Effect of storage duration on the rheological properties of goose liquid egg products and eggshell membranes. Poult. Sci. 2016, 95, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Lannes, S.C.S.; Medeiros, M.L.; Gioielli, L.A. Rheological properties of cupuassu and cocoa fats. Grasas Y Aceites 2004, 55, 115–121. [Google Scholar] [CrossRef]
- Fernandes, V.A.; Müller, A.J.; Sandoval, A.J. Thermal, structural and rheological characteristics of dark chocolate with different compositions. J. Food Eng. 2013, 116, 97–108. [Google Scholar] [CrossRef]
- Hřivna, L.; Machálková, L.; Burešová, I.; Nedomová, Š.; Gregor, T. Texture, color, and sensory changes occurring in chocolate bars with filling during storage. Food Sci. Nutr. 2021, 9, 4863–4873. [Google Scholar] [CrossRef]
- de Jesus Silva, G.; Gonçalves, B.R.F.; de Jesus, J.C.; Vidigal, M.C.T.R.; Minim, L.A.; Ferreira, S.O.; Ferrão, S.P.B. Study of the structural properties of goat′s milk chocolates with different concentrations of cocoa mass. J. Texture Stud. 2019, 50, 547–555. [Google Scholar] [CrossRef]
- Eduardo, M.F.; Correa De Mello, K.G.P.; Polakiewicz, B.; Da Silva Lannes, S.C. Evaluation of chocolate milk beverage formulated with modified chitosan. J. Agric. Sci. Technol. 2014, 16, 1301–1312. [Google Scholar]
- Sikora, M.; Adamczyk, G.; Krystyjan, M. Thixotropy as a measure of liquid food producs. Food Sci. Technol. Qual. 2011, 18, 5–14. [Google Scholar] [CrossRef]
- Servais, C.; Ranc, H.; Roberts, I.D. Determination of chocolate viscosity. J. Texture Stud. 2003, 34, 467–497. [Google Scholar] [CrossRef]
- Vlaev, S.D.; Staykov, P.; Popov, R. Pressure distribution at impeller blades of some radial flow impellers in saccharose and xanthan gum solutions: A CFD visualization approach. Food Bioprod. Process. 2004, 82, 13–20. [Google Scholar] [CrossRef]
- Žitný, R.; Landfeld, A.; Skočilas, J.; Štancl, J.; Flegl, V.; Zachariášová, M.; Houška, M. Hydraulic characteristic of collagen. Czech J. Food Sci. 2015, 33, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Kumbár, V.; Strnková, J.; Nedomová, Š.; Buchar, J. Fluid dynamics of liquid egg products. J. Biol. Phys. 2015, 41, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumbár, V.; Polcar, A.; Votava, J. Physical and mechanical properties of bioethanol and gasoline blends. Listy Cukrov. A Reparske 2015, 131, 112–115. [Google Scholar]
- Devakar, M.; Raje, A. Modelling and analysis of the unsteady flow and heat transfer of immiscible micropolar and newtonian fluids through a pipe of circular cross section. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 1–18. [Google Scholar] [CrossRef]
- Arosemena, A.A.; Andersson, H.I.; Solsvik, J. Turbulent channel flow of generalized newtonian fluids at a low reynolds number. J. Fluid Mech. 2021, 908, A43. [Google Scholar] [CrossRef]
- Nedomová, Š.; Kumbár, V.; Pytel, R.; Buchar, J. Mechanical properties of sugar beet root during storage. Int. Agrophys. 2017, 31, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.; Radhakrishnan, A.K.T.; van Lier, J.; Clemens, F. A wall boundary condition for the simulation of a turbulent non-newtonian domestic slurry in pipes. Water 2018, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Baranovskii, E.S. A novel 3D model for non-newtonian fluid flows in a pipe network. Math. Methods Appl. Sci. 2021, 44, 3827–3839. [Google Scholar] [CrossRef]
- Clauss, J. Taming turbulent piping-system flow. HPAC Heat. Pip. AirCond. Eng. 2009, 81, 36–43. [Google Scholar]
- Xue, H.; Fung, Y.C. Persistence of asymmetry in nonaxisymmetric entry flow in a circular cylindrical tube and its relevance to arterial pulse wave diagnosis. J. Biomech. Eng. 1989, 111, 37–41. [Google Scholar] [CrossRef]
- Jirkovsky, L.; Boot, L.M. Numerical tests of a new molecule-dependent momentum transport equation. Phys. A Stat. Mech. Appl. 2008, 387, 5012–5016. [Google Scholar] [CrossRef]
- Edomwonyi-Otu, L.C. Distortion of velocity profiles of water flow with heavy molecular weight polymers. Defect Diffus. Forum 2019, 392, 228–238. [Google Scholar] [CrossRef]
- Simpson, M.M.; Janna, W.S. Newtonian and non-newtonian fluids: Velocity profiles, viscosity data, and laminar flow friction factor equations for flow in a circular duct. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Lake Buena Vista, FL, USA, 13–19 November 2009; Volume 9, pp. 173–180. [Google Scholar] [CrossRef]
- Wiklund, J.; Stading, M.; Trägårdh, C. Monitoring liquid displacement of model and industrial fluids in pipes by in-line ultrasonic rheometry. J. Food Eng. 2010, 99, 330–337. [Google Scholar] [CrossRef]
- Das, B.; Bishop, J.J.; Kim, S.; Meiselman, H.J.; Johnson, P.C.; Popel, A.S. Red blood cell velocity profiles in skeletal muscle venules at low flow rates are described by the casson model. Clin. Hemorheol. Microcirc. 2007, 36, 217–233. [Google Scholar]
- Ardakani, H.A.; Mitsoulis, E.; Hatzikiriakos, S.G. Capillary flow of milk chocolate. J. Non-Newton. Fluid Mech. 2014, 210, 56–65. [Google Scholar] [CrossRef]
- Pegoraro, P.R.; Marangoni, M.; Gut, J.A.W. Residence time distribution models derived from non-ideal laminar velocity profiles in tubes. Chem. Eng. Technol. 2012, 35, 1593–1603. [Google Scholar] [CrossRef]
- Song, K.; Al-Salaymeh, A.; Jovanovic, J.; Rauh, C.; Delgado, A. Experimental in situ investigations of turbulence under high pressure. Ann. N. Y. Acad. Sci. 2010, 1189, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zelený, P.; Růžička, V. The design of the 3D printer for use in gastronomy. Mod. Mach.(MM) Sci. J. 2017, 1744–1747. [Google Scholar] [CrossRef]
- Rando, P.; Ramaioli, M. Food 3D printing: Effect of heat transfer on print stability of chocolate. J. Food Eng. 2021, 294, 110415. [Google Scholar] [CrossRef]
- Walker, J.H. Bulk chocolate handling. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2009; pp. 247–260. [Google Scholar] [CrossRef]



| Chocolate Mass | Nutritional Information Per 100 g | Composition | ||||
|---|---|---|---|---|---|---|
| Energy Value | Fats | Carbohydrates | Proteins | Salt | ||
| Of Which Saturated Fatty Acids | Of Which Sugars | |||||
| EDC | 2576.0 kJ | 53.0 g | 14.6 g | 15.0 g | 0.2 g | 100% cocoa components |
| 20.0 g | 1.0 g | |||||
| DC | 2264.6 kJ | 35.0 g | 46.9 g | 5.7 g | <0.005 g | 55.5% cocoa components, sugar, cocoa butter (35.0%), emulsifier: soy lecithin (E322), aroma: natural vanilla |
| 21.6 g | 44.0 g | |||||
| MC | 2297.2 kJ | 33.3 g | 55.6 g | 5.8 g | 0.206 g | 36.5% cocoa components, sugar, cocoa butter (29.0%), whole milk powder, lactose, whey powder, emulsifier: soy lecithin (E322), aroma: natural vanilla |
| 20.4 g | 54.7 g | |||||
| WC | 2360.5 kJ | 35.1 g | 56.1 g | 6.4 g | 0.234 g | 28% cocoa components, sugar, cocoa butter (28%), whole milk powder, emulsifier: soy lecithin (E322), aroma: natural vanilla |
| 21.3 g | 56.1 g | |||||
| RC | 2356.0 kJ | 35.9 g | 49.6 g | 9.3 g | 0.270 g | 47.3% cocoa components, sugar, cocoa butter, whole milk powder, emulsifier: soy lecithin (E322), acidity regulator: citric acid, aroma: natural vanilla |
| 21.5 g | 48.5 g | |||||
| Melting Temperature, °C | Cooling Temperature, °C | Working Temperature, °C | |
|---|---|---|---|
| EDC | 55 | 28.5–29.5 | 29.5–30.5 |
| DC | 45–50 | 28–29 | 31–32 |
| MC | 40–45 | 27–28 | 30–31 |
| WC | 40–42 | 25–26 | 28–29 |
| RC | 45 | 27 | 28.5–29.5 |
| 1→500 s−1 | T, °C | Bingham Model | Herschel–Bulkley Model | Casson Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| τ0 (Pa) | ηB (Pa·s) | SSE | R2 | τ0 (Pa) | K (Pa·sn) | n (−) | SSE | R2 | τ0 (Pa) | ηC (Pa·s) | SSE | R2 | ||
| EDC | 36 | 22.27 | 1.572 | 19,340 | 0.9914 | 0.870 | 4.688 | 0.8185 | 1415 | 0.9994 | 5.865 | 1.276 | 6045 | 0.9973 |
| 38 | 23.21 | 1.456 | 17,460 | 0.9909 | 3.002 | 4.415 | 0.8158 | 1506 | 0.9992 | 6.704 | 1.168 | 5314 | 0.9972 | |
| 40 | 19.79 | 1.360 | 10,640 | 0.9937 | 4.306 | 3.498 | 0.8429 | 825 | 0.9995 | 5.355 | 1.115 | 2844 | 0.9983 | |
| 42 | 18.22 | 1.291 | 8090 | 0.9946 | 4.684 | 3.122 | 0.8531 | 487 | 0.9997 | 4.886 | 1.068 | 1888 | 0.9987 | |
| 44 | 18.62 | 1.225 | 7402 | 0.9946 | 5.820 | 2.951 | 0.8537 | 587 | 0.9996 | 5.297 | 1.006 | 1768 | 0.9987 | |
| DC | 36 | 32.92 | 1.462 | 5272 | 0.9973 | 22.28 | 2.788 | 0.8925 | 319 | 0.9998 | 9.959 | 1.133 | 1012 | 0.9995 |
| 38 | 31.68 | 1.389 | 5744 | 0.9967 | 20.48 | 2.814 | 0.8825 | 322 | 0.9998 | 10.050 | 1.075 | 886 | 0.9995 | |
| 40 | 27.73 | 1.257 | 2731 | 0.9981 | 20.29 | 2.150 | 0.9105 | 225 | 0.9998 | 8.695 | 0.989 | 1007 | 0.9993 | |
| 42 | 22.68 | 1.035 | 1700 | 0.9982 | 17.96 | 1.581 | 0.9293 | 626 | 0.9994 | 7.543 | 0.827 | 1705 | 0.9982 | |
| 44 | 30.08 | 1.101 | 3728 | 0.9966 | 20.86 | 2.284 | 0.8785 | 144 | 0.9999 | 11.891 | 0.835 | 300 | 0.9997 | |
| MC | 36 | 26.29 | 2.018 | 4058 | 0.9989 | 18.29 | 2.927 | 0.9380 | 1001 | 0.9997 | 3.724 | 1.718 | 2505 | 0.9993 |
| 38 | 21.51 | 1.702 | 835 | 0.9997 | 18.48 | 2.026 | 0.9709 | 373 | 0.9999 | 2.945 | 1.478 | 2644 | 0.9990 | |
| 40 | 21.26 | 1.612 | 1116 | 0.9995 | 17.40 | 2.032 | 0.9614 | 371 | 0.9998 | 3.277 | 1.387 | 2225 | 0.9990 | |
| 42 | 24.84 | 1.486 | 1022 | 0.9995 | 20.59 | 1.954 | 0.9544 | 148 | 0.9999 | 5.199 | 1.233 | 2054 | 0.9990 | |
| 44 | 22.07 | 1.429 | 979 | 0.9995 | 19.80 | 1.670 | 0.9740 | 718 | 0.9996 | 4.137 | 1.210 | 3246 | 0.9982 | |
| WC | 36 | 22.95 | 1.802 | 4749 | 0.9984 | 13.17 | 2.961 | 0.9173 | 338 | 0.9999 | 3.802 | 1.528 | 930 | 0.9997 |
| 38 | 21.04 | 1.606 | 3125 | 0.9987 | 13.29 | 2.509 | 0.9256 | 314 | 0.9999 | 3.743 | 1.364 | 915 | 0.9996 | |
| 40 | 18.43 | 1.264 | 1541 | 0.9990 | 13.88 | 1.776 | 0.9433 | 450 | 0.9997 | 3.937 | 1.072 | 1266 | 0.9991 | |
| 42 | 24.24 | 1.472 | 6122 | 0.9969 | 12.67 | 2.936 | 0.8851 | 310 | 0.9998 | 6.033 | 1.199 | 736 | 0.9996 | |
| 44 | 24.27 | 1.393 | 3762 | 0.9979 | 15.84 | 2.410 | 0.9087 | 562 | 0.9997 | 6.141 | 1.135 | 951 | 0.9995 | |
| RB | 36 | 16.07 | 1.385 | 1543 | 0.9991 | 11.05 | 1.951 | 0.9429 | 323 | 0.9998 | 2.709 | 1.205 | 853 | 0.9995 |
| 38 | 14.40 | 1.258 | 564 | 0.9996 | 11.32 | 1.593 | 0.9606 | 96 | 0.9999 | 2.410 | 1.104 | 703 | 0.9995 | |
| 40 | 11.68 | 1.123 | 368 | 0.9997 | 9.80 | 1.323 | 0.9726 | 190 | 0.9998 | 1.848 | 1.002 | 784 | 0.9993 | |
| 42 | 14.21 | 1.071 | 312 | 0.9997 | 12.07 | 1.302 | 0.9675 | 83 | 0.9999 | 2.944 | 0.929 | 808 | 0.9992 | |
| 44 | 16.21 | 1.020 | 237 | 0.9997 | 14.98 | 1.149 | 0.9801 | 159 | 0.9998 | 3.857 | 0.868 | 1579 | 0.9983 | |
| 500→1 s−1 | T, °C | Bingham Model | Herschel–Bulkley Model | Casson Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| τ0 (Pa) | ηB (Pa·s) | SSE | R2 | τ0 (Pa) | K (Pa·sn) | n (−) | SSE | R2 | τ0 (Pa) | ηC (Pa·s) | SSE | R2 | ||
| EDC | 36 | 17.98 | 1.491 | 15,120 | 0.9925 | 0 | 4.071 | 0.8330 | 1310 | 0.9994 | 4.477 | 1.239 | 5004 | 0.9975 |
| 38 | 19.46 | 1.406 | 8048 | 0.9955 | 8.258 | 2.827 | 0.8837 | 2416 | 0.9986 | 4.428 | 1.175 | 3709 | 0.9979 | |
| 40 | 18.12 | 1.319 | 6449 | 0.9959 | 8.750 | 2.480 | 0.8949 | 2410 | 0.9985 | 4.110 | 1.110 | 3481 | 0.9978 | |
| 42 | 13.43 | 1.279 | 5338 | 0.9964 | 3.092 | 2.594 | 0.8823 | 623 | 0.9996 | 2.882 | 1.102 | 1660 | 0.9989 | |
| 44 | 15.84 | 1.201 | 4332 | 0.9967 | 9.894 | 1.896 | 0.9238 | 2592 | 0.9980 | 3.344 | 1.029 | 3594 | 0.9972 | |
| DC | 36 | 21.94 | 1.462 | 2087 | 0.9989 | 15.46 | 2.210 | 0.9312 | 128 | 0.9999 | 4.563 | 1.224 | 706 | 0.9996 |
| 38 | 21.26 | 1.380 | 2599 | 0.9985 | 14.18 | 2.213 | 0.9213 | 279 | 0.9998 | 4.801 | 1.149 | 761 | 0.9996 | |
| 40 | 15.86 | 1.276 | 4457 | 0.9970 | 7.97 | 2.231 | 0.9069 | 1776 | 0.9988 | 3.541 | 1.086 | 1334 | 0.9991 | |
| 42 | 20.85 | 1.016 | 1342 | 0.9986 | 18.84 | 1.232 | 0.9680 | 1134 | 0.9988 | 6.225 | 0.830 | 3254 | 0.9965 | |
| 44 | 18.38 | 1.098 | 1160 | 0.9989 | 13.54 | 1.655 | 0.9316 | 63 | 0.9999 | 4.938 | 0.913 | 503 | 0.9995 | |
| MC | 36 | 19.30 | 1.854 | 2625 | 0.9992 | 16.41 | 2.161 | 0.9744 | 2213 | 0.9993 | 2.077 | 1.644 | 3570 | 0.9988 |
| 38 | 15.28 | 1.665 | 219 | 0.9999 | 15.55 | 1.638 | 1.0030 | 216 | 0.9999 | 1.309 | 1.516 | 2461 | 0.9990 | |
| 40 | 17.22 | 1.583 | 278 | 0.9999 | 17.28 | 1.577 | 1.0010 | 278 | 0.9999 | 1.814 | 1.419 | 3277 | 0.9985 | |
| 42 | 18.93 | 1.461 | 1528 | 0.9992 | 22.00 | 1.170 | 1.0370 | 997 | 0.9995 | 2.134 | 1.303 | 7279 | 0.9962 | |
| 44 | 11.85 | 1.436 | 2491 | 0.9987 | 8.95 | 1.748 | 0.9672 | 2086 | 0.9989 | 1.415 | 1.296 | 2011 | 0.9989 | |
| WC | 36 | 17.60 | 1.658 | 1943 | 0.9992 | 16.17 | 1.806 | 0.9857 | 1840 | 0.9993 | 1.953 | 1.477 | 3757 | 0.9985 |
| 38 | 11.47 | 1.575 | 2314 | 0.9990 | 6.76 | 2.095 | 0.9524 | 1274 | 0.9994 | 1.276 | 1.427 | 956 | 0.9996 | |
| 40 | 12.44 | 1.231 | 178 | 0.9999 | 11.13 | 1.367 | 0.9824 | 91 | 0.9999 | 1.737 | 1.102 | 854 | 0.9994 | |
| 42 | 14.97 | 1.440 | 408 | 0.9998 | 12.63 | 1.688 | 0.9735 | 134 | 0.9999 | 1.950 | 1.281 | 1107 | 0.9994 | |
| 44 | 10.92 | 1.385 | 2312 | 0.9987 | 6.88 | 1.832 | 0.9533 | 1542 | 0.9991 | 1.418 | 1.248 | 1280 | 0.9993 | |
| RB | 36 | 12.630 | 1.373 | 1029 | 0.9994 | 12.301 | 1.407 | 0.9959 | 1023 | 0.9994 | 1.233 | 1.254 | 2768 | 0.9984 |
| 38 | 9.364 | 1.255 | 157 | 0.9999 | 9.067 | 1.285 | 0.9960 | 152 | 0.9999 | 0.856 | 1.162 | 895 | 0.9994 | |
| 40 | 9.158 | 1.108 | 61 | 0.9999 | 8.859 | 1.138 | 0.9955 | 56 | 0.9999 | 1.035 | 1.018 | 755 | 0.9993 | |
| 42 | 9.287 | 1.067 | 77 | 0.9999 | 9.355 | 1.060 | 1.0010 | 77 | 0.9999 | 1.109 | 0.979 | 876 | 0.9991 | |
| 44 | 9.391 | 1.017 | 35 | 0.9999 | 9.561 | 1.001 | 1.0030 | 33 | 0.9999 | 1.231 | 0.929 | 862 | 0.9991 | |
| Model | Coefficient of Determination R2 | Sum of Squared Estimate of Errors SSE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | MIN | MAX | SD | Mean | Median | MIN | MAX | SD | |
| Bingham | 0.9980 | 0.9989 | 0.9909 | 0.9999 | 0.0023 | 3581 | 2200 | 35 | 19,340 | 4318 |
| Herschel–Bulkley | 0.9996 | 0.9998 | 0.9980 | 0.9999 | 0.0004 | 733 | 372 | 33 | 2592 | 729 |
| Casson | 0.9988 | 0.9991 | 0.9962 | 0.9997 | 0.0008 | 2034 | 1457 | 300 | 7279 | 1547 |
| Temperature, °C | EDC, (Pa·s−1) | DC, (Pa·s−1) | MC, (Pa·s−1) | WC, (Pa·s−1) | RC, (Pa·s−1) |
|---|---|---|---|---|---|
| 36 | 12 814 | 7521 | 25 787 | 21 694 | 3625 |
| 38 | 9531 | 6771 | 7953 | 8817 | 3147 |
| 40 | 6554 | 3705 | 6156 | 7657 | 3266 |
| 42 | 3901 | 3446 | 6892 | 9063 | 3238 |
| 44 | 4924 | 6318 | 4501 | 8363 | 3952 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumbár, V.; Kouřilová, V.; Dufková, R.; Votava, J.; Hřivna, L. Rheological and Pipe Flow Properties of Chocolate Masses at Different Temperatures. Foods 2021, 10, 2519. https://doi.org/10.3390/foods10112519
Kumbár V, Kouřilová V, Dufková R, Votava J, Hřivna L. Rheological and Pipe Flow Properties of Chocolate Masses at Different Temperatures. Foods. 2021; 10(11):2519. https://doi.org/10.3390/foods10112519
Chicago/Turabian StyleKumbár, Vojtěch, Veronika Kouřilová, Renáta Dufková, Jiří Votava, and Luděk Hřivna. 2021. "Rheological and Pipe Flow Properties of Chocolate Masses at Different Temperatures" Foods 10, no. 11: 2519. https://doi.org/10.3390/foods10112519






