Correlation of Mercury Occurrence with Age, Elemental Composition, and Life History in Sea-Run Food Fish from the Canadian Arctic Archipelago’s Lower Northwest Passage
Abstract
:1. Introduction
2. Materials and Methods
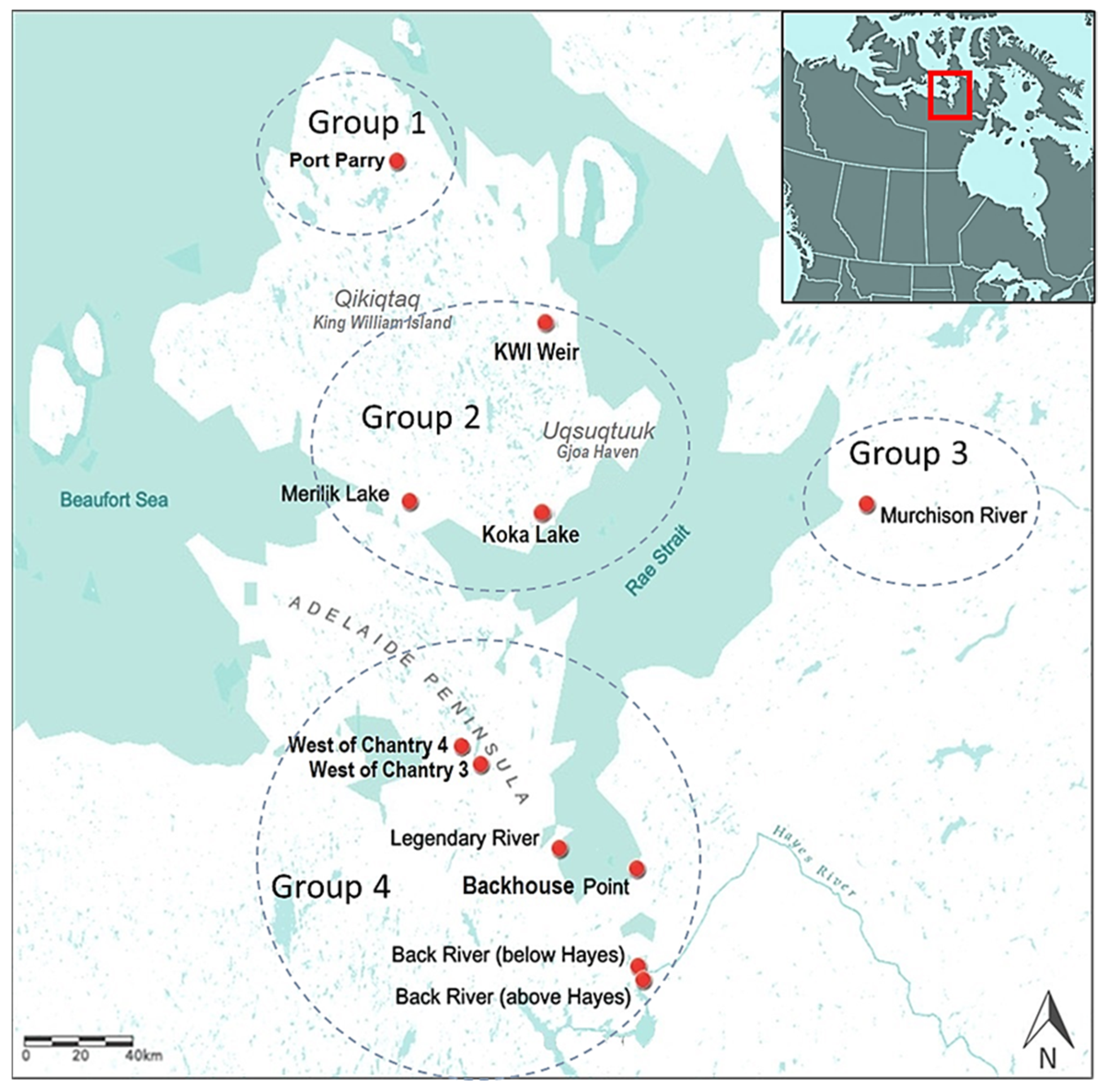
2.1. Sample Collection
2.2. Life History Analysis
2.3. Elemental Analysis
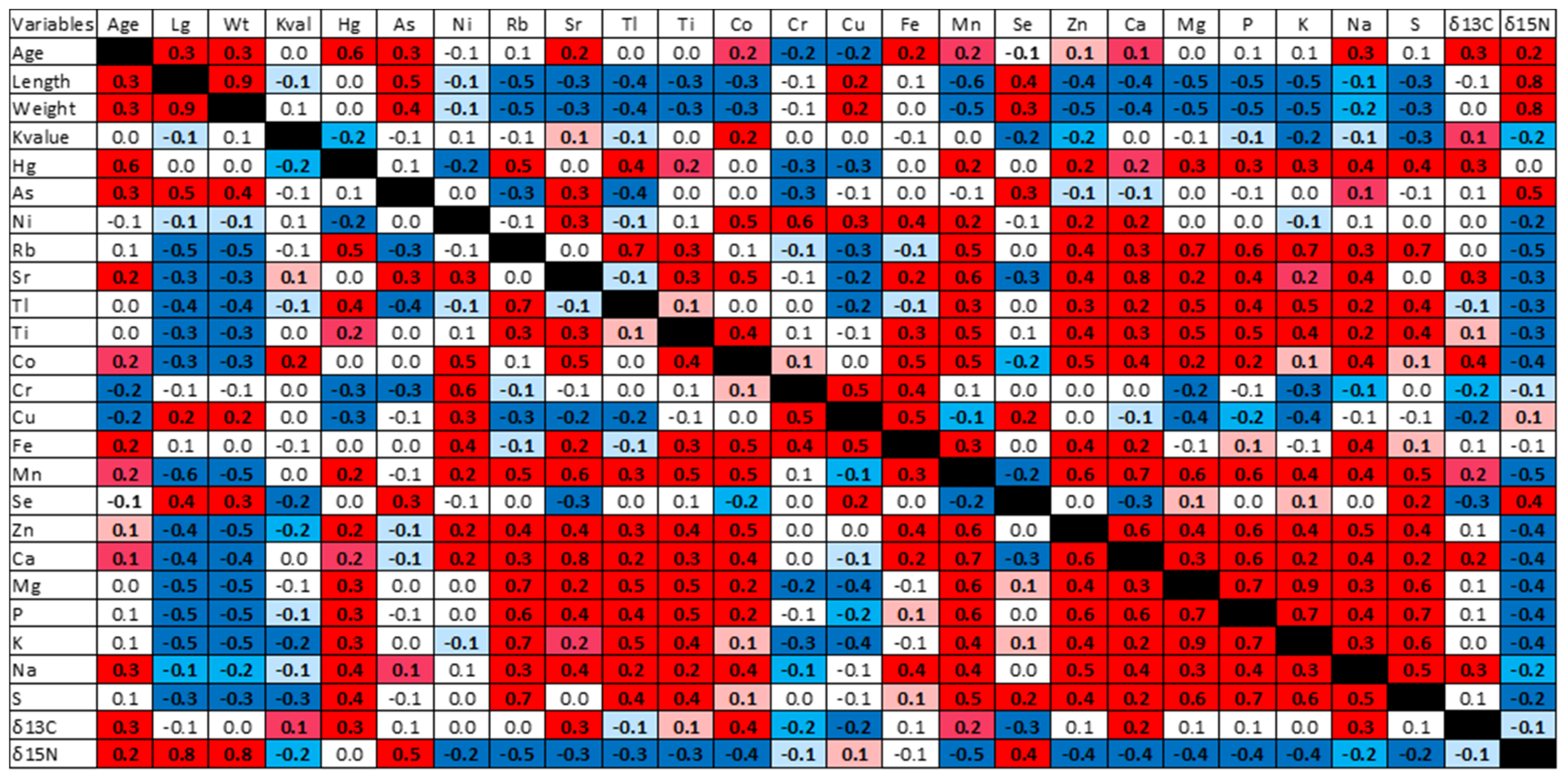
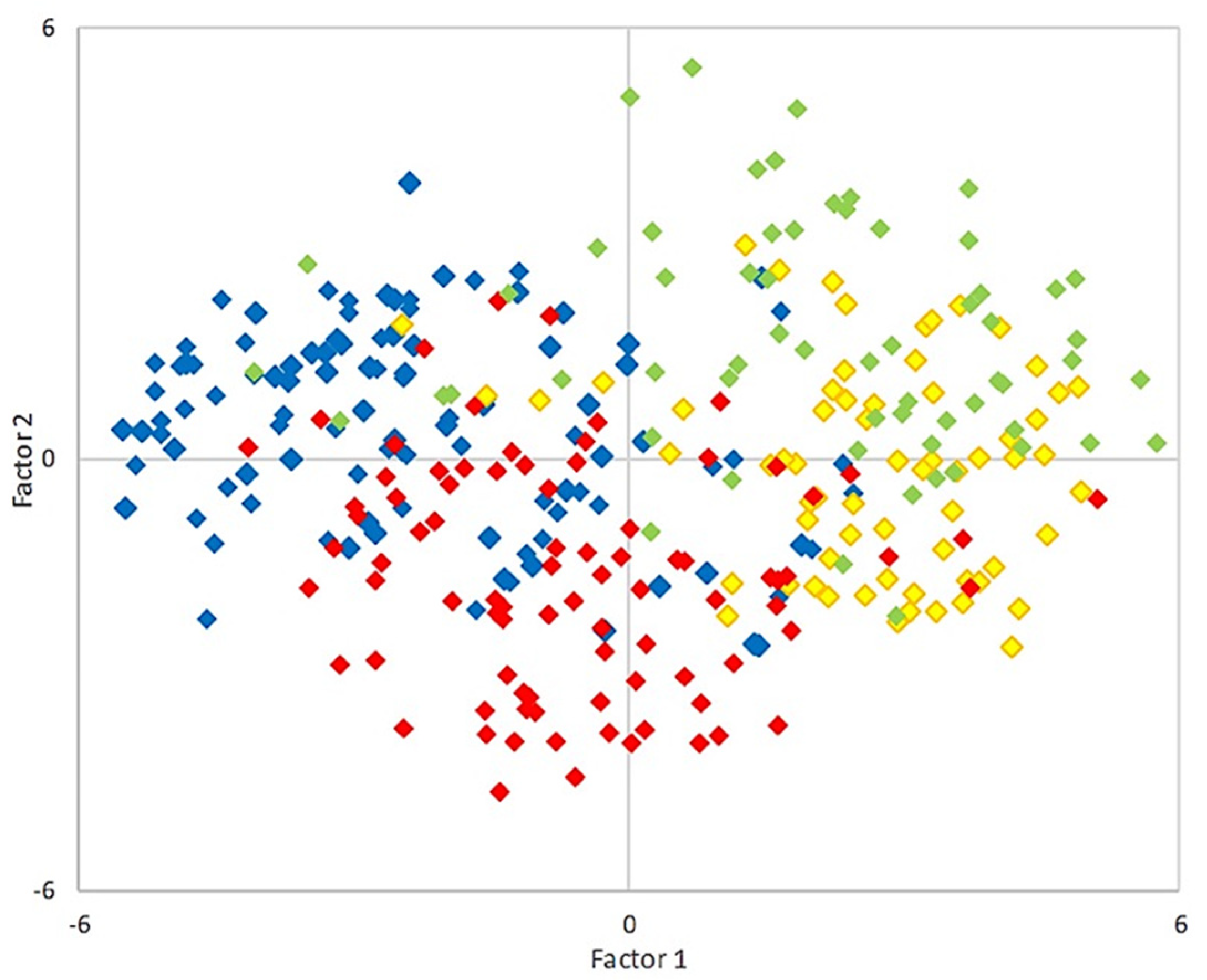
2.4. Data Analysis
3. Results and Discussion
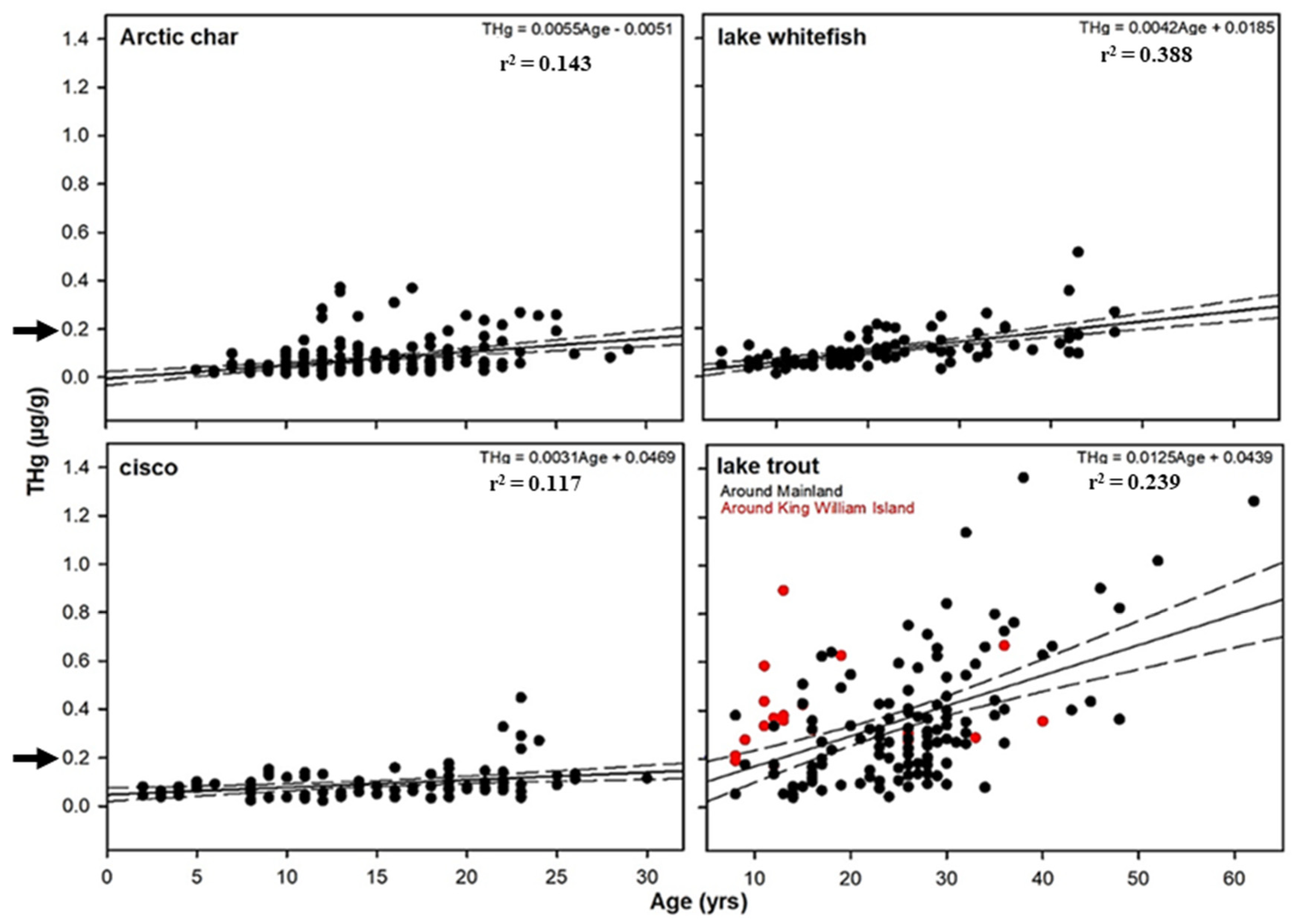
3.1. Comparison of THg Concentrations in Relation to Life History Characteristics
3.2. THg Concentrations with Respect to Selenium and Other Elements, Age, and Body Condition
3.3. THg Levels in East Kitikmeot Salmonids and Commercial and Subsistence Advisories
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietz, R.; Outridge, P.M.; Hobson, K.A. Anthropogenic contributions to mercury levels in present-day Arctic animals—A review. Sci. Total Environ. 2009, 407, 6120–6131. [Google Scholar] [CrossRef]
- Steffen, A.; Douglas, T.; Amyot, M.; Ariya, P.; Aspmo, K.; Berg, T.; Bottenheim, J.; Brooks, S.; Cobbett, F.; Dastoor, A.; et al. A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow. Atmos. Chem. Phys. 2008, 8, 1445–1482. [Google Scholar] [CrossRef] [Green Version]
- Durnford, D.; Dastoor, A.; Figueras-Nieto, D.; Ryjkov, A. Long range transport of mercury to the Arctic and across Canada. Atmos. Chem. Phys. 2010, 10, 6063–6086. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jocob, D.J.; Horowitz, H.M.; Chen, L.; Amos, H.M.; Krabbenhoft, D.P.; Slemr, F.; St. Louis, V.L.; Sunderland, M. Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. PNAS 2016, 113, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, W.H.; Anlauf, K.G.; Barrie, L.A.; Lu, J.Y.; Steffan, A.; Schneeberger, D.R.; Berg, T. Arctic springtime depletion of mercury. Nature 1998, 394, 331–332. [Google Scholar] [CrossRef]
- Chételat, J.; Amyot, M.; Arp, P.; Blais, J.M.; Depew, D.; Emmerton, C.A.; Evans, M.; Gamberg, M.; Gantner, N.; Girard, C.; et al. Mercury in freshwater ecosystems of the Canadian Arctic: Recent advances on its cycling and fate. Sci. Total Environ. 2015, 509–510, 41–66. [Google Scholar] [CrossRef] [Green Version]
- Bloom, N.S. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Swanson, H.K.; Kidd, K.A. Mercury concentrations in Arctic food fishes reflect the presence of anadromous Arctic charr (Salvelinus alpinus), species, and life history. Environ. Sci. Technol. 2010, 44, 3286–3292. [Google Scholar] [CrossRef]
- Bodaly, R.A.; Rudd, J.W.M.; Fudge, R.J.P.; Kelly, C.A. Mercury concentrations in fish related to size of remote Canadian shield lakes. Can. J. Fish. Aquat. Sci. 1993, 50, 980–987. [Google Scholar] [CrossRef]
- Evans, M.S.; Muir, D.; Lockhart, W.L.; Stern, G.; Ryan, M.; Roach, P. Persistent organic pollutants and metals in the freshwater biota of the Canadian subarctic and Arctic: An overview. Sci. Total Environ. 2005, 351, 94–147. [Google Scholar] [CrossRef]
- Stern, G.; Loseto, L.; MacDonald, R.W.; Wang, F.; Zdanowicz, C.; Outridge, P.M.; Cole, A.; Hintelmann, H.; Steffen, A. How does climate change influence Arctic mercury? AMAP Assess. Mercury Arct. 2011, 4, 67–83. [Google Scholar] [CrossRef]
- Lockhart, W.L.; Uthe, J.F.; Kenney, A.R.; Mehrle, P.M. Methylmercury in northern pike (Esox lucius): Distribution, elimination, and some biochemical characteristics of contaminated fish. J. Fish. Res. Board Can. 1972, 29, 1519–1523. [Google Scholar] [CrossRef]
- Trudel, M.; Rasmussen, J.B. Bioenergetics and mercury dynamics in fish: A modelling perspective. Can. J. Fish. Aquat. Sci. 2006, 63, 1890–1902. [Google Scholar] [CrossRef]
- Kidd, K.A.; Hesslein, R.H.; Fedge, R.J.P.; Hallard, K.A. The influence of trophic level as measured by δ 15 N on mercury concentrations in freshwater organisms. Water Air Soil Pollut. 1995, 80, 1011–1015. [Google Scholar] [CrossRef]
- Stafford, C.P.; Haines, T.A. Mercury contamination and growth rate in two piscivore populations. Environ. Toxicol. Chem. 2001, 20, 2099–2101. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, M.; Lucotte, M.; Garceau, S.; Laliberté, D. Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lakes. Environ. Res. 2005, 98, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Nislow, K.H.; Chen, C.Y.; Folt, C.L. Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. Trans. Am. Fish. Soc. 2010, 139, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, V.K.; Das, P.; Li, P.; Lougheed, S.C.; Moniz, K.; Schott, S.; Qitsualik, J.; Koch, I. Identification of Arctic food fish species for anthropogenic contaminant testing using geography and genetics. Foods 2020, 9, 1824. [Google Scholar] [CrossRef]
- Berry, M.J.; Ralston, N.V. Mercury toxicity and the mitigating role of selenium. EcoHealth 2008, 5, 456–459. [Google Scholar] [CrossRef]
- Sobolev, N.; Aksenov, A.; Sorokinaa, T.; Chashchin, V.; Ellingsen, D.G.; Nieboer, E.; Varakinaa, Y.; Veselkina, E.; Kotsur, D.; Thomassen, Y. Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Sonesten, L. Fish mercury levels in lakes–adjusting for Hg and fish-size covariation. Environ. Pollut. 2003, 125, 255–265. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. A review of the lifespans and mortality rates of fish in nature, and their relation to growth and other physiological characteristics. In The Lifespan of Animals; Wolstenholme, G.E.W., O’Connor, M., Eds.; Ciba Foundation Colloquia on Ageing; Wiley: NewYork, NY, USA, 1959; Volume V, pp. 142–177. [Google Scholar]
- Purchase, C.F. and Brown, J.A. Interpopulation differences in growth rates and food conversion efficiencies of young Grand Banks and Gulf of Maine Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 2000, 57, 2223–2229. [Google Scholar] [CrossRef]
- Beaudoin, C.P.; Tonn, W.M.; Prepas, E.E.; Wassenaar, L. Individual specialization and trophic adaptability of northern pike (Esox lucius): An isotope dietary analysis. Oceologia 1999, 120, 286–396. [Google Scholar] [CrossRef]
- Roux, M.J.; Tallman, R.F.; Lewis, C.W. Small-scale Arctic char Salvelinus alpinus fisheries in Canada’s Nunavut: Management challenges and options. J. Fish. Biol. 2011, 79, 1625–1647. [Google Scholar] [CrossRef]
- Chételat, J.; Shao, Y.; Richardson, M.C.; MacMillan, G.A.; Amyot, M.; Drevnick, P.E.; Gill, H.; Köck, G.; Muir, D.C.G. Diet influences on growth and mercury concentrations of two salmonid species from lakes in the eastern Canadian Arctic. Environ. Pollut. 2021, 268, 115820. [Google Scholar] [CrossRef]
- Casselman, J.M. Determination of age and growth. In The Biology of Fish Growth; Weatherly, A.H., Gill, H.S., Eds.; Academic Press: Cambridge, MA, USA, 1987; pp. 209–242. [Google Scholar]
- Scott, W.B.; Crossman, E.J. Freshwater fishes of Canada. J. Fish. Res. Board Can. 1973, 184, 966. [Google Scholar]
- Swanson, H.K.; Kidd, K.A.; Babaluk, J.A.; Wastle, R.J.; Yang, P.P.; Halden, N.M.; Reist, J.D. Anadromy in Arctic populations of lake trout (Salvelinus namaycush): Ototith microchemistry, stable isotopes, and comparisons with Arctic char (Salvelinus alpinus). Can. J. Fish. Aquat. Sci. 2010, 67, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, W.L.; Stern, G.A.; Low, G.; Hendzel, M.; Boila, G.; Roach, P.; Evans, M.S.; Billeck, B.N.; DeLaronde, J.; Friesen, S.; et al. A history of total mercury in edible muscle of fish from lakes in northern Canada. Sci. Total Environ. 2005, 351–352, 427–463. [Google Scholar] [CrossRef] [PubMed]
- Depew, D.C.; Burgess, N.M.; Anderson, M.R.; Baker, R.; Bhavsar, S.P.; Bodaly, R.A.; Eckley, C.S.; Evans, M.S.; Gantner, N.; Graydon, J.A.; et al. An overview of mercury concentrations in freshwater fish species: A national fish mercury dataset for Canada. Can. J. Fish. Aquat. Sci. 2013, 70, 1–16. [Google Scholar] [CrossRef]
- Evans, M.; Muir, D.; Magsaga, K.; Harris, L.; McLennan, D.; Keating, J.; Wang, X. Temporal Trends and Spatial Variations in Persistent Organic Pollutants and Metals in Sea-Run Arctic char from Cambridge Bay, Nunavut. 2017. Available online: http://pubs.aina.ucalgary.ca/ncp/83652.pdf (accessed on 17 August 2021).
- Wu, Y.; Lougheed, D.R.; Lougheed, S.C.; Moniz, K.; Walker, V.K.; Colautti, R.I. baRcodeR: An open-source R package for sample labelling. Methods Ecol. Evol. 2020, 11, 980–985. [Google Scholar] [CrossRef]
- ArcGIS on Line 2020. Available online: https://www.arcgis.com/index.html (accessed on 4 April 1990).
- Casselman, J.M. A Techniques Manual Outlining General Procedures for Preparing Thin Sections of Fish Otoliths for Age and Growth Interpretation; Special Publication of AFishESci Inc.: Mallorytown, ON, Canada, 2015; p. 30. [Google Scholar]
- Campana, S.E.; Casselman, J.M.; Jones, C.M. Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arctic species. Can. J. Fish. Aquat. Sci. 2008, 65, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Casselman, J.M.; Jones, C.M.; Campana, S.E. Bomb radiocarbon age validation for the long-lived, unexploited Arctic fish species Coregonus clupeaformis. Mar. Freshw. Res. 2019, 70, 1–8. [Google Scholar] [CrossRef]
- Rogers, S.M. Designatable units at an Appropriate Scale for the Lake Whitefish, Coregonus clupeaformis in Canada. Committee on the Status of Endangered Wildlife in Canada. 2008. Available online: http://cosewic.ca/images/cosewic/pdf/Lake_Whitefish_Special_Report_2008_e.pdf (accessed on 6 August 2020).
- US Environmental Protection Agency. Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, Revision 0. Washington, DC, 1998. Available online: https://www.epa.gov/esam/epa-method-7473-sw-846-mercury-solids-and-solutions-thermal-decomposition-amalgamation-and (accessed on 6 August 2020).
- Bloom, N. Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapour atomic fluorescence detection. Can. J. Fish. Aquat. Sci. 1989, 46, 1131–1140. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Method 1630: Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry; EPA-821-R-01–020; US Environmental Protection Agency: Washington, DC, USA, 2001.
- Cabana, G.; Rasmussen, J.B. Comparison of aquatic food chains using nitrogen isotopes. Proc. Natl. Acad. Sci. USA 1996, 93, 10844–10847. [Google Scholar] [CrossRef] [Green Version]
- France, R.L. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol. Oceanogr. 1995, 7, 1310–1313. [Google Scholar] [CrossRef]
- France, R.L. Critical examination of stable isotope analysis as a means for tracing carbon pathways in stream ecosystems. Can. J. Fish. Aquat. Sci. 1995, 52, 651–656. [Google Scholar] [CrossRef]
- Fulton, T.W. The rate of growth of fishes. 20th Annu. Rep. Fish. Board Scotl. 1902, 3, 326–446. [Google Scholar]
- Anderson-Sprecher, R. Model comparisons and R2. Am. Stat. 1994, 48, 113–117. [Google Scholar]
- Swanson, H.; Gantner, N.; Kidd, K.A.; Muir, D.C.G.; Reist, J.D. Comparison of mercury concentrations in landlocked, resident, and sea-run fish (Salvelinus spp.) from Nunavut, Canada. Environ. Toxicol. Chem. 2011, 30, 1459–1467. [Google Scholar] [CrossRef]
- Evans, M.S.; Muir, D.; Keating, J.; Wang, X. Anadromous char as an alternate food choice to marine animals: A synthesis of mercury concentrations and population features. Sci. Total Environ. 2015, 509–510, 175–194. [Google Scholar] [CrossRef]
- Hrenchuk, L.E.; Blanchfield, P.J.; Paterson, M.J.; Hintelmann, H.H. Dietary and waterborne mercury accumulation by yellow perch: A field experiment. Environ. Sci. Technol. 2012, 46, 509–516. [Google Scholar] [CrossRef]
- Sandheinrich, M.B.; Drevnick, P. Relationship among mercury concentration, growth rate, and condition of northern pike: A tautology resolved? Environ. Toxicol Chem. 2016, 35, 2910–2915. [Google Scholar] [CrossRef]
- Power, M.; Klein, G.M.; Guiguer, K.R.R.A.; Kwan, M.K.H. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J. Appl. Ecol. 2002, 39, 819–830. [Google Scholar] [CrossRef]
- Casadevall, M.; Rodríguez-Prieto, C.; Torres, J. The importance of the age when evaluating mercury pollution in fishes: The case of Diplodus sargus (Pisces, Sparidae) in the NW Mediterranean. AIMS Environ. Sci. 2017, 4, 17–26. [Google Scholar] [CrossRef]
- Cyr, A.P.; López, J.A.; Wooller, M.J.; Whiting, A.; Gerlach, R.; O’Hara, T. Ecological drivers of mercury concentrations in fish species in subsistence harvests from Kotzebue Sound, Alaska. Environ. Res. 2019, 177, 108622. [Google Scholar] [CrossRef] [PubMed]
- Element, G.; Engel, K.; Neufeld, J.D.; Casselman, J.M.; van Coeverden de Groot, P.; Greer, C.W.; Walker, V.K. Seasonal habitat drives intestinal microbiome composition in anadromous Arctic char (Salvelinus alpinus). Environ. Microbiol. 2020, 22, 3112–3125. [Google Scholar] [CrossRef] [PubMed]
- Element, G.; Engel, K.; Neufeld, J.; Casselman, J.; van Coeverden de Groot, P.; Walker, V.K. Distinct intestinal microbial communities of two sympatric anadromous arctic salmonids and the effects of migration and feeding. Arct. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Gantner, N.; Power, M.; Babaluk, J.A.; Reist, J.D.; Kőck, G.; Lockhart, L.W.; Solomon, K.R.; Muir, D.G. Temporal trends of mercury, cesium, potassium, selenium, and thallium in Arctic char (Salvelinus alpinus) from Lake Hazen, Nunavut, Canada: Effects of trophic position, size, and age. Environ. Toxicol. Chem. 2009, 28, 254–263. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W.; Gunn, J.M.; Tong, J.; Alarie, Y.; Delonchap, T.; Lang, C.-Y. The effect of selenium on mercury assimilation by freshwater organisms. Can. J. Fish. Aquat. Sci. 2006, 63, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.-Y.; Ye, X.; Chen, Y.-W.; Belzile, N. Inverse relationships between selenium and mercury in tissues of young walleye (Stizosedion vitreum) from Canadian boreal lakes. Sci. Total Environ. 2010, 408, 1676–1683. [Google Scholar] [CrossRef]
- Beckvar, N.; Dillon, T.M.; Read, L.B. Approaches for linking whole-body fish tissue residues of mercury or DDT to biological effects thresholds. Environ. Toxicol. Chem. 2005, 24, 2094–2105. [Google Scholar] [CrossRef] [Green Version]
- Sandheinrich, M.B.; Bhavsar, S.P.; Bodaly, R.A.; Drevnick, P.E.; Paul, E.A. Ecological risk of methylmercury to piscivorous fish of the Great Lakes region. Ecotoxicology 2011, 20, 1577–1587. [Google Scholar] [CrossRef]
- Depew, D.C.; Basu, N.; Murray, N.; Burgess, N.M.; Campbell, L.M.; Devlin, W.; Drevnick, P.E.; Murphy, C.A.; Sandheinrich, M.B.; Wiener, J.G. Toxicity of dietary methylmercury to fish: Derivation of ecologically meaningful threshold concentrations. Environ. Toxicol. Chem. 2012, 31, 1536–1547. [Google Scholar] [CrossRef] [Green Version]
- Fuchsman, P.C.; Henning, M.H.; Sorensen, M.T.; Brown, L.E.; Bock, M.J.; Beals, C.D.; Lyndall, J.L.; Magar, V.S. Critical perspectives on mercury toxicity reference values for protection of fish. Environ. Toxicol. Chem. 2016, 35, 529–549. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Currin, C.A.; Wainright, S.C.; Able, K.W.; Weinstein, M.P.; Fuller, C.M. Determination of food web support and trophic position of the mummichog, Fundulus heteroclitus, in New Jersey smooth cordgrass (Spartina alterniflora), common reed (Phragmites australis), and restored salt marshes. Estuaries 2003, 26, 495–510. [Google Scholar] [CrossRef]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- Saunders, L.H.; Power, G. The Arctic char, Salvelinus alpinus (Linnaeus), of Matamek Lake, Quebec. Nat. Can. 1969, 96, 919–934. [Google Scholar]
- Bjornsson, B. Diel changes in the feeding behaviour of Arctic Char (Salvelinus alpinus) and Brown Trout (Salmo trutta) in Ellidavatn, a small lake in Southwest Iceland. Limnologica 2001, 31, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Eloranta, A.P.; Nieminen, P.; Kahilainen, K.K. Trophic interactions between introduced lake trout (Salvelinus namaycush) and native Arctic Charr (S. alpinus) in a large Fennoscandian subarctic lake. Ecol. Freshw. Fish. 2015, 24, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Vander, Z.M.J. and Rasmussen, J.B. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 1999, 80, 1395–1404. [Google Scholar]
- Hoffman, J.C.; Sierszen, M.E.; Cotter, A.M. Fish tissue lipid-C: N relationships for correcting δ13C values and estimating lipid content in aquatic food-web studies. Rapid Comm. Mass Spectrom. 2015, 29, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Fagan, K.-A.; Koops, M.A.; Arts, M.T.; Power, M. Assessing the utility of C:N ratios for predicting lipid content in fishes. Can. J. Fish. Aquat. Sci. 2011, 68, 374–385. [Google Scholar] [CrossRef]
- McConnaughey, T.; McRoy, C.P. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Mar. Biol. 1979, 53, 257–262. [Google Scholar] [CrossRef]
- Has-Schön, E.; Bogut, I.; Strelec, I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chena, L.; Denga, X.; Liang, G.; Giesye, J.P.; Rao, Q.; Wen, Z.; Wu, Y.; Chena, J.; Xie, P. Spatial and interspecies differences in concentrations of eight trace elements in wild freshwater fishes at different trophic levels from middle and eastern China. Sci. Total Environ. 2019, 672, 883–892. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Jeitner, C.; Pittfield, T.; Donio, M. Heavy metals in fish from the Aleutians: Interspecific and locational differences. Environ. Res. 2014, 131, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.-S.; Harris, L.N.; Tallman, R.F.; Taylor, E.B. The interplay between dispersal and gene flow in anadromous Arctic char (Salvelinus alpinus): Implications for potential for local adaptation. Can. J. Fish. Aquat. Sci. 2013, 70, 1327–1338. [Google Scholar] [CrossRef] [Green Version]
- Coad, B.W.; Reist, J.D. (Eds.) Marine Fishes of Arctic Canada; Canadian Museum of Nature University of Toronto Press: Toronto, ON, Canada, 2018; ISBN 978-1-4426-4710-7. [Google Scholar]
- Hard, J.J.; Gross, M.R.; Heino, M.; Hilborn, R.; Kope, R.G.; Law, R.; Reynolds, J.D. Evolutionary consequences of fishing and their implications for salmon. Evol. Appl. 2008, 1, 388–408. [Google Scholar] [CrossRef] [Green Version]
- St. Pierre, K.A.; Zolkos, S.; Shakil, S.; Tank, S.E.; St. Louis, V.L.; Kokelj, S.V. Unprecedented increases in total and methyl mercury concentrations downstream of retrogressive thaw slumps in the western Canadian Arctic. Environ. Sci. Technol. 2018, 52, 14099–14109. [Google Scholar] [CrossRef] [PubMed]
- Health Canada 2020 (Originally Posted 2018). Health Canada’s Maximum Levels for Chemical Contaminants in Foods. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/maximum-levels-chemical-contaminants-foods.html. (accessed on 5 August 2020).
- Kristofferson, A.H.; Leroux, D.R.; Orr, J.R. A Biological Assessment of Arctic Char, Salvelinus alpinus (L.), Stocks in the Gjoa Haven-Pelly Bay Area of the Northwest Territories, 1979–1980; Western Region Department of Fisheries and Oceans: Winnipeg, MB, Canada, 1982; p. 51. [Google Scholar]
- Verbrugge, L. A Risk Management Strategy to Optimize the Public’s Health on Behalf of the Alaska Scientific Advisory Committee for Fish Consumption Section of Epidemiology Division of Public Health Department of Health and Social Services, State of Alaska. 2007. Available online: http://epibulletins.dhss.alaska.gov/Document/Display?DocumentId=262 (accessed on 20 July 2020).
- US Environmental Protection Agency. Water Quality Control Plan for Inland Surface Waters, Enclosed Bays, and Estuaries of California–Tribal and Subsistence Fishing Beneficial Uses and Mercury Provisions. 2017. Available online: https://www.epa.gov/sites/production/files/2017-07/documents/ca_hg_approval_letter_with_enclosures_signed_071417.pdf (accessed on 20 July 2020).
- NEIWPCC. A Regional Commission (USA) 2020. Available online: https://neiwpcc.org/our-programs/nps/mercury/# (accessed on 10 July 2020).
- Government of Alberta. Recommended Fish Consumption Limits in Alberta. 2019. Available online: https://mywildalberta.ca/fishing/advisories-corrections-closures/documents/FishConsumptionLimitsAlberta-Jun2019.pdf (accessed on 10 July 2020).
- Girard, C.; Charette, T.; Leclerc, M.; Shapiro, B.J.; Amyot, M. Cooking and co-ingested polyphenols reduce in vitro methylmercury bioaccessibility from fish and may alter exposure in humans. Sci. Total Environ. 2018, 616–617, 863–874. [Google Scholar] [CrossRef] [PubMed]
| Site (English Name) | Site (Inuktitut Name) | GPS Location | Water Type and Fishing Gear |
|---|---|---|---|
| Port Parry | Tununik | N 69°33′28.764″ W 97°26′13.884″ | fresh/nets |
| Koka Lake | Koka | N 68°32′5.1″ W 96°12′45.899″ | fresh/nets |
| Backhouse Point | Kautakshat | N 67°27′27.2″ W 95°21′38.6″ | brackish/nets |
| Legendary River | Kuuktaq | N 67°31′17.8″ W 96°26′21.8″ | brackish/nets |
| Merilik Lake | Malirualik | N 68°34′25.98″ W 97°19′36.72″ | fresh/nets |
| Murchison River | Kuuk | N 68°25′35.5″ W 93°19′11.6″ | fresh/nets |
| Back River | Amurat | N 66°57′30.70″ W 95°18′5.20″ | fresh/nets |
| KWI Weir | Iqalugmiat | N 68°55′58.08″ W 96° 13′10.2″ | fresh/spears/nets |
| West of Chantry 3 | Panigtogaruk | N 67°48′41.7096″ W 97°2′37.457″ | fresh/nets |
| West of Chantry 4 | Tahuaruak | N 67°52′10.4736″ W 96°43′7.3128″ | fresh/nets |
| Species | ||||
|---|---|---|---|---|
| Variable | Arctic Char | Lake Trout | Lake Whitefish | Cisco spp. |
| Sample size (n) | 197 | 136 | 100 | 98 |
| Fork length (mm) | 624 ± 18 | 638 ± 20 | 398 ± 10 | 334 ± 16 |
| Body weight (g) | 3086 ± 247 | 3308 ± 186 | 935 ± 79 | 504 ± 66 |
| Condition factor (K) | 1.155 ± 0.037 | 1.163 ± 0.028 | 1.409 ± 0.043 | 1.162 ± 0.057 |
| Age: | ||||
| Range | 5–29 | 8–62 | 4–47 | 2–30 |
| Mean ± 95% CI | 14.2 ± 0.6 | 25.4 ± 1.6 | 21.3 ± 2.2 | 14.8 ± 1.4 |
| Linear growth (mm∙year−1) | 47.1 ± 1.9 | 27.8 ± 1.4 | 23.8 ± 2.8 | 28.5 ± 2.9 |
| Mercury content (µg∙g−1 ww) | 0.073 ± 0.009 | 0.350 ± 0.042 | 0.092 ± 0.012 | 0.092 ± 0.013 |
| Selenium content (µg∙g−1 ww) | 0.44 ± 0.02 | 0.32 ± 0.02 | 0.34 ± 0.06 | 0.31 ± 0.02 |
| δ13C content (‰) | −25.1 ± 0.4 | −23.9 ± 0.7 | −21.3 ± 0.6 | −25.3 ± 0.5 |
| δ15N content (‰) | 14.0 ± 0.3 | 14.3 ± 0.3 | 11.3 ± 0.3 | 11.8 ± 0.2 |
| Assayed for δ15N & δ15N (n=) | 102 | 79 | 55 | 65 |
| Species | n | Age (years) | Fork Length (mm) | Round Weight (g) | Condition Factor (K) | Linear Growth (mm∙year−1) | Selenium (µg∙g−1 ww) | δ13C (‰) | δ15N (‰) |
|---|---|---|---|---|---|---|---|---|---|
| Arctic char | 197 | 0.143 ** | ns | ns | −0.097 ** | −0.042 ** | ns | 0.120 ** | −0.083 ** |
| Lake trout | 136 | 0.300 ** | 0.088 ** | ns | −0.145 ** | ns | ns | 0.098 ** | ns |
| Lake whitefish | 100 | 0.388 ** | 0.113 ** | 0.074 ** | ns | ns | ns | ns | ns |
| Cisco spp. | 98 | 0.117 ** | ns | ns | ns | ns | 0.139 ** | ns | ns |
| Species | n | Age (years) | Fork Length (mm) | Round Weight (g) | Condition Factor (K) | Linear Growth (mm∙year−1) | δ13C (‰) | δ15N (‰) |
|---|---|---|---|---|---|---|---|---|
| Arctic char | 197 | 0.058 ** | 0.149 ** | 0.055 ** | −0.063 ** | 0.111 ** | −0.162 ** | 0.053 * |
| Lake trout | 136 | ns | 0.195 ** | 0.289 ** | 0.099 ** | 0.127 ** | ns | 0.458 ** |
| Lake whitefish | 100 | ns | ns | ns | ns | ns | ns | ns |
| Cisco spp. | 98 | −0.054 * | ns | −0.055 * | ns | ns | ns | ns |
| Isotopic Carbon–δ13C | |||||||
| Species | n | Age (years) | Fork Length (mm) | Round Weight (g) | Condition Factor (K) | Linear Growth (mm∙year−1) | δ15N (‰) |
| Arctic char | 102 | ns | ns | −0.032 a | ns | −0.073 ** | ns |
| Lake trout | 79 | 0.122 ** | 0.086 ** | ns | −0.082 * | ns | 0.097 b |
| Lake whitefish | 55 | 0.206 ** | 0.069 c | 0.067 d | ns | ns | ns |
| Cisco spp. | 65 | ns | ns | ns | 0.109 ** | ns | −0.105 ** |
| Isotopic Nitrogen–δ15N | |||||||
| Species | n | Age (years) | Fork Length (mm) | Round Weight (g) | Condition Factor (K) | Linear Growth (mm∙year−1) | |
| Arctic char | 102 | 0.193 ** | 0.567 ** | 0.437 ** | ns | 0.406 ** | |
| Lake trout | 79 | 0.109 ** | 0.456 ** | 0.502 ** | ns | 0.377 ** | |
| Lake whitefish | 55 | 0.191 ** | 0.111 * | 0.084 ** | ns | ns | |
| Cisco spp. | 65 | 0.253 ** | 0.617 ** | 0.414 ** | ns | 0.088 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, I.; Das, P.; McPhedran, B.E.; Casselman, J.M.; Moniz, K.L.; van Coeverden de Groot, P.; Qitsualik, J.; Muir, D.; Schott, S.; Walker, V.K. Correlation of Mercury Occurrence with Age, Elemental Composition, and Life History in Sea-Run Food Fish from the Canadian Arctic Archipelago’s Lower Northwest Passage. Foods 2021, 10, 2621. https://doi.org/10.3390/foods10112621
Koch I, Das P, McPhedran BE, Casselman JM, Moniz KL, van Coeverden de Groot P, Qitsualik J, Muir D, Schott S, Walker VK. Correlation of Mercury Occurrence with Age, Elemental Composition, and Life History in Sea-Run Food Fish from the Canadian Arctic Archipelago’s Lower Northwest Passage. Foods. 2021; 10(11):2621. https://doi.org/10.3390/foods10112621
Chicago/Turabian StyleKoch, Iris, Pranab Das, Bronte E. McPhedran, John M. Casselman, Kristy L. Moniz, Peter van Coeverden de Groot, James Qitsualik, Derek Muir, Stephan Schott, and Virginia K. Walker. 2021. "Correlation of Mercury Occurrence with Age, Elemental Composition, and Life History in Sea-Run Food Fish from the Canadian Arctic Archipelago’s Lower Northwest Passage" Foods 10, no. 11: 2621. https://doi.org/10.3390/foods10112621







