Microstructural and Texturizing Properties of Partially Pectin-Depleted Cell Wall Material: The Role of Botanical Origin and High-Pressure Homogenization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blanching of the Fruit and Vegetable Tissues
2.3. Generation of the Alcohol-Insoluble Residues and Acid-Unextractable Fractions
2.4. Chemical Characterization
2.4.1. Analysis of the Uronic Acid Content of the Alcohol-Insoluble Residues and Acid-Unextractable Fractions
2.4.2. Analysis of the Neutral Monosaccharide Composition of the Acid-Unextractable Fractions
2.4.3. Analysis of the Degree of Methyl Esterification of the Acid-Unextractable Fractions
2.5. Preparation of the Suspensions of the Acid-Unextractable Fractions and High-Pressure Homogenization
2.6. Characterization of the Microstructural Properties of the Acid-Unextractable Fractions in Suspension
2.6.1. Microscopic Visualization
2.6.2. Particle Size Distribution Analysis
2.7. Characterization of the Functional Properties of the Acid-Unextractable Fractions in Suspension
2.7.1. Rheological Analysis
2.7.2. Measurement of Water Binding Capacity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization
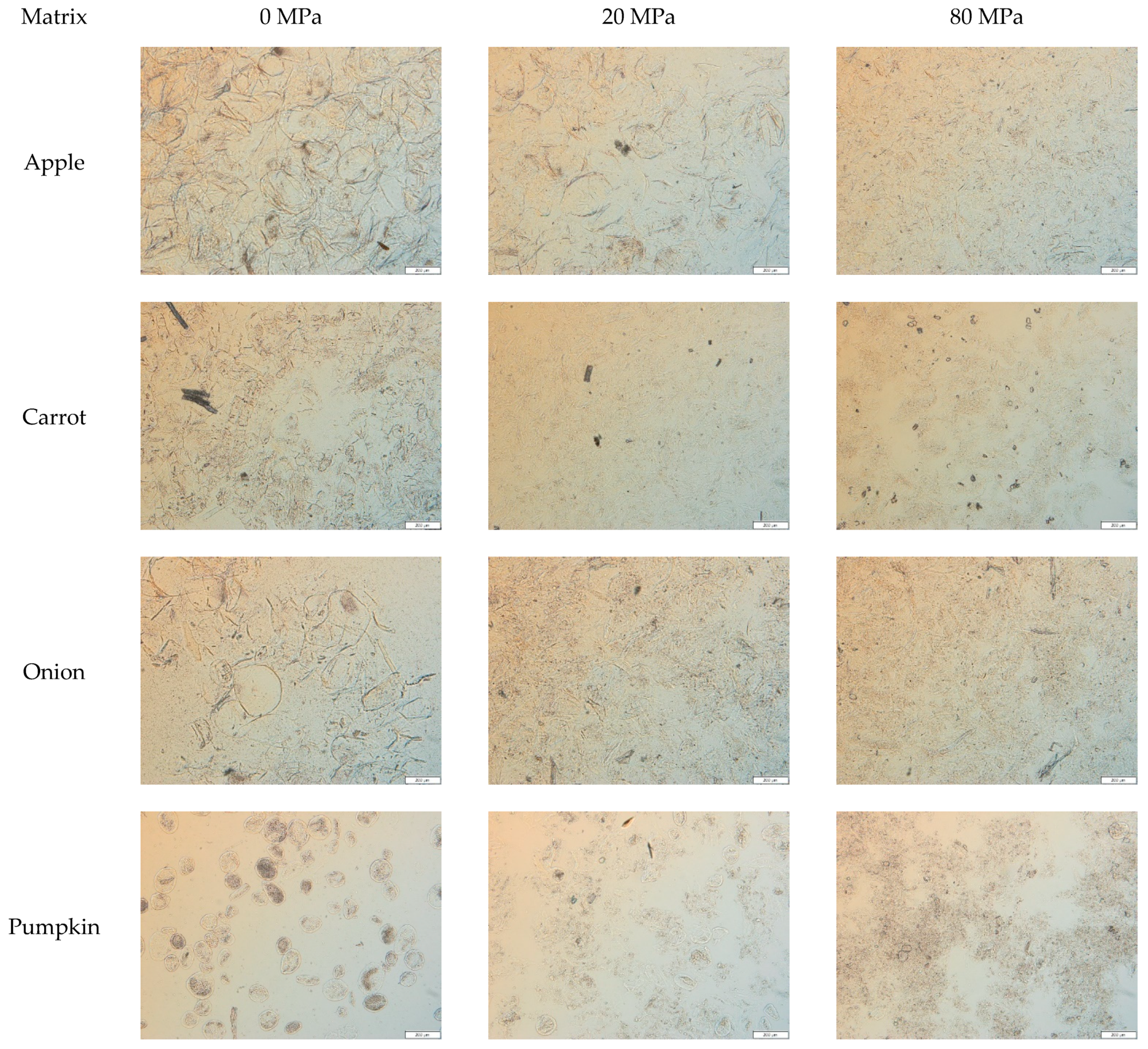
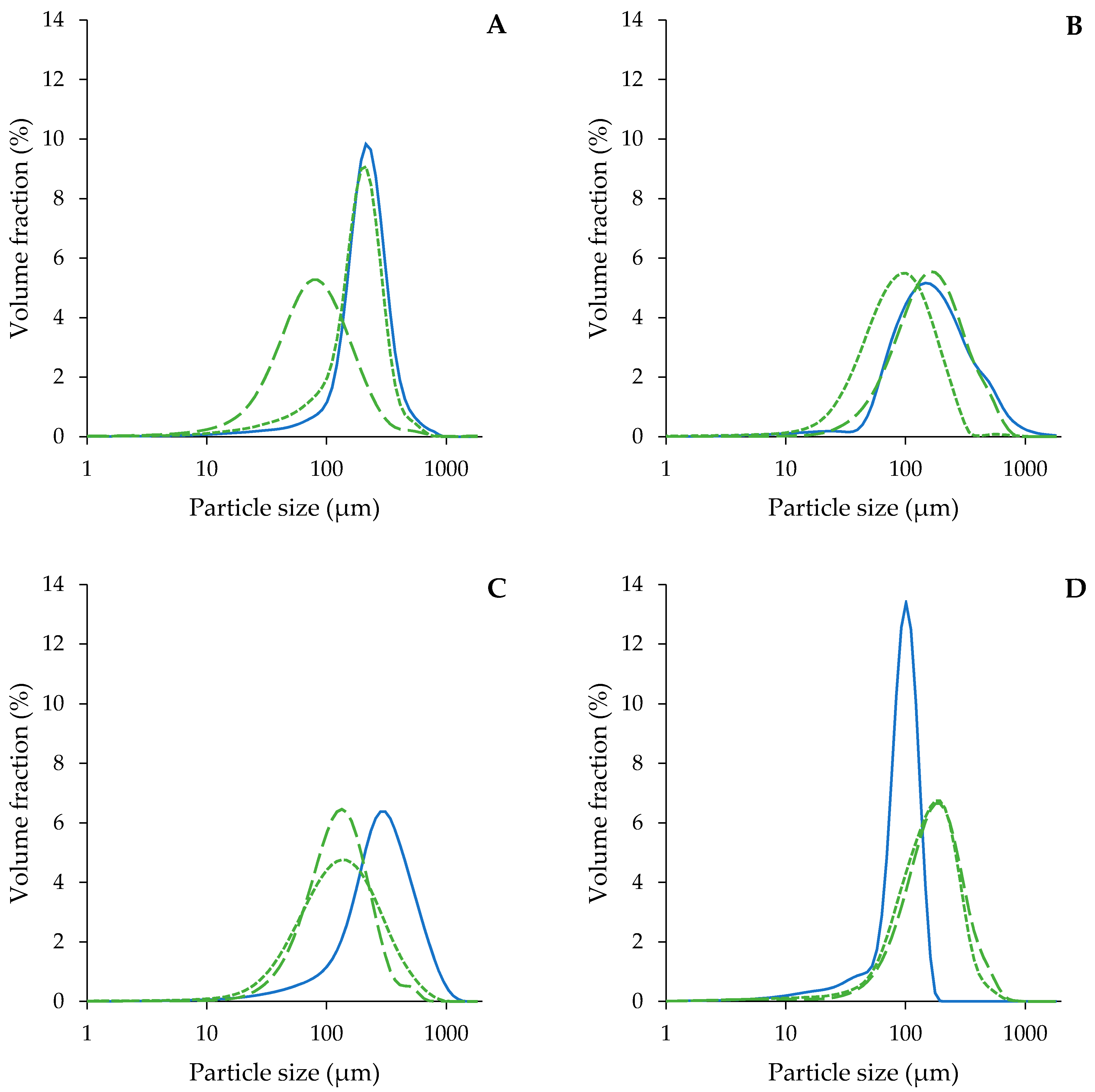
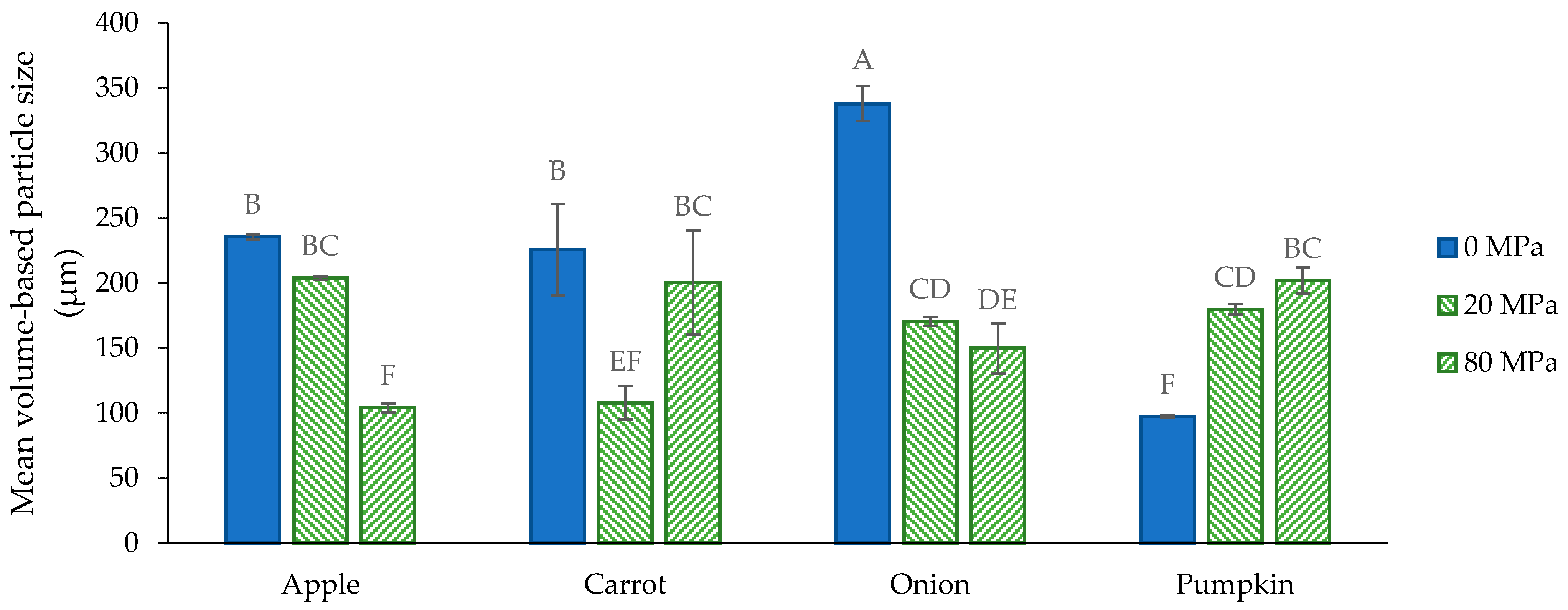
3.2. Microstructural Characterization
3.2.1. Microstructure of the Acid-Unextractable Fractions in Suspension before High-Pressure Homogenization
3.2.2. Microstructure of the Acid-Unextractable Fractions in Suspension after High-Pressure Homogenization and the Effect of Pressure Level
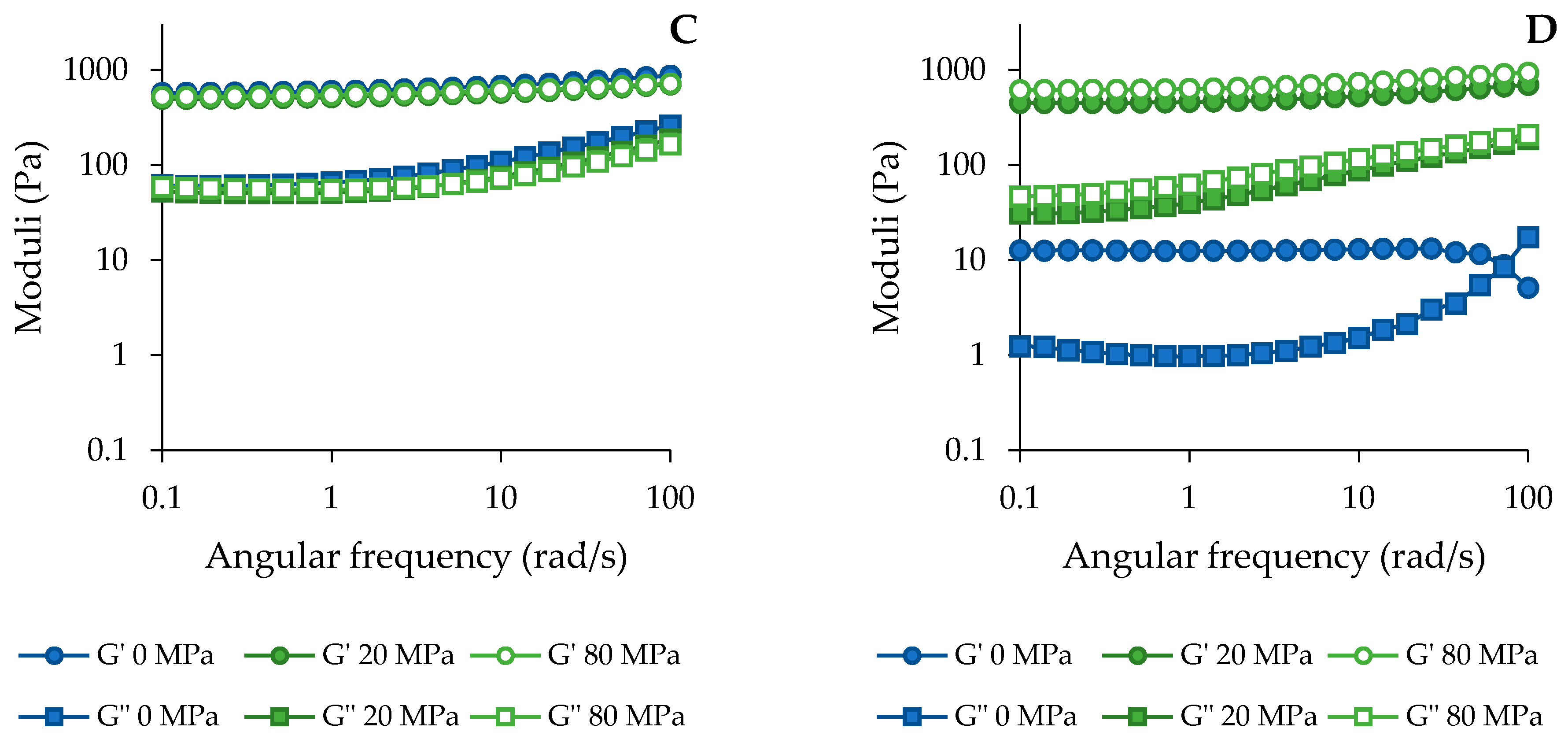
3.3. Functional Characterization
3.3.1. General Considerations
3.3.2. Functional Properties of the Acid-Unextractable Fractions in Suspension before High-Pressure Homogenization
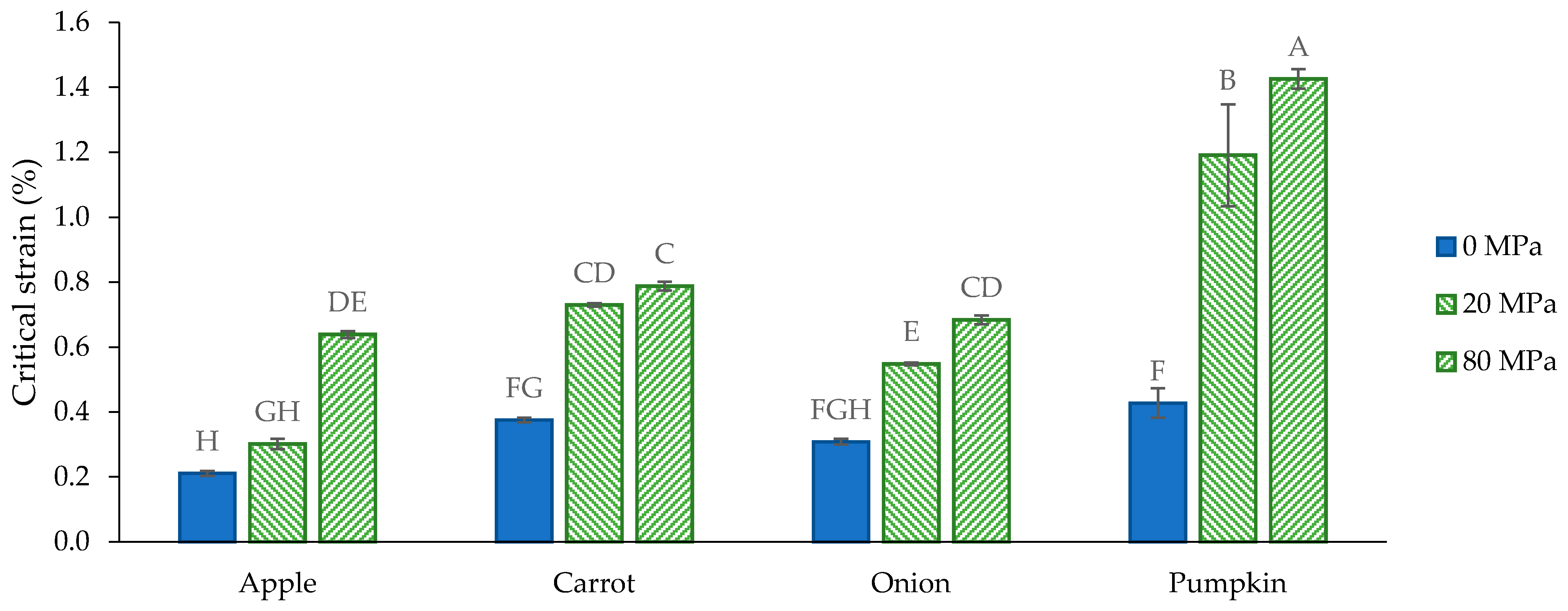
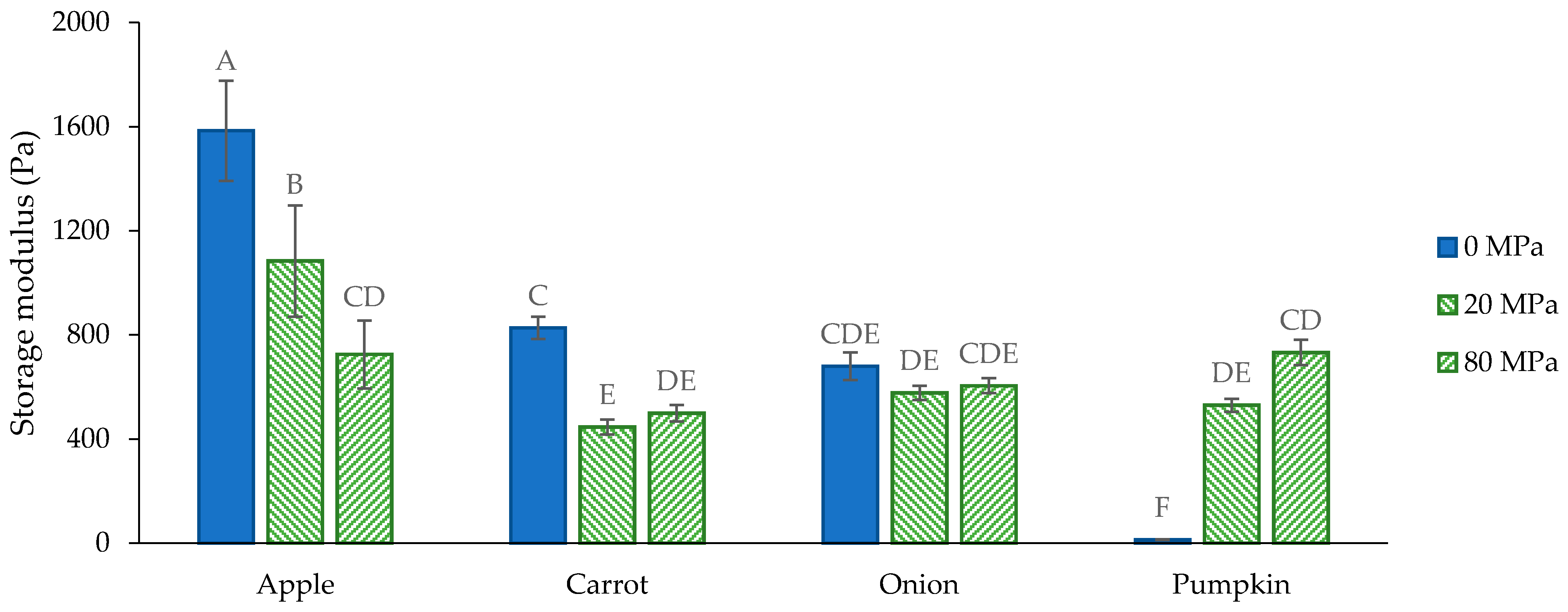
3.3.3. Functional Properties of the Acid-Unextractable Fractions in Suspension after High-Pressure Homogenization and the Effect of Pressure Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcUF | acid-unextractable fraction |
| AIR | alcohol-insoluble residue |
| Ara | arabinose |
| CWM | cell wall material |
| DM | degree of methyl esterification |
| Fuc | fucose |
| Gal | galactose |
| Glc | glucose |
| HPH | high-pressure homogenization |
| PSD | particle size distribution |
| Man | mannose |
| Rha | rhamnose |
| UA | uronic acid |
| WBC | water binding capacity |
| Xyl | xylose |
| d4,3 | mean volume-based particle size |
| G′ | storage modulus |
| G″ | loss modulus |
Appendix A

References
- Lopez-Sanchez, P.; Nijsse, J.; Blonk, H.C.G.; Bialek, L.; Schumm, S.; Langton, M. Effect of mechanical and thermal treatments on the microstructure and rheological properties of carrot, broccoli and tomato dispersions. J. Sci. Food Agric. 2011, 91, 207–217. [Google Scholar] [CrossRef]
- Moelants, K.R.N.; Cardinaels, R.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. A Review on the relationships between processing, food structure, and rheological properties of plant-tissue-based food suspensions. Compr. Rev. Food Sci. Food Saf. 2014, 13, 241–260. [Google Scholar] [CrossRef]
- Waldron, K.W.; Parker, M.L.; Smith, A.C. Plant Cell Walls and Food Quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Wall Structure and Wall Loosening. A Look Backwards and Forwards. Plant Physiol. 2001, 125, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Popper, Z.A.; Fry, S.C. Widespread Occurrence of a Covalent Linkage between Xyloglucan and Acidic Polysaccharides in Suspension-cultured Angiosperm Cells. Ann. Bot. 2005, 96, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Zykwinska, A.W.; Ralet, M.-C.J.; Garnier, C.D.; Thibault, J.-F.J. Evidence for In Vitro Binding of Pectin Side Chains to Cellulose. Plant Physiol. 2005, 139, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zykwinska, A.; Thibault, J.-F.; Ralet, M.-C. Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. J. Exp. Bot. 2007, 58, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.J. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Ricci, J.; Delalonde, M.; Wisniewski, C.; Dahdouh, L. Role of dispersing and dispersed phases in the viscoelastic properties and the flow behavior of fruit juices during concentration operation: Case of orange juice. Food Bioprod. Process. 2021, 126, 121–129. [Google Scholar] [CrossRef]
- Leverrier, C.; Almeida, G.; Menut, P.; Cuvelier, G. Design of Model Apple Cells Suspensions: Rheological Properties and Impact of the Continuous Phase. Food Biophys. 2017, 12, 383–396. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Chapara, V.; Schumm, S.; Farr, R. Shear Elastic Deformation and Particle Packing in Plant Cell Dispersions. Food Biophys. 2012, 7, 1–14. [Google Scholar] [CrossRef]
- Moelants, K.R.N.; Cardinaels, R.; Jolie, R.P.; Verrijssen, T.A.J.; Van Buggenhout, S.; Zumalacarregui, L.M.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Relation between particle properties and rheological characteristics of carrot-derived suspensions. Food Bioprocess Technol. 2013, 6, 1127–1143. [Google Scholar] [CrossRef]
- Appelqvist, I.A.M.; Cochet-Broch, M.; Poelman, A.A.M.; Day, L. Morphologies, volume fraction and viscosity of cell wall particle dispersions particle related to sensory perception. Food Hydrocoll. 2015, 44, 198–207. [Google Scholar] [CrossRef]
- Hemar, Y.; Lebreton, S.; Xu, M.; Day, L. Small-deformation rheology investigation of rehydrated cell wall particles–xanthan mixtures. Food Hydrocoll. 2011, 25, 668–676. [Google Scholar] [CrossRef]
- Bengtsson, H.; Tornberg, E. Physicochemical characterization of fruit and vegetable fiber suspensions. I: Effect of homogenization. J. Texture Stud. 2011, 42, 268–280. [Google Scholar] [CrossRef]
- Bengtsson, H.; Wikberg, J.; Tornberg, E. Physicochemical characterization of fruit and vegetable fiber suspensions. II: Effect of variations in heat treatmens. J. Texture Stud. 2011, 42, 281–290. [Google Scholar] [CrossRef]
- Diamante, L.; Umemoto, M. Rheological Properties of Fruits and Vegetables: A Review. Int. J. Food Prop. 2015, 18, 1191–1210. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Farr, R. Power Laws in the Elasticity and Yielding of Plant Particle Suspensions. Food Biophys. 2012, 7, 15–27. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications beyond Microbial Inactivation. Food Eng. Rev. 2020, 1–19. [Google Scholar] [CrossRef]
- Salehi, F. Physico-chemical and rheological properties of fruit and vegetable juices as affected by high pressure homogenization: A review. Int. J. Food Prop. 2020, 23, 1136–1149. [Google Scholar] [CrossRef]
- Atencio, S.; Bernaerts, T.; Liu, D.; Reineke, K.; Hendrickx, M.; Van Loey, A. Impact of processing on the functionalization of pumpkin pomace as a food texturizing ingredient. Innov. Food Sci. Emerg. Technol. 2021, 69, 102669. [Google Scholar] [CrossRef]
- Willemsen, K.L.D.D.; Panozzo, A.; Moelants, K.; Debon, S.J.J.; Desmet, C.; Cardinaels, R.; Moldenaers, P.; Wallecan, J.; Hendrickx, M.E.G. Physico-chemical and viscoelastic properties of high pressure homogenized lemon peel fiber fraction suspensions obtained after sequential pectin extraction. Food Hydrocoll. 2017, 72, 358–371. [Google Scholar] [CrossRef] [Green Version]
- Koubala, B.B.; Kansci, G.; Mbome, L.I.; Crépeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Chaula, D.; Van Loey, A.M.; Hendrickx, M.E. Unravelling process-induced pectin changes in the tomato cell wall: An integrated approach. Food Chem. 2012, 132, 1534–1543. [Google Scholar] [CrossRef]
- McFeeters, R.F.; Armstrong, S.A. Measurement of pectin methylation in plant cell walls. Anal. Biochem. 1984, 139, 212–217. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1977, 1, 361–365. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method of quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Yeats, T.; Vellosillo, T.; Sorek, N.; Ibáñez, A.; Bauer, S. Rapid Determination of Cellulose, Neutral Sugars, and Uronic Acids from Plant Cell Walls by One-step Two-step Hydrolysis and HPAEC-PAD. Bio-Protocol 2016, 6, e1978. [Google Scholar] [CrossRef] [Green Version]
- Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods 2021, 10, 1064. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Charleer, L.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stability during gastrointestinal conditions effects lipid digestion kinetics. Food Chem. 2018, 246, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, K.L.D.D.; Panozzo, A.; Moelants, K.; Cardinaels, R.; Wallecan, J.; Moldenaers, P.; Hendrickx, M. Effect of pH and salts on microstructure and viscoelastic properties of lemon peel acid insoluble fiber suspensions upon high pressure homogenization. Food Hydrocoll. 2018, 82, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Auffret, A.; Ralet, M.-C.; Guillon, F.; Barry, J.-L.; Thibault, J.-F. Effect of Grinding and Experimental Conditions on the Measurement of Hydration Properties of Dietary Fibres. LWT Food Sci. Technol. 1994, 27, 166–172. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Voragen, A.G.J.; Thibault, J.F.; Pilnik, W. Studies on Apple Protopectin: I. Extraction of Insoluble Pectin by Chemical Means. Carbohydr. Polym. 1990, 12, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Massiot, P.; Baron, A.; Drilleau, J.F. Characterisation and enzymatic hydrolysis of cell-wall polysaccharides from different tissue zones of apple. Carbohydr. Polym. 1994, 25, 145–154. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Psonka-Antonczyk, K.M.; Stokke, B.T. The relation of apple texture with cell wall nanostructure studied using an atomic force microscope. Carbohydr. Polym. 2013, 92, 128–137. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Selvendran, R.R. Structural features of cell-wall polysaccharides of onion Allium cepa. Carbohydr. Res. 1986, 157, 183–199. [Google Scholar] [CrossRef]
- De Escalada Pla, M.F.; Ponce, N.M.; Wider, M.E.; Stortz, C.A.; Rojas, A.M.; Gerschenson, L.N. Chemical and biochemical changes of pumpkin (Cucumis moschata, Duch) tissue in relation to osmotic stress. J. Sci. Food Agric. 2005, 85, 1852–1860. [Google Scholar] [CrossRef]
- Fry, S.C. Cross-Linking of Matrix Polymers in the Growing Cell Walls of Angiosperms. Annu. Rev. Plant Physiol. 1986, 37, 165–186. [Google Scholar] [CrossRef]
- Massiot, P.; Rouau, X.; Thibault, J.-F. Characterisation of the extractable pectins and hemicelluloses of the cell wall of carrot. Carbohydr. Res. 1988, 172, 229–242. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.H.E.; Van Audenhove, J.; Bernaerts, T.; de Wilde d’Estmael, H.; Hendrickx, M.E.; Van Loey, A.M. Structural and emulsion stabilizing properties of pectin rich extracts obtained from different botanical sources. Food Res. Int. 2021, 141, 110087. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. Characterisation of cell wall polysaccharide profiles of apricots (Prunus armeniaca L.), peaches (Prunus persica L.), and pumpkins (Cucurbita sp.) for the evaluation of fruit product authenticity. Food Chem. 2008, 106, 421–430. [Google Scholar] [CrossRef]
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G.J. A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr. Res. 1995, 279, 265–279. [Google Scholar] [CrossRef]
- Christiaens, S.; Uwibambe, D.; Uyttebroek, M.; Van Droogenbroeck, B.; Van Loey, A.M.; Hendrickx, M.E. Pectin characterisation in vegetable waste streams: A starting point for waste valorisation in the food industry. LWT Food Sci. Technol. 2015, 61, 275–282. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Parker, C.C.; Smith, A.C.; Waldron, K.W. Characterization and Distribution of Phenolics in Carrot Cell Walls. J. Agric. Food Chem. 2008, 56, 8558–8564. [Google Scholar] [CrossRef]
- Mankarios, A.T.; Hall, M.A.; Jarvis, M.C.; Threlfall, D.R.; Friend, J. Cell wall polysaccharides from onions. Phytochemistry 1980, 19, 1731–1733. [Google Scholar] [CrossRef]
- Golovchenko, V.V.; Khramova, D.S.; Ovodova, R.G.; Shashkov, A.S.; Ovodov, Y.S. Structure of pectic polysaccharides isolated from onion Allium cepa L. using a simulated gastric medium and their effect on intestinal absorption. Food Chem. 2012, 134, 1813–1822. [Google Scholar] [CrossRef]
- Sen, S.K.; Chatterjee, B.P.; Rao, C.V.N. A galactan from onion (Allium cepa Linn.) pectic substance. J. Chem. Soc. C Org. 1971, 1788–1791. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.H.E.; Bernaerts, T.; Verheyen, D.; Hendrickx, M.E.; Van Loey, A.M. Investigating the role of the different molar mass fractions of a pectin rich extract from onion towards its emulsifying and emulsion stabilizing potential. Food Hydrocoll. 2021, 117, 106735. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.-C.; Thibault, J.-F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Lecain, S.; Ng, A.; Parker, M.L.; Smith, A.C.; Waldron, K.W. Modification of cell-wall polymers of onion waste—Part I. Effect of pressure-cooking. Carbohydr. Polym. 1999, 38, 59–67. [Google Scholar] [CrossRef]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Vetter, S.; Kunzek, H. The influence of the sequential extractions on the structure and the properties of single cell materials from apples. Eur. Food Res. Technol. 2003, 217, 392–400. [Google Scholar] [CrossRef]
- Waldron, K.W.; Smith, A.C.; Parr, A.J.; Ng, A.; Parker, M.L. New approaches to understanding and controlling cell separation in relation to fruit and vegetable texture. Trends Food Sci. Technol. 1997, 8, 213–221. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.; Kristiani, K.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Minimizing quality changes of cloudy apple juice: The use of kiwifruit puree and high pressure homogenization. Food Chem. 2018, 249, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, S.; Van Buggenhout, S.; Chaula, D.; Moelants, K.; David, C.C.; Hofkens, J.; Van Loey, A.M.; Hendrickx, M.E. In situ pectin engineering as a tool to tailor the consistency and syneresis of carrot purée. Food Chem. 2012, 133, 146–155. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Svelander, C.; Bialek, L.; Schumm, S.; Langton, M. Rheology and Microstructure of Carrot and Tomato Emulsions as a Result of High-Pressure Homogenization Conditions. J. Food Sci. 2011, 76, E130–E140. [Google Scholar] [CrossRef]
- Day, L.; Xu, M.; Øiseth, S.K.; Hemar, Y.; Lundin, L. Control of morphological and rheological properties of carrot cell wall particle dispersions through processing. Food Bioprocess Technol. 2010, 3, 928–934. [Google Scholar] [CrossRef]
- Houben, K.; Christiaens, S.; Ngouémazong, D.E.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. The Effect of Endogenous Pectinases on the Consistency of Tomato–Carrot Purée Mixes. Food Bioprocess Technol. 2014, 7, 2570–2580. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Jernstedt, J.A.; Slaughter, D.C.; Barrett, D.M. Microscopic Quantification of Cell Integrity in Raw and Processed Onion Parenchyma Cells. J. Food Sci. 2010, 75, E402–E408. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Muñoz, L.; Renard, C.M.G.C.; Symoneaux, R.; Biau, N.; Cuvelier, G. Structural parameters that determine the rheological properties of apple puree. J. Food Eng. 2013, 119, 619–626. [Google Scholar] [CrossRef]
- Mayor, L.; Pissarra, J.; Sereno, A.M. Microstructural changes during osmotic dehydration of parenchymatic pumpkin tissue. J. Food Eng. 2008, 85, 326–339. [Google Scholar] [CrossRef]
- Wang, X.; Lee, J.; Wang, Y.-W.; Huang, Q. Composition and Rheological Properties of β-Lactoglobulin/Pectin Coacervates: Effects of Salt Concentration and Initial Protein/Polysaccharide Ratio. Biomacromolecules 2007, 8, 992–997. [Google Scholar] [CrossRef]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional colloids from proteins and polysaccharides for food applications. Trends Food Sci. Technol. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Sankaran, A.K.; Nijsse, J.; Bialek, L.; Bouwens, L.; Hendrickx, M.E.; Van Loey, A.M. Effect of enzyme homogenization on the physical properties of carrot cell wall suspensions. Food Bioprocess Technol. 2015, 8, 1377–1385. [Google Scholar] [CrossRef]
- Gundurao, A.; Ramaswamy, H.S.; Ahmed, J. Effect of soluble solids concentration and temperature on thermo-physical and rheological properties of mango puree. Int. J. Food Prop. 2011, 14, 1018–1036. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Gehin-Delval, C. Physicochemical properties of cell wall materials from apple, kiwifruit and tomato. Eur. Food Res. Technol. 2008, 227, 607–618. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, P.; Li, H.; Adhikari, B.P.; Li, D. Rheological Behavior of Tomato Fiber Suspensions Produced by High Shear and High Pressure Homogenization and Their Application in Tomato Products. Int. J. Anal. Chem. 2018, 5081938. [Google Scholar] [CrossRef]
- Kunzek, H.; Kabbert, R.; Gloyna, D. Aspects of material science in food processing: Changes in plant cell walls of fruits and vegetables. Zeitschrift Leb. Forsch. A 1999, 208, 233–250. [Google Scholar] [CrossRef]
- Pickardt, C.; Dongowski, G.; Kunzek, H. The influence of mechanical and enzymatic disintegration of carrots on the structure and properties of cell wall materials. Eur. Food Res. Technol. 2004, 219, 229–239. [Google Scholar] [CrossRef]
- Kotcharian, A.; Kunzek, H.; Dongowski, G. The influence of variety on the enzymatic degradation of carrots and on functional and physiological properties of the cell wall materials. Food Chem. 2004, 87, 231–245. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Bayod, E.; Willers, E.P.; Tornberg, E. Rheological and structural characterization of tomato paste and its influence on the quality of ketchup. LWT Food Sci. Technol. 2008, 41, 1289–1300. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterisation of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid, Semisolid, and Solid Foods, 3rd ed.; Barbosa-Canovas, G.V., Ed.; Food Engineering Series; Springer: New York, NY, USA, 2014; ISBN 978-1-4614-9229-0. [Google Scholar]
- Moelants, K.R.N.; Cardinaels, R.; Jolie, R.P.; Verrijssen, T.A.J.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Rheology of Concentrated Tomato-Derived Suspensions: Effects of Particle Characteristics. Food Bioprocess Technol. 2014, 7, 248–264. [Google Scholar] [CrossRef]
- Chaplin, M.F. Fibre and water binding. Proc. Nutr. Soc. 2003, 62, 223–227. [Google Scholar] [CrossRef]
- López, G.; Ros, G.; Rincón, F.; Periago, M.J.; Martínez, M.C.; Ortuño, J. Relationship between Physical and Hydration Properties of Soluble and Insoluble Fiber of Artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- Thebaudin, J.Y.; Lefebvre, A.C.; Harrington, M.; Bourgeois, C.M. Dietary fibres: Nutritional and technological interest. Trends Food Sci. Technol. 1997, 8, 41–48. [Google Scholar] [CrossRef]
- Ulbrich, M.; Flöter, E. Impact of high pressure homogenization modification of a cellulose based fiber product on water binding properties. Food Hydrocoll. 2014, 41, 281–289. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Wallecan, J.; Christiaens, S.; Debon, S.J.J.; Desmet, C.; Van Loey, A.; Hendrickx, M.; Mazoyer, J. Influence of high-pressure homogenization on functional properties of orange pulp. Innov. Food Sci. Emerg. Technol. 2015, 30, 51–60. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U. Pectin-water interactions in foods–From powder to gel. Food Hydrocoll. 2018, 78, 109–119. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Hatakeyama, H.; Hatakeyama, T. Influence of pectin modification on water binding properties. Food Hydrocoll. 2012, 27, 494–502. [Google Scholar] [CrossRef]
- Den Ouden, F.W.C.; Van Vliet, T. Particle Size Distribution in Tomato Concentrate and Effects on Rheological Properties. J. Food Sci. 1997, 62, 565–567. [Google Scholar] [CrossRef]
- Leverrier, C.; Almeida, G.; Espinosa-Muñoz, L.; Cuvelier, G. Influence of Particle Size and Concentration on Rheological Behaviour of Reconstituted Apple Purees. Food Biophys. 2016, 11, 235–247. [Google Scholar] [CrossRef]
- Willemsen, K.L.D.D.; Panozzo, A.; Moelants, K.; Wallecan, J.; Hendrickx, M. Towards improved understanding of the viscoelastic properties of functionalized lemon peel fibers in suspension based on microstructure, hydration value and swelling volume. J. Food Eng. 2020, 278, 109950. [Google Scholar] [CrossRef]


| Apple | Carrot | Onion | Pumpkin | |
|---|---|---|---|---|
| Monosaccharide composition (mg/g) | ||||
| Fuc | 7.1 ± 0.9 | <d.l. | 0.6 ± 0.2 | <d.l. |
| Rha | 7.0 ± 0.6 | 8.4 ± 0.3 | 3.5 ± 0.1 | 4.7 ± 0.2 |
| Ara | 24.7 ± 0.8 | 15.9 ± 0.1 | 4.9 ± 0.3 | 3.8 ± 0.8 |
| Gal | 56.9 ± 3.6 | 56.6 ± 4.6 | 77.6 ± 3.9 | 52.5 ± 4.7 |
| Glc | 454.7 ± 14.7 | 382.8 ± 11.4 | 425.7 ± 16.6 | 470.7 ± 19.3 |
| Xyl | 70.3 ± 6.5 | 17.3 ± 0.7 | 36.8 ± 1.6 | 35.3 ± 5.9 |
| Man | 24.7 ± 1.5 | 25.8 ± 2.0 | 17.4 ± 0.8 | 17.1 ± 3.9 |
| UA | 143.2 ± 4.9 | 193.5 ± 9.0 | 102.6 ± 4.0 | 91.4 ± 3.5 |
| Other compositional and structural properties | ||||
| DM (%) | 37.0 ± 2.4 | 38.4 ± 1.8 | 40.4 ± 4.3 | 53.4 ± 3.5 |
| Contribution of the pectin backbone (UA + Rha) (mol%) | 17.6 ± 0.7 | 27.2 ± 1.3 | 14.8 ± 0.7 | 13.3 ± 0.6 |
| Ratio of Glc to UA (−) | 3.4 ± 0.2 | 2.1 ± 0.1 | 4.5 ± 0.2 | 5.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Audenhove, J.; Bernaerts, T.; Putri, N.I.; Okello, E.O.; Van Rooy, L.; Van Loey, A.M.; Hendrickx, M.E. Microstructural and Texturizing Properties of Partially Pectin-Depleted Cell Wall Material: The Role of Botanical Origin and High-Pressure Homogenization. Foods 2021, 10, 2644. https://doi.org/10.3390/foods10112644
Van Audenhove J, Bernaerts T, Putri NI, Okello EO, Van Rooy L, Van Loey AM, Hendrickx ME. Microstructural and Texturizing Properties of Partially Pectin-Depleted Cell Wall Material: The Role of Botanical Origin and High-Pressure Homogenization. Foods. 2021; 10(11):2644. https://doi.org/10.3390/foods10112644
Chicago/Turabian StyleVan Audenhove, Jelle, Tom Bernaerts, Novita I. Putri, Erick O. Okello, Luisa Van Rooy, Ann M. Van Loey, and Marc E. Hendrickx. 2021. "Microstructural and Texturizing Properties of Partially Pectin-Depleted Cell Wall Material: The Role of Botanical Origin and High-Pressure Homogenization" Foods 10, no. 11: 2644. https://doi.org/10.3390/foods10112644
APA StyleVan Audenhove, J., Bernaerts, T., Putri, N. I., Okello, E. O., Van Rooy, L., Van Loey, A. M., & Hendrickx, M. E. (2021). Microstructural and Texturizing Properties of Partially Pectin-Depleted Cell Wall Material: The Role of Botanical Origin and High-Pressure Homogenization. Foods, 10(11), 2644. https://doi.org/10.3390/foods10112644







