Comparing Taste Detection Thresholds across Individuals Following Vegan, Vegetarian, or Omnivore Diets
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Overview of the Study
2.3. Stimuli
2.4. Detection Threshold Tests
2.5. Data Analysis
2.5.1. Detection Threshold Estimation
2.5.2. Statistical Analysis
3. Results
3.1. Participant Characteristics
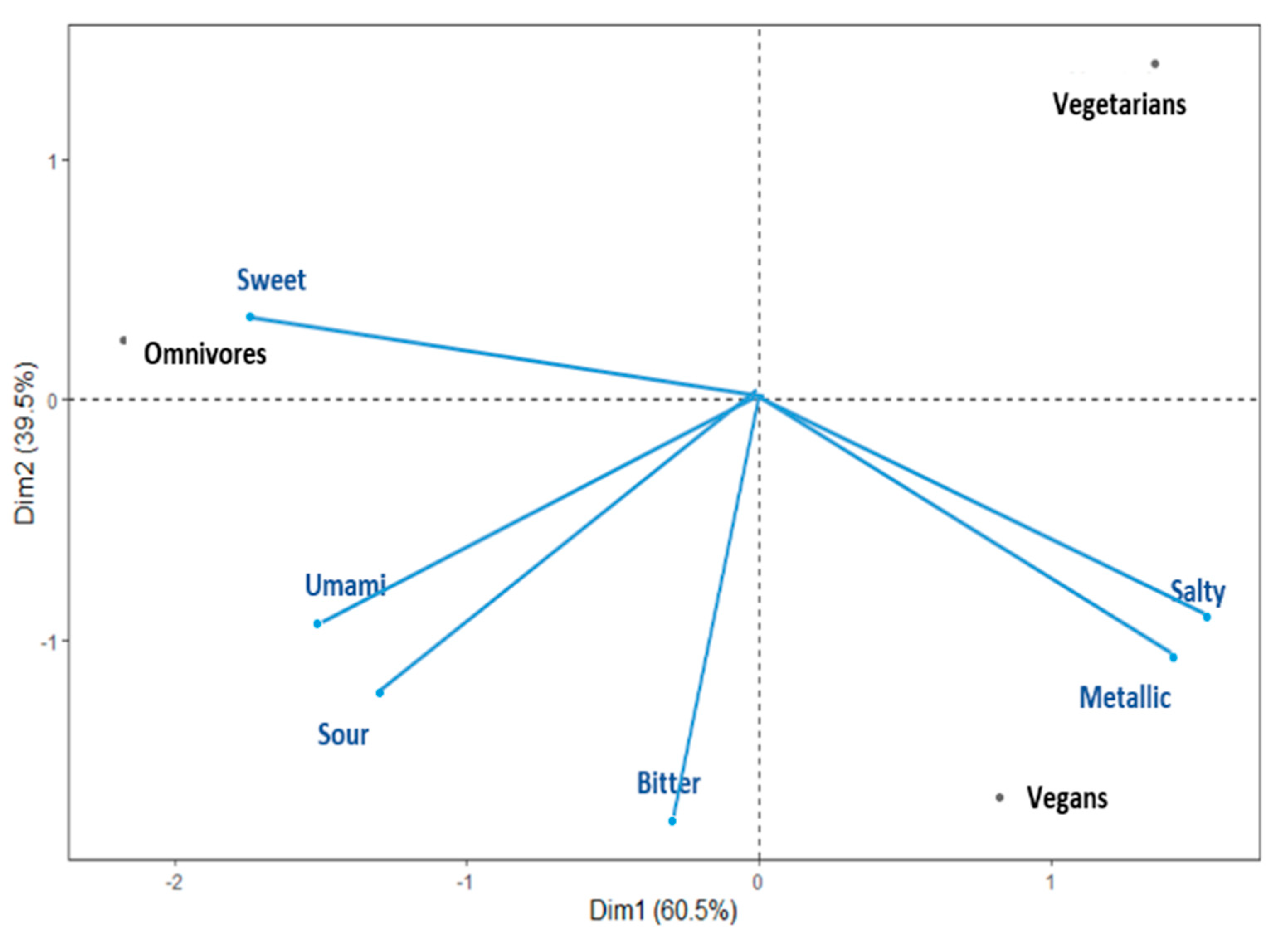
3.2. Comparing Taste Sensitivity Patterns across the Vegan, Vegetarian, and Omnivore Groups
3.3. Profiling Taste Sensitivities for Vegan, Vegetarian, and Omnivore Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puputti, S.; Hoppu, U.; Sandell, M. Taste sensitivity is associated with food consumption behavior but not with recalled pleasantness. Foods 2019, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.E.; Keast, R.S.J. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, L.P.; Bolhuis, D.P.; Toress, S.J.; Keast, R.S. Dietary fat restriction increases fat taste sensitivity in people with obesity. Obesity 2016, 24, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Ohara, I.; Tabuchi, R.; Kimura, M.; Itokowa, Y. Decline of taste sensitivity in protein deficient adult rats. Physiol. Behav. 1995, 57, 921–926. [Google Scholar] [CrossRef]
- Juan, W.; Yamini, S.; Britten, P. Food intake patterns of self-identified vegetarians among the US population, 2007–2010. Procedia Food Sci. 2015, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, G.B.; Clari, R.; Vigotti, F.N.; Leone, F.; Attini, R.; Cabiddu, G.; Mauro, G.; Castelluccia, N.; Colombi, N.; Capizzi, I.; et al. Vegan–vegetarian diets in pregnancy: Danger or panacea? A systematic narrative review. Int. J. Obstet. Gynaecol. 2015, 122, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, C.T.; Temple, N.; Woodside, J.W. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Dorard, G.; Mathieu, S. Vegetarian and omnivorous diets: A cross-sectional study of motivation, eating disorders, and body shape perception. Appetite 2021, 156, 104972. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, D.L. Gender differences in vegetarian identity: How men and women construe meatless dieting. Food Qual. Prefer. 2020, 81, 103859. [Google Scholar] [CrossRef]
- Sanchez-Sabate, R.; Sabaté, J. Consumer attitudes towards environmental concerns of meat consumption: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 1220. [Google Scholar] [CrossRef] [Green Version]
- Roper, J.E. Dietary Intakes and Major Food Sources of Vitamin B12 among New Zealand Adolescent Males and Females. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2021. [Google Scholar]
- Liem, D.G.; Russell, C.G. The influence of taste liking on the consumption of nutrient rich and nutrient poor foods. Front. Nutr. 2019, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Gargari, G.; Koirala, R.; Laureati, M.; Riso, P.; Guglielmetti, S.; Pagliarini, E. New insights into the relationship between taste perception and oral microbiota composition. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawless, H.T. A simple alternative analysis for threshold data determined by ascending forced-choice methods of limits. J. Sens. Stud. 2010, 25, 332–346. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Discrimination Testing. In Sensory Evaluation of Food; Springer: Berlin/Heidelberg, Germany, 2010; pp. 79–100. [Google Scholar]
- Chamoun, E.; Liu, A.A.; Duizer, L.M.; Darlington, G.; Duncan, A.M.; Haines, J.; Ma, D.W. Taste sensitivity and taste preference measures are correlated in healthy young adults. Chem. Senses 2019, 44, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Veček, N.N.; Mucalo, L.; Dragun, R.; Miličević, T.; Pribisalić, A.; Patarčić, I.; Hayward, C.; Polašek, O.; Kolčić, I. The association between salt taste perception, mediterranean diet and metabolic syndrome: A cross-sectional study. Nutrients 2020, 12, 1164. [Google Scholar] [CrossRef]
- Wise, P.M.; Nattress, L.; Flammer, L.J.; Beauchamp, G.K. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am. J. Clin. Nutr. 2016, 103, 50–60. [Google Scholar] [CrossRef]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.E.; Newman, L.P.; Keast, R.S.J. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef]
- Heinze, J.M.; Costanzo, A.; Baselier, I.; Fritsche, A.; Frank-Podlech, S.; Keast, R. Detection thresholds for four different fatty stimuli are associated with increased dietary intake of processed high-caloric food. Appetite 2018, 123, 7–13. [Google Scholar] [CrossRef]
- Trachootham, D.; Satoh-Kuriwada, S.; Lam-Ubol, A.; Promkam, C.; Chotechuang, N.; Sasano, T.; Shoji, N. Differences in taste perception and spicy preference: A Thai–Japanese cross-cultural study. Chem. Senses 2018, 43, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Lee, H.M. Frequent consumption of certain fast foods may be associated with an enhanced preference for salt taste. J. Hum. Nutr. Diet. 2009, 22, 475–480. [Google Scholar] [CrossRef]
- Kim, H.; Han, S.N.; Song, K.; Lee, H. Lifestyle, dietary habits and consumption pattern of male university students according to the frequency of commercial beverage consumptions. Nutr. Res. Pract. 2011, 5, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, H.; Kessler, C.S.; Sundberg, T.; Leach, M.J.; Schumann, D.; Adams, J.; Lauche, R. Characteristics of Americans choosing vegetarian and vegan diets for health reasons. J. Nutr. Educ. Behav. 2017, 49, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Dagnelie, P.C.; Mariotti, F. Vegetarian diets: Definitions and Pitfalls in Interpreting Literature on Health Effects of Vegetarianism. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–10. [Google Scholar]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Rosell, M.S. Health effects of vegetarian and vegan diets. Proc. Nutr. Soc. 2006, 65, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-E.; Yen, C.H.; Huang, M.C.; Cheng, C.H.; Huang, Y.C. Dietary intake and nutritional status of vegetarian and omnivorous preschool children and their parents in Taiwan. Nutr. Res. 2008, 28, 430–436. [Google Scholar] [CrossRef]
- Ball, M.J.; Bartlett, M.A. Dietary intake and iron status of Australian vegetarian women. Am. J. Clin. Nutr. 1999, 70, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciuris, C.; Lynch, H.M.; Wharton, C.; Johnston, C.S. A comparison of dietary protein digestibility, based on DIAAS scoring, in vegetarian and non-vegetarian athletes. Nutrients 2019, 11, 3016. [Google Scholar] [CrossRef] [Green Version]
- Lau, E.; Kwok, T.; Woo, J.; Ho, S.C. Bone mineral density in Chinese elderly female vegetarians, vegans, lacto-vegetarians and omnivores. Eur. J. Clin. Nutr. 1998, 52, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Janelle, K.C.; Barr, S.I. Nutrient intakes and eating behavior see of vegetarian and nonvegetarian women. J. Am. Diet. Assoc. 1995, 95, 180–189. [Google Scholar] [CrossRef]
- Cliceri, D.; Spinelli, S.; Dinnella, C.; Prescott, J.; Monteleone, E. The influence of psychological traits, beliefs and taste responsiveness on implicit attitudes toward plant-and animal-based dishes among vegetarians, flexitarians and omnivores. Food Qual. Prefer. 2018, 68, 276–291. [Google Scholar] [CrossRef]
- Choi, S. Comparisons of 6-n-propylthiouracil (prop) sensitivity, food liking and food intake between vegetarian and non-vegetarian women. J. Obes. Weight Loss Ther. 2015, 5, 2. [Google Scholar]
- Bryant, C.J. We can’t keep meating like this: Attitudes towards vegetarian and vegan diets in the United Kingdom. Sustainability 2019, 11, 6844. [Google Scholar] [CrossRef] [Green Version]
- Abbilardo, T.; Chairani, S.; Nasution, N. Differences in Taste Sensitivity between Vegans and Non-Vegetarians in Palembang, Indonesia. J. Indones. Dent. Assoc. 2020, 3, 24–30. [Google Scholar] [CrossRef]
- North, M.; Kothe, E.; Klas, A.; Ling, M. How to define “Vegan”: An exploratory study of definition preferences among omnivores, vegetarians, and vegans. Food Qual. Prefer. 2021, 93, 104246. [Google Scholar] [CrossRef]
- Larsson, C.L.; Klock, K.S.; Åstrøm, A.N.; Haugejorden, O.; Johansson, G. Food habits of young Swedish and Norwegian vegetarians and omnivores. Public Health Nutr. 2001, 4, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Vidal, V.A.; Paglarini, C.S.; Freitas, M.Q.; Coimbra, L.O.; Esmerino, E.A.; Pollonio, M.A.; Cruz, A.G. Q Methodology: An interesting strategy for concept profile and sensory description of low sodium salted meat. Meat Sci. 2020, 161, 108000. [Google Scholar] [CrossRef]
- Zanardi, E.; Ghidini, S.; Conter, M.; Ianieri, A. Mineral composition of Italian salami and effect of NaCl partial replacement on compositional, physico-chemical and sensory parameters. Meat Sci. 2010, 86, 742–747. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Defining and Calculating Individual and Group Sensory Thresholds for Forced-Choice Data Sets of Intermediate Size; Standard practice E1432–04; American Society for Testing and Materials: Philadelphia, PA, USA, 2004. [Google Scholar]
- Peng, M.; Jaeger, S.R.; Hautus, M.J. Determining odour detection thresholds: Incorporating a method-independent definition into the implementation of ASTM E679. Food Qual. Prefer. 2012, 25, 95–104. [Google Scholar] [CrossRef]
- Giguère, J.-F.; de Moura Piovesana, P.; Proulx-Belhumeur, A.; Doré, M.; de Lemos Sampaio, K.; Gallani, M.C. Reliability of a simple method for determining salt taste detection and recognition thresholds. Chem. Senses 2016, 41, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Ambaldhage, V.K.; Puttabuddi, J.H.; Nunsavath, P.N.; Tummuru, Y.R. Taste disorders: A review. J. Indian Acad. Oral Med. Radiol. 2014, 26, 69. [Google Scholar] [CrossRef]
- Riera, C.E.; Vogel, H.; Simon, S.A.; Coutre, J.L. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R626–R634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zygler, A.; Wasik, A.; Namieśnik, J. Analytical methodologies for determination of artificial sweeteners in foodstuffs. TrAC Trends Anal. Chem. 2009, 28, 1082–1102. [Google Scholar] [CrossRef]
- Hajnal, A.; Covasa, M.; Bello, N.T. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1675–R1686. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Yan, J.; Suo, Y.; Li, J.; Wang, Q.; Lv, B. Nutritional status alters saccharin intake and sweet receptor mRNA expression in rat taste buds. Brain Res. 2010, 1325, 53–62. [Google Scholar] [CrossRef]
- Ahart, Z.C.; Martin, L.E.; Kemp, B.R.; Dutta Banik, D.; Roberts, S.G.; Torregrossa, A.M.; Medler, K.F. Differential Effects of Diet and Weight on Taste Responses in Diet-Induced Obese Mice. Obesity 2020, 28, 284–292. [Google Scholar] [CrossRef]
- Liu, G.; Walter, L.; Tang, S.; Tan, X.; Shi, F.; Pan, H.; Roos, C.; Liu, Z.; Li, M. Differentiated adaptive evolution, episodic relaxation of selective constraints, and pseudogenization of umami and sweet taste genes TAS1Rs in catarrhine primates. Front. Zool. 2014, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Drewnowski, A. The science and complexity of bitter taste. Nutr. Rev. 2001, 59, 163–169. [Google Scholar] [CrossRef]
- Petty, S.; Salame, C.; Mennella, J.A.; Pepino, M.Y. Relationship between sucrose taste detection thresholds and preferences in children, adolescents, and adults. Nutrients 2020, 12, 1918. [Google Scholar] [CrossRef]
- Lim, J.; Lawless, H.T. Detection thresholds and taste qualities of iron salts. Food Qual. Prefer. 2006, 17, 513–521. [Google Scholar] [CrossRef]
- Joseph, P.V.; Reed, D.R.; Mennella, J.A. Individual differences among children in sucrose detection thresholds: Relationship with age, gender, and bitter taste genotype. Nurs. Res. 2016, 65, 3. [Google Scholar] [CrossRef]

| Taste | Prototypic Compound | Supplier | Purity | Concentration Range (g·L−1) | Dilution Factor | |
|---|---|---|---|---|---|---|
| Lowest | Highest | |||||
| Sweet | Sucrose | Chelsea, New Zealand | 97% | 1.13 × 10−1 | 8.00 | 2.34 |
| Bitter | Caffeine | Simple Nutritions, Turkey | 98% | 6.40 × 10−5 | 0.20 | 5.00 |
| Salty | Sodium Chloride | Cerebos, New Zealand | 96% | 1.20 × 10−3 | 1.30 | 4.30 |
| Metallic | Iron II Sulphate Heptahydrate | PipingRock, England | 99% | 4.00 × 10−5 | 0.04 | 4.00 |
| Umami | Monosodium Glutamate (MSG) | Miwon, Korea | 99% | 9.20 × 10−2 | 0.70 | 1.50 |
| Sour | Citric Acid | Hansells, New Zealand | 98% | 5.00 × 10−2 | 1.00 | 1.80 |
| Taste Stimuli | Vegans | Vegetarians | Omnivores | F Statistic | p Value |
|---|---|---|---|---|---|
| Sweet | 1.205 ± 2.08 A | 1.63 ± 2.15 A | 2.33 ± 2.49 A | 1.94 | 0.151 |
| Salty | 0.210 ± 0.47 A | 0.211 ± 0.62 A | 0.182 ± 0.44 A | 0.02 | 0.979 |
| Bitter | 0.107 ± 0.16 A | 0.049 ± 0.13 B | 0.085 ± 0.16 A | 8.37 | <0.001 * |
| Sour | 0.101 ± 0.11 A | 0.084 ± 0.04 A | 0.101 ± 0.02 A | 0.86 | 0.429 |
| Metallic | 0.025 ± 0.01 A | 0.022 ± 0.01 A | 0.016 ± 0.01 A | 1.04 | 0.359 |
| Umami | 0.230 ± 0.13 A | 0.205 ± 0.14 A | 0.237 ± 0.22 A | 0.32 | 0.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil Mozhdehi, F.; Abeywickrema, S.; Bremer, P.J.; Peng, M. Comparing Taste Detection Thresholds across Individuals Following Vegan, Vegetarian, or Omnivore Diets. Foods 2021, 10, 2704. https://doi.org/10.3390/foods10112704
Jalil Mozhdehi F, Abeywickrema S, Bremer PJ, Peng M. Comparing Taste Detection Thresholds across Individuals Following Vegan, Vegetarian, or Omnivore Diets. Foods. 2021; 10(11):2704. https://doi.org/10.3390/foods10112704
Chicago/Turabian StyleJalil Mozhdehi, Fatemeh, Sashie Abeywickrema, Phil James Bremer, and Mei Peng. 2021. "Comparing Taste Detection Thresholds across Individuals Following Vegan, Vegetarian, or Omnivore Diets" Foods 10, no. 11: 2704. https://doi.org/10.3390/foods10112704
APA StyleJalil Mozhdehi, F., Abeywickrema, S., Bremer, P. J., & Peng, M. (2021). Comparing Taste Detection Thresholds across Individuals Following Vegan, Vegetarian, or Omnivore Diets. Foods, 10(11), 2704. https://doi.org/10.3390/foods10112704






