Impact of the Freeze-Drying Conditions Applied to Obtain an Orange Snack on Energy Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Formulation
2.2. Freeze-Drying Conditions
2.3. Water Content
2.4. Power Consumption and Temperature Record
2.5. Statistical Analysis
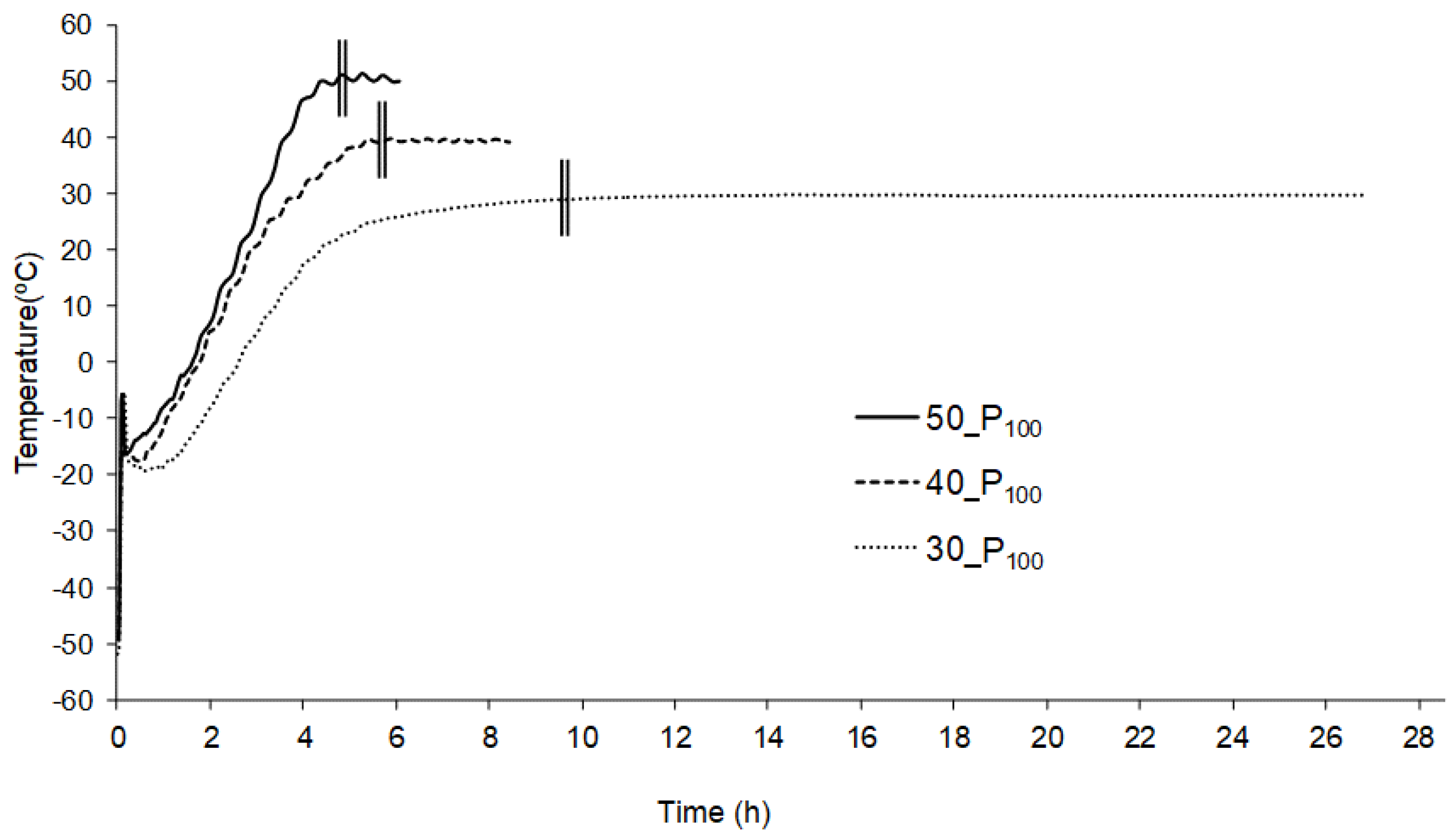
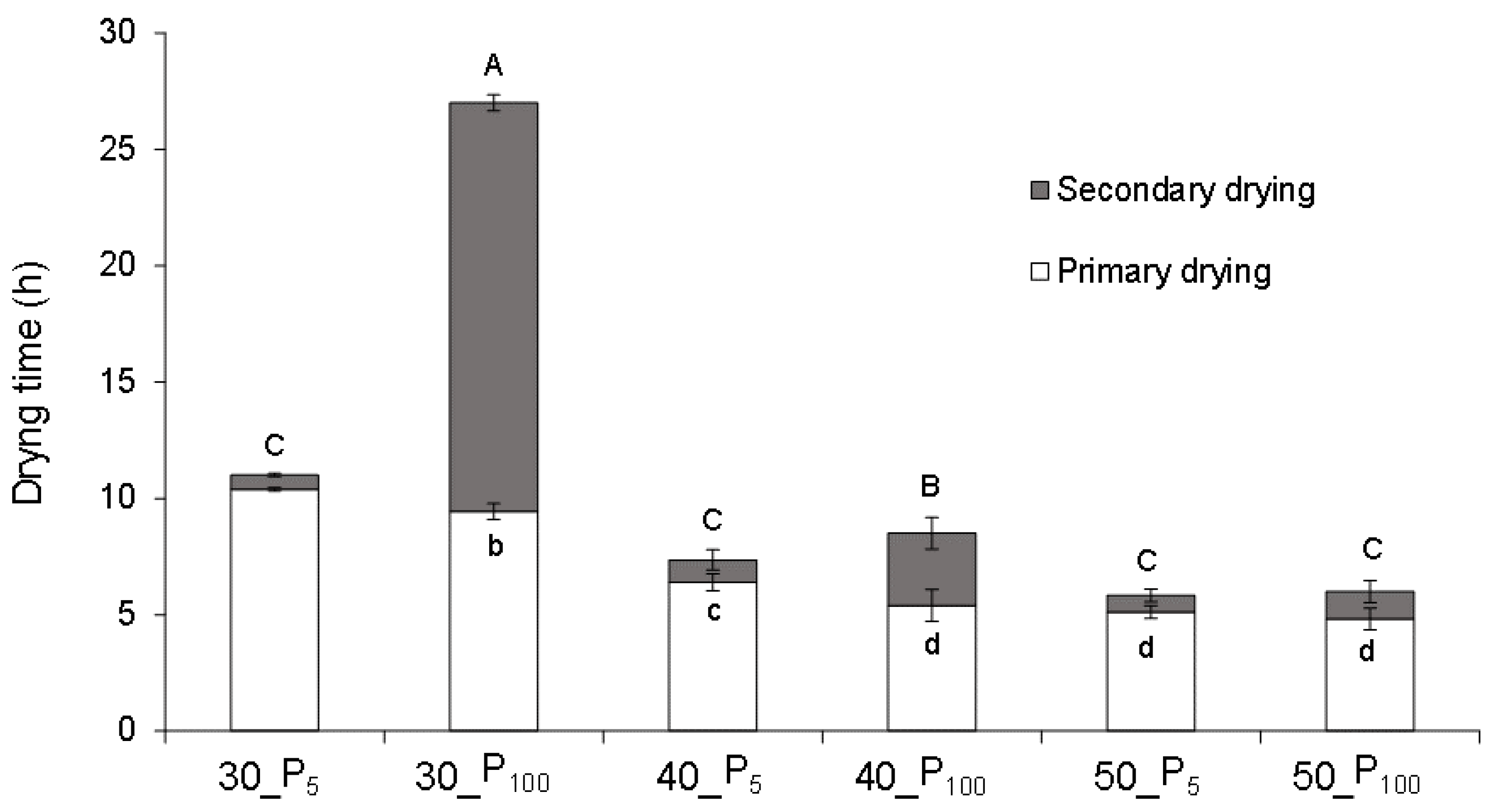
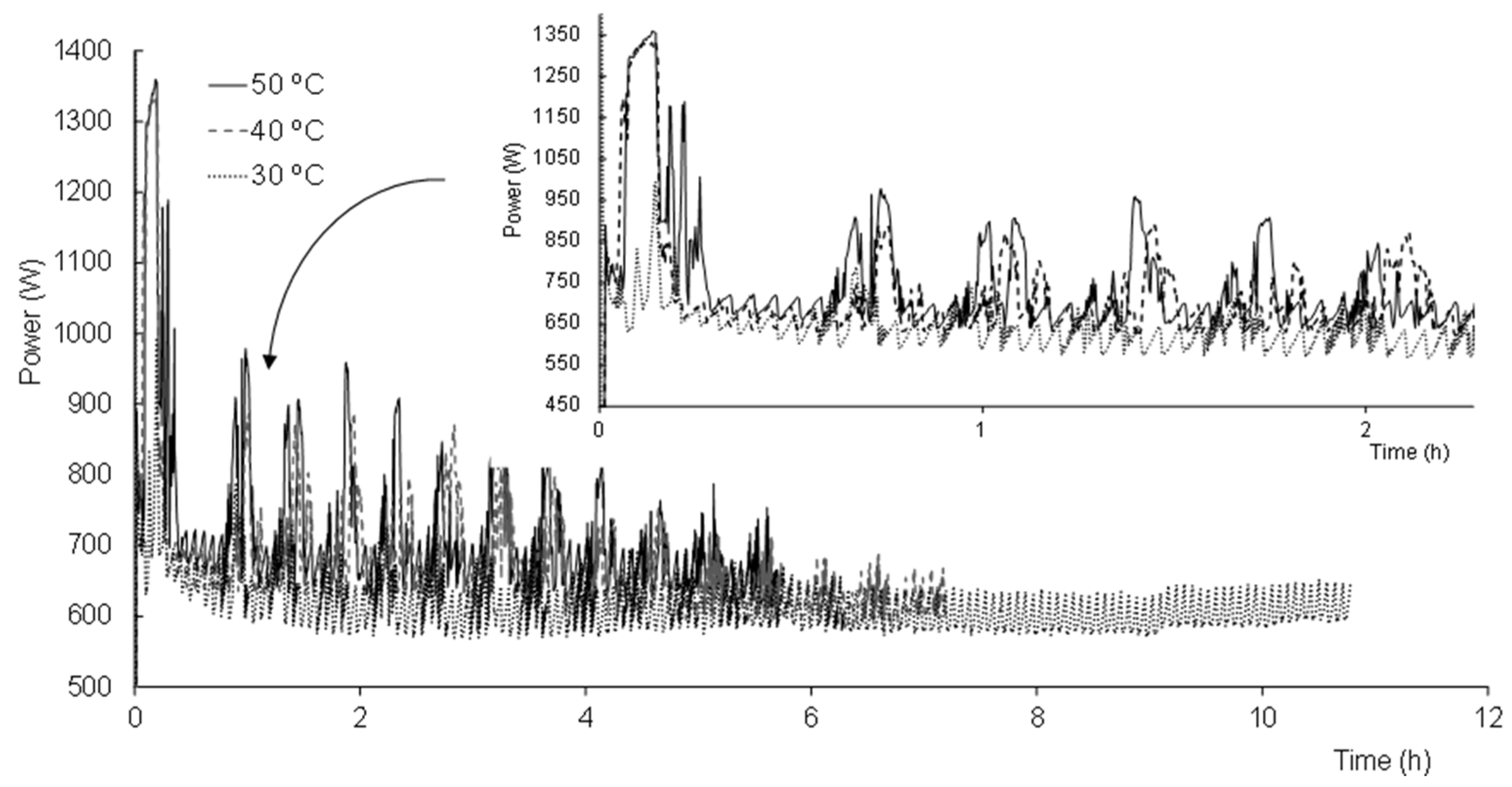
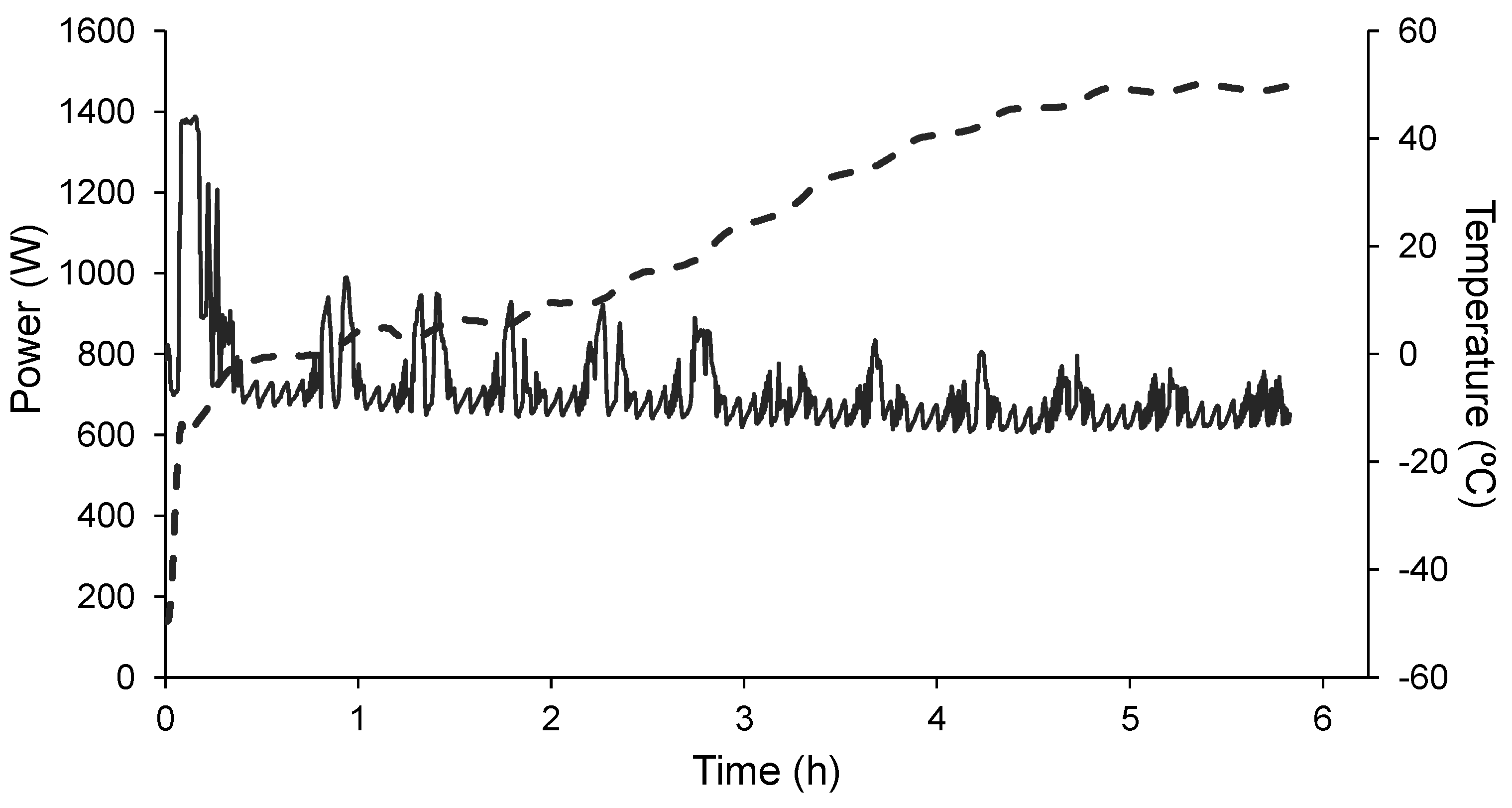
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Navarrete, N.; Camacho, M.M.; Martínez-Lahuerta, J.J. Bioactive compounds in fruits and their effects on health. Rev. Esp. Nutrición Hum. Diet. 2008, 12, 64–68. [Google Scholar]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Increasing Fruit and Vegetable Consumption Becomes a Global Priority. Available online: http://www.fao.org/english/newsroom/focus/2003/fruitveg1.htm (accessed on 10 October 2021).
- Hui, Y.H.; Clary, C.; Farid, M.M.; Fasina, O.O.; Noomhorm, A.; Welti-Chanes, J. Food Drying, Science and Technology; DEStech Publications Inc.: Lancaster, PA, USA, 2008. [Google Scholar]
- Liapis, A.I.; Bruttini, R. Freeze Drying. In Handbook of Industrial Drying; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 257–283. [Google Scholar]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Marczak, W.; Lenart, A.; Janowicz, M. Production of innovative freeze-dried vegetable snack with hydrocolloids in terms of technological process and carbon footprint calculation. Food Hydrocoll. 2020, 108, 105993. [Google Scholar] [CrossRef]
- Egas-Astudillo, L.A.; Martínez-Navarrete, N.; Camacho, M.M. Impact of biopolymers added to a grapefruit puree and freeze-drying shelf temperature on process time reduction and product quality. Food Bioprod. Process. 2020, 120, 143–150. [Google Scholar] [CrossRef]
- Leiton-Ramírez, Y.M.; Ayala-Aponte, A.; Ochoa-Martínez, C.I. Physicochemical properties of guava snacks as affected by drying technology. Processes 2020, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Uscanga, M.A.; Salvador, A.; Camacho, M.M.; Martínez-Navarrete, N. Impact of freeze-drying shelf temperature on the bioactive compounds, physical properties and sensory evaluation of a product based on orange juice. Int. J. Food Sci. 2021, 56, 5409–5416. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.M.; Martínez-Navarrete, N. The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods 2020, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Uscanga, M.A.; Camacho, M.M.; Salgado, M.A.; Martínez-Navarrete, N. Influence of an orange product composition on the characteristics of the obtained freeze-dried cake and powder as related to their consumption pattern. Food Bioprocess Technol. 2020, 13, 1368–1379. [Google Scholar] [CrossRef]
- Flink, J.M. Energy analysis in dehydration processes. Food Technol. 1977, 31, 77–79. [Google Scholar]
- Camacho, M.M.; Casanova, M.A.; Fenollosa, L.; Ribal, J.; Martínez-Lahuerta, J.J.; Martínez-Navarrete, N. Economic feasibility of freeze-drying to obtain powdered fruit. In Proceedings of the 21st International Drying Symposium, Valencia, Spain, 18–21 September 2018; Cárcel, J.A., Clemente, G., Mulet, A., Eds.; Universitat Politècnica de València: Valencia, Spain, 2019. [Google Scholar]
- Telis, V.R.N.; Martínez-Navarrete, N. Biopolymer Engineering in Food Processing; Telis, V.R.N., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 279–325. [Google Scholar]
- Hammami, C.; René, F.; Marin, M. Determination of freeze-drying process variables for strawberries. J. Food Eng. 1997, 32, 133–154. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Martínez-Navarrete, N.; Salvador, A.; Oliva, C.; Camacho, M.M. Influence of biopolymers and freeze-drying shelf temperature on the quality of a mandarin snack. LWT 2019, 99, 57–61. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; García-Martínez, E.; Martínez-Navarrete, N. Protective capacity of gum Arabic, maltodextrin, different starches, and fibers on the bioactive compounds and antioxidant activity of an orange puree (Citrus sinensis (L.) Osbeck) against freeze-drying and in vitro digestion. Food Chem. 2021, 357, 129724. [Google Scholar] [CrossRef]
- Zainol, M.; Khairi, M.; Abdul-Hamid, A.; Abu Bakar, F.; Pak Dek, M.S. Effect of different drying methods on the degradation of selected flavonoids in Centella asiatica. Int. Food Res. J. 2009, 16, 531–537. [Google Scholar]
- Silva-Espinoza, M.A.; Camacho, M.M.; Martínez-Navarrete, N. Use of different biopolymers as carriers for purposes of obtaining a freeze-dried orange snack. LWT 2020, 127, 109415. [Google Scholar] [CrossRef]
- Fischer, K. Neues Verfahren zur maßanalytischen Bestimmung des Wassergehaltes von Flüssigkeiten und festen Körpern. Angew. Chem. 1935, 48, 394–396. [Google Scholar] [CrossRef]
- Verhoff, J.C.; Barendrecht, E. Mechanism and reaction rate of the Karl Fischer titration reaction. Part I. Potentiometric measurements. J. Electroanal. Chem. 1976, 71, 305–315. [Google Scholar] [CrossRef]
- Statgraphics Centurion, v. XVII.II (32 bit); Statpoint Technologies, INC.: Herndon, VA, USA, 2018; Available online: https://www.statgraphics.com/centurion-overview (accessed on 10 October 2021).
- Raharitsifa, N.; Ratti, C. Foam-mat freeze-drying of apple juice part 1: Experimental data and ANN simulations. J. Food Process. Eng. 2010, 33, 268–283. [Google Scholar] [CrossRef]
- Ratti, C. Handbook of Food Powders: Processes and Properties; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 57–84. [Google Scholar]
- Barresi, A.A.; Pisano, R.; Fissore, D.; Rasetto, V.; Velardi, S.A.; Vallan, A. Monitoring of the primary drying of a lyophilization process in vials. Chem. Eng. Process. Process. Intensif. 2009, 48, 408–423. [Google Scholar] [CrossRef] [Green Version]
- Barbosa-Cánovas, G.V.; Vega-Mercado, H. Food Dehydration; Acribia SA: Zaragoza, Spain, 2000. [Google Scholar]
- Koganti, V.R.; Shalaev, E.Y.; Berry, M.R.; Osterberg, T.; Youssef, M.; Hiebert, D.N. Investigation of design space for freeze-drying: Use of modeling for primary drying segment of a freeze-drying cycle. AAPS PharmSciTech 2011, 12, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Pikal, M.J.; Shah, S.; Roy, M.L.; Putman, R. The secondary drying stage of freeze drying: Drying kinetics as a function of temperature and chamber pressure. Int. J. Pharm. 1990, 60, 203–207. [Google Scholar] [CrossRef]
- Tang, X.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Searles, J.A.; Aravapalli, S.; Hodge, C. Effects of Chamber Pressure and Partial Pressure of Water Vapor on Secondary Drying in Lyophilization. AAPS PharmSciTech 2017, 18, 2808–2813. [Google Scholar] [CrossRef]
- Saravacos, G.D.; Stinchfield, R.M. Effect of temperature and pressure on the sorption of water vapor by freeze-dried food materials. J. Food Sci. 1965, 30, 779–786. [Google Scholar] [CrossRef]
- Tang, X.; Nail, S.L.; Pikal, M.J. Freeze-drying process design by manometric temperature measurement: Design of a smart freeze-dryer. Pharm. Res. 2005, 22, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Mujumdar, A.S.; Lim, R.X. Comparison of the effect of microwave freeze drying and microwave vacuum drying upon the process and quality characteristics of potato/banana re-structured chips. Int. J. Food Sci. Technol. 2011, 46, 570–576. [Google Scholar] [CrossRef]
| Shelf Temperature | Pressure | Water Content (g Water/100 g Orange Snack) | Total Power Consumed (kWh) |
|---|---|---|---|
| 30 °C | P5 | 3.7 ± 0.2 a | 6.83 ± 0.06 b |
| P100 | 3.8 ± 0.13 a | 16.49 ± 0.12 a | |
| 40 °C | P5 | 3.6 ± 0.5 a | 5.03 ± 0.12 d |
| P100 | 3.8 ± 0.4 a | 5.72 ± 0.06 c | |
| 50 °C | P5 | 3.6 ± 0.3 a | 4.14 ± 0.05 e |
| P100 | 3.80 ± 0.12 a | 4.23 ± 0.09 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Espinoza, M.A.; Camacho, M.d.M.; Martínez-Monzó, J.; Martínez-Navarrete, N. Impact of the Freeze-Drying Conditions Applied to Obtain an Orange Snack on Energy Consumption. Foods 2021, 10, 2756. https://doi.org/10.3390/foods10112756
Silva-Espinoza MA, Camacho MdM, Martínez-Monzó J, Martínez-Navarrete N. Impact of the Freeze-Drying Conditions Applied to Obtain an Orange Snack on Energy Consumption. Foods. 2021; 10(11):2756. https://doi.org/10.3390/foods10112756
Chicago/Turabian StyleSilva-Espinoza, Marilú Andrea, María del Mar Camacho, Javier Martínez-Monzó, and Nuria Martínez-Navarrete. 2021. "Impact of the Freeze-Drying Conditions Applied to Obtain an Orange Snack on Energy Consumption" Foods 10, no. 11: 2756. https://doi.org/10.3390/foods10112756
APA StyleSilva-Espinoza, M. A., Camacho, M. d. M., Martínez-Monzó, J., & Martínez-Navarrete, N. (2021). Impact of the Freeze-Drying Conditions Applied to Obtain an Orange Snack on Energy Consumption. Foods, 10(11), 2756. https://doi.org/10.3390/foods10112756







