Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Grape Pomace Powders
2.2. Preparation of Breadsticks
2.3. Accelerated Oxidation Test of Breadsticks with OXITEST
Study of Oxidation Kinetic
2.4. Thermodynamic Study
2.5. Total Phenol Content, Antioxidant Activities FRAP and ABTS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Accelerated Oxidation Test with OXITEST and Kinetic Study
3.2. Thermodynamic Study
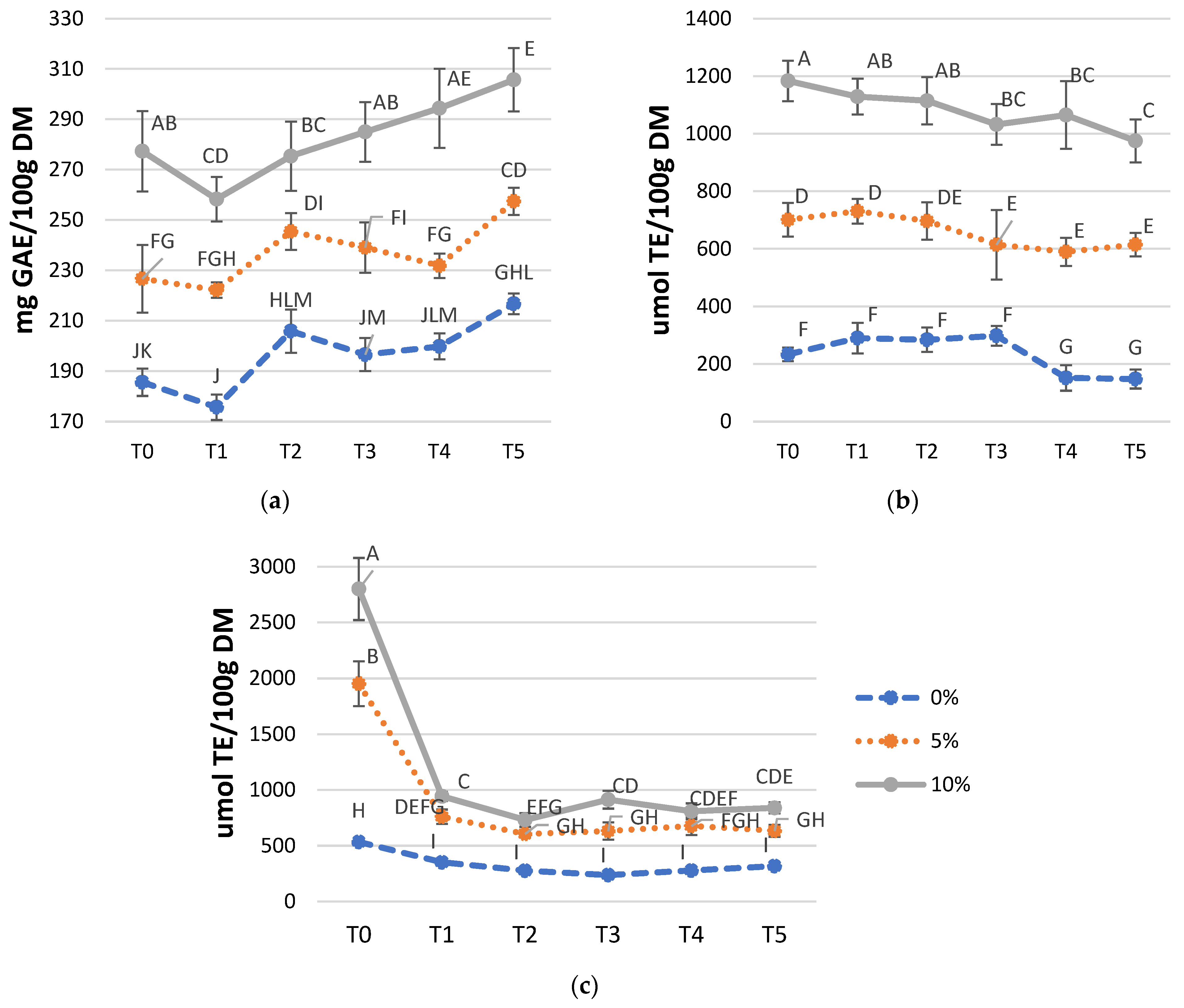
3.3. Total Phenol Content (TPC) and Antioxidant Activities (FRAP and ABTS) in Fortified Breadsticks at Room Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Food Losses and Food waste. Save Food Congress; FAO: Rome, Italy, 2011. [Google Scholar]
- ONU 70/1. Transforming Our World: The 2030 Agenda for Sustainable Development; ONU: New York, NY, USA, 2015. [Google Scholar]
- Bianchi, F.; Tolve, R.; Rainero, G.; Bordiga, M.; Brennan, C.S.; Simonato, B. Technological, nutritional, and sensory properties of pasta fortified with agro-industrial by-products: A review. Int. J. Food Sci. Technol. 2021, 56, 4356–4366. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT 2019, 114. [Google Scholar] [CrossRef]
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders. Int. J. Food Sci. Technol. 2019, 54, 952–958. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Rocchetti, G.; Rizzi, C.; Cervini, M.; Rainero, G.; Bianchi, F.; Giuberti, G.; Lucini, L.; Simonato, B. Impact of Grape Pomace Powder on the Phenolic Bioaccessibility and on In Vitro Starch Digestibility of Wheat Based Bread. Foods 2021, 10, 507. [Google Scholar] [CrossRef]
- Sattar, D.-S.; Ali, T.M.; Abbas, T.; Hasnain, A. Textural, Bioactive and Sensory Attributes of Breadsticks Containing Germinated and Non-Germinated Legumes. J. Food Chem. Nanotechnol. 2018, 04, 51–56. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive carbonyl species in vivo: Generation and dual biological effects. Sci. World J. 2014, 2014, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Kreps, F.; Dubaj, T.; Krepsová, Z. Accelerated oxidation method and simple kinetic model for predicting thermooxidative stability of edible oils under storage conditions. Food Packag. Shelf Life 2021, 29. [Google Scholar] [CrossRef]
- Souza, A.L.; Martínez, F.P.; Ferreira, S.B.; Kaiser, C.R. A complete evaluation of thermal and oxidative stability of chia oil: The richest natural source of α-linolenic acid. J. Therm. Anal. Calorim. 2017, 130, 1307–1315. [Google Scholar] [CrossRef]
- Caruso, M.C.; Galgano, F.; Colangelo, M.A.; Condelli, N.; Scarpa, T.; Tolve, R.; Favati, F. Evaluation of the oxidative stability of bakery products by OXITEST method and sensory analysis. Eur. Food Res. Technol. 2017, 243, 1183–1191. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; Methods 925.10, 65.17, 974.24, 992.16; AOAC: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Akcicek, A.; Bozkurt, F.; Akgül, C.; Karasu, S. Encapsulation of olive pomace extract in rocket seed gum and chia seed gum nanoparticles: Characterization, antioxidant activity and oxidative stability. Foods 2021, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Lante, A.; Bernardi, M.; Cappiello, F.; Galgano, F.; Caruso, M.C.; Favati, F. Comparison of OXITEST and RANCIMAT methods to evaluate the oxidative stability in frying oils. Eur. Food Res. Technol. 2018, 244, 747–755. [Google Scholar] [CrossRef]
- Comandini, P.; Verardo, V.; Maiocchi, P.; Caboni, M.F. Accelerated oxidation: Comparative study of a new reactor with oxidation stability instrument. Eur. J. Lipid Sci. Technol. 2009, 111, 933–940. [Google Scholar] [CrossRef]
- Xie, H.K.; Zhou, D.Y.; Liu, Z.Y.; Li, D.Y.; Tan, Z.F.; Dong, X.F.; Liu, X.Y.; Shahidi, F.; Zhu, B.W. Effects of natural phenolics on shelf life and lipid stability of freeze-dried scallop adductor muscle. Food Chem. 2019, 295, 423–431. [Google Scholar] [CrossRef]
- Mora, L.; Limbo, S.; Maiocchi, P. Application of the oxitest method to estimate the kinetic parameters in soybean oil under accelerated storage conditions. Ital. J. Food Sci. 2011, 23, 72–74. [Google Scholar]
- Yasantha, A.; Ki-Whan, L.; Fereidoon, S.; Min Soo, H.; Hung-Tae, K.; Jung-Suck, L.; Jeon, Y. Antioxidant efficacy of extracts of an edible red alga in linoleic acid and fish oil. J. Food Lipids 2003, 10, 313–327. [Google Scholar]
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of Grape Pomace Addition on the Technological, Sensory, and Nutritional Properties of Durum Wheat Pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef] [Green Version]
- Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G.; Simonato, B. Breadsticks fortification with red grape pomace: Effect on nutritional, technological, and sensory properties. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat bread fortification by grape pomace powder: Nutritional, technological, antioxidant, and sensory properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B.; Selamat, J.; Yusoff, M.S.A. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133–1138. [Google Scholar] [CrossRef]
- Spacino, K.R.; Borsato, D.; Buosi, G.M.; Chendynski, L.T. Determination of kinetic and thermodynamic parameters of the B100 biodiesel oxidation process in mixtures with natural antioxidants. Fuel Process. Technol. 2015, 137, 366–370. [Google Scholar] [CrossRef]
- de Sousa, L.S.; Garcia, M.A.S.; Santos, E.C.P.; do Nascimento Silva, J.; de Castro, A.G.; de Moura, C.V.R.; de Moura, E.M. Study of the kinetic and thermodynamic parameters of the oxidative degradation process of biodiesel by the action of antioxidants using the Rancimat and PetroOXY methods. Fuel 2019, 238, 198–207. [Google Scholar] [CrossRef]
- Gülmez, Ö.; Şahin, S. Evaluation of oxidative stability in hazelnut oil treated with several antioxidants: Kinetics and thermodynamics studies. LWT 2019, 111, 478–483. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Zagotto, A.; Alberton, A.; Lante, A.; Zagotto, G.; Ribaudo, G.; Rizzi, C. Monitoring the antioxidant activity of an eco-friendly processed grape pomace along the storage. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.A.R.; Hongsprabhas, P.; Thottiam Vasudevan, R. In vitro and in vivo inhibition of maillard reaction products using amino acids, modified proteins, vitamins, and genistein: A review. J. Food Biochem. 2019, 43, 1–17. [Google Scholar] [CrossRef]
- Zocca, F.; Lomolino, G.; Curioni, A.; Spettoli, P.; Lante, A. Detection of pectinmethylesterase activity in presence of methanol during grape pomace storage. Food Chem. 2007, 102, 59–65. [Google Scholar] [CrossRef]
- Chamorro, S.; Goñi, I.; Hervert-Hernández, D.; Viveros, A.; Brenes, A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Kollathova, R.; Hanušovsky, O.; Galik, B.; Biro, D.; Šimko, M.; Juraček, M.; Rolinec, M.; Puntigam, R.; Slama, J.A.; Gierus, M. Fatty acid profile analysis of grape by-products from Slovakia and Austria. Acta Fytotech. Zootech. 2020, 23, 78–84. [Google Scholar] [CrossRef]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between total antioxidant capacity, polyphenol and fatty acid content of native grape seed and pomace of four different grape varieties in hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef]
- Gao, F.; Birch, J. Oxidative stability, thermal decomposition, and oxidation onset prediction of carrot, flax, hemp, and canola seed oils in relation to oil composition and positional distribution of fatty acids. Eur. J. Lipid Sci. Technol. 2016, 118, 1042–1052. [Google Scholar] [CrossRef]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
| Samples | IP (min) | Rate of Oxidation k (1/Day) | ||||
|---|---|---|---|---|---|---|
| 90 °C | 100 °C | 110 °C | 90 °C | 100 °C | 110 °C | |
| B0 | 1465.5 ± 48.8 a | 747.5 ± 29.0 b | 419.5 ± 7.8 f | 0.2053 ± 0.0012 a | 0.4132 ± 0.0025 d | 0.7710 ± 0.0047 g |
| B5 | 776.5 ± 44.5 b | 386.5 ± 6.4 d | 218 ± 4.2 g | 0.2307 ± 0.0015 b | 0.4890 ± 0.0027 e | 0.8201 ± 0.0052 h |
| B10 | 698.5 ± 9.2 c | 331.5 ± 7.8 e | 201 ± 8.5 h | 0.3010 ± 0.0020 c | 0.5185 ± 0.0032 f | 0.9295 ± 0.0064 i |
| Samples | R2 | Shelf-Life 25 °C (days) | Shelf-Life 20 °C (days) |
|---|---|---|---|
| B0 | 0.9981 | 58 | 80 |
| B5 | 0.9967 | 33 | 45 |
| B10 | 0.9870 | 27 | 36 |
| Samples | Ea (kJ/mol) | ΔH (kJ/mol) | ΔS (J/K mol) | ΔG 90 °C (kJ/mol) |
|---|---|---|---|---|
| B0 | 77.53 ± 2.65 a | 74.43 ± 2.65 a | −127.36 ± 7.20 a | 120.68 ± 1.04 a |
| B5 | 78.22 ± 0.96 a | 75.12 ± 0.96 a | −122.45 ± 2.73 a | 119.58 ± 1.95 a |
| B10 | 56.99 ± 1.52 b | 53.89 ± 1.52 b | −177.36 ± 3.84 b | 118.30 ± 2.91 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, F.; Lomuscio, E.; Rizzi, C.; Simonato, B. Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders. Foods 2021, 10, 2815. https://doi.org/10.3390/foods10112815
Bianchi F, Lomuscio E, Rizzi C, Simonato B. Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders. Foods. 2021; 10(11):2815. https://doi.org/10.3390/foods10112815
Chicago/Turabian StyleBianchi, Federico, Elisabetta Lomuscio, Corrado Rizzi, and Barbara Simonato. 2021. "Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders" Foods 10, no. 11: 2815. https://doi.org/10.3390/foods10112815
APA StyleBianchi, F., Lomuscio, E., Rizzi, C., & Simonato, B. (2021). Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders. Foods, 10(11), 2815. https://doi.org/10.3390/foods10112815