Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spontaneous and Inoculated Fermentation of the Chickpea Flours
2.3. Determination of BAs and GABA by HPLC
2.3.1. Extraction Procedure
2.3.2. Derivatization with Dansyl Chloride (DNS–Cl)
2.3.3. HPLC Analyses
2.4. Folin–Ciocalteu Assays of Antioxidants
2.5. Enzymatic Degradation of BA
2.5.1. Enzymatic Degradation of BA in the Sourdough Suspension
2.5.2. Enzymatic Degradation of BA in the Dough
2.6. Influence of Baking on the Content of BA in Bread Prepared from Sourdough
2.7. Measurement of pH and Determination of Dry Weight
2.8. Statistical Analysis
3. Results and Discussion
3.1. Formation of Nutritionally Undesirable Biogenic Amines in the Sourdoughs of the Chickpea Flours
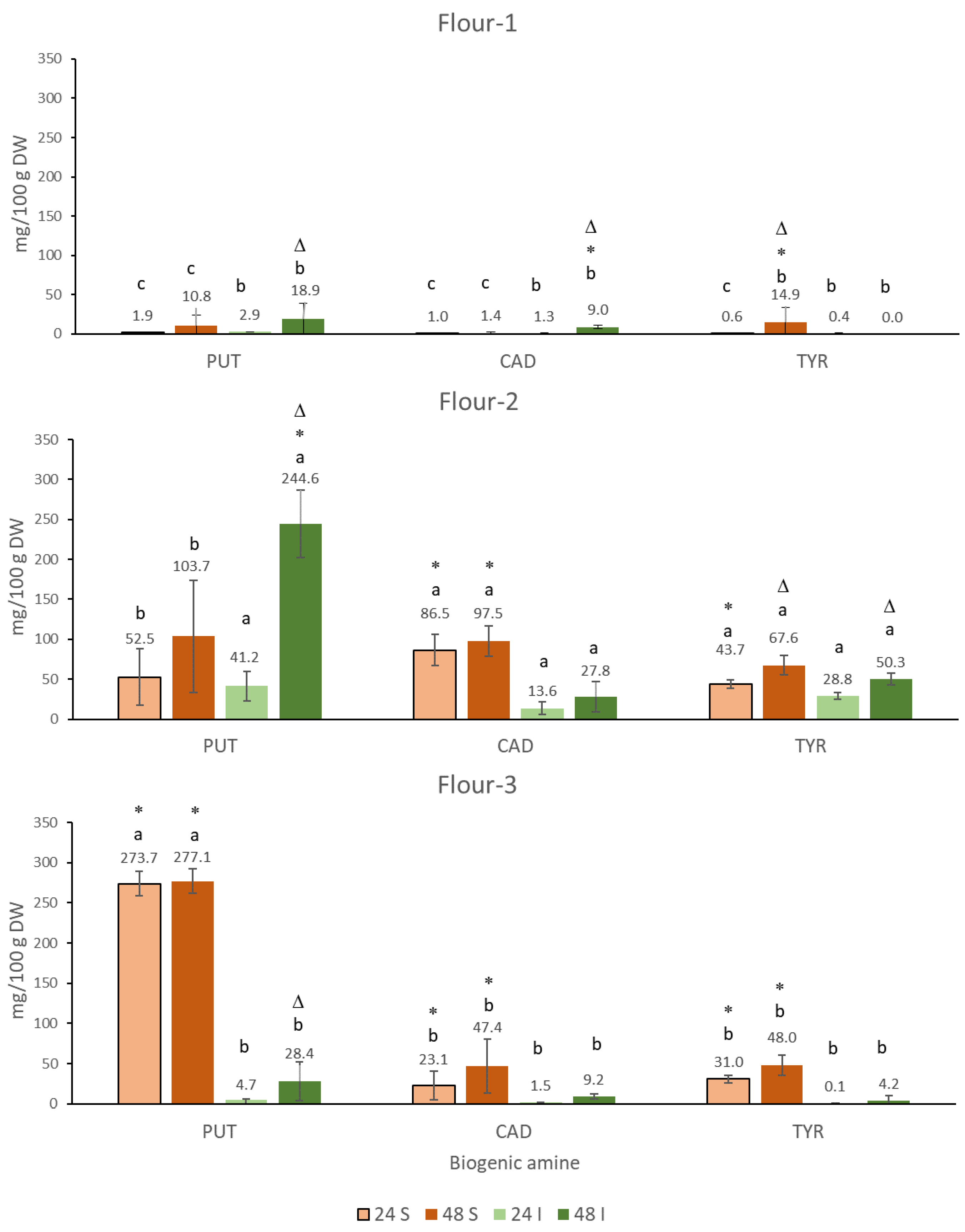
3.1.1. Putrescine (PUT)
3.1.2. Cadaverine (CAD)
3.1.3. Tyramine (TYR)
3.1.4. Tryptamine (TRM), Phenethylamine (PEA), and Histamine (HIS)
3.2. Nutritionally Desirable BAs and GABA in the Sourdoughs of the Chickpea Flours
3.2.1. Spermidine (SPD) and Spermine (SPM)
3.2.2. Gamma-Aminobutyric Acid (GABA)
3.3. Antioxidant Content of the Sourdoughs of the Chickpea Flours
3.4. Application of Lyophilized Fenugreek Sprouts for the Reduction of Nutritionally Undesirable Biogenic Amines in the Sourdoughs and Bread
3.5. Influence of Baking on the Stability of Biogenic Amines in Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


| Slope of Calibration Curves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GABA | TRP | PEA | PUT | CAD | HIS | IS2 | TYR | SPD | SPM | |
| UV (mAU × min × L/mg) | 7.6 | 16.2 | 17.9 | 38.9 | 35.1 | 28.2 | 29.4 | 30.5 | 33.7 | 29.1 |
| FL (signal × min × L/mg) | / | 229 | 245 | 540 | 594 | / | 552 | 88.8 | 535 | 561 |
| Flour | 24 S | 48 S | 24 I | 48 I | |
|---|---|---|---|---|---|
| Flour-1 | 6.12 | 5.78 | 5.02 | 5.27 | 5.34 |
| Flour-2 | 6.10 | 4.74 | 4.34 | 5.18 | 4.95 |
| Flour-3 | 6.17 | 5.67 | 5.09 | 5.04 | 5.00 |
References
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Licandro, H.; Ho, P.H.; Nguyen, T.K.C.; Petchkongkaew, A.; Van Nguyen, H.; Chu-Ky, S.; Nguyen, T.V.A.; Lorn, D.; Waché, Y. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control 2020, 110, 106957. [Google Scholar] [CrossRef]
- Reese, A.T.; Madden, A.A.; Joossens, M.; Lacaze, G.; Dunn, R.R. Influences of Ingredients and Bakers on the Bacteria and Fungi in Sourdough Starters and Bread. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Microorganisms Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of Sourdough Technology: From Production to Marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Wendin, K.; Mustafa, A.; Ortman, T.; Gerhardt, K. Consumer Awareness, Attitudes and Preferences towards Heritage Cereals. Foods 2020, 9, 742. [Google Scholar] [CrossRef]
- Suo, B.; Chen, X.; Wang, Y. Recent research advances of lactic acid bacteria in sourdough: Origin, diversity, and function. Curr. Opin. Food Sci. 2021, 37, 66–75. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Cleary, L.J.; Andersson, R.; Brennan, C.S. The behaviour and susceptibility to degradation of high and low molecular weight barley b-glucan in wheat bread during baking and in vitro digestion. Food Chem. 2007, 102, 889–897. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2018, 302, 103–113. [Google Scholar] [CrossRef]
- Katina, K.; Hartikainen, K.; Poutanen, K. Process-Induced changes in rye foods-rye baking. In Rye and Health; Elsevier Inc.: Rochester, MN, USA, 2014; pp. 7–21. ISBN 9780128122884. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA). Int. J. Food Microbiol. 2009, 137, 236–245. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J.; Yildiz, F. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Selvaraj, D.; Nile, A.S.; Ramalingam, S.; Kai, G.; Nile, S.H. Indian pulses: A review on nutritional, functional and biochemical properties with future perspectives. Trends Food Sci. Technol. 2019, 88, 228–242. [Google Scholar] [CrossRef]
- Rawal, V.; Navarro, D.K. The Global Economy of Pulses; FAO: Rome, Italy, 2019; ISBN 9789251097304. [Google Scholar]
- Schettino, R.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Microorganisms Extension of the Shelf-Life of Fresh Pasta Using Chickpea Flour Fermented with Selected Lactic Acid Bacteria. Microorganisms 2020, 8, 1322. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, M.; Bavaro, A.R.; Lonigro, S.L.; Pontonio, E.; Conte, A.; Padalino, L.; Minisci, A.; Lavermicocca, P.; Valerio, F. Lactobacillus plantarum ITM21B fermentation product and chickpea flour enhance the nutritional profile of salt reduced bakery products. Int. J. Food Sci. Nutr. 2019, 70, 701–713. [Google Scholar] [CrossRef]
- Sayaslan, A.; Şahin, N. Effects of fermented-chickpea liquor (chickpea yeast) on whole-grain wheat flour bread properties. Qual. Assur. Saf. Crop. Foods 2018, 10, 183–192. [Google Scholar] [CrossRef]
- Shrivastava, C.; Chakraborty, S. Bread from wheat flour partially replaced by fermented chickpea flour: Optimizing the formulation and fuzzy analysis of sensory data. LWT—Food Sci. Technol. 2018, 90, 215–223. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, L.; Chen, Y.; Zhang, S.; Rui, X.; Dong, M. Comparative study of the effects of fermented and non-fermented chickpea flour addition on quality and antioxidant properties of wheat bread. CyTA—J. Food 2016, 14, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, C.G.; Calasso, M.; Campanella, D.; De Angelis, M.; Gobbetti, M. Use of sourdough fermentation and mixture of wheat, chickpea, lentil and bean flours for enhancing the nutritional, texture and sensory characteristics of white bread. Int. J. Food Microbiol. 2014, 180, 78–87. [Google Scholar] [CrossRef]
- Kahraman, G.; Harsa, S.; Lucisano, M.; Cappa, C. Physicochemical and rheological properties of rice-based gluten-free blends containing differently treated chickpea flours. LWT 2018, 98, 276–282. [Google Scholar] [CrossRef]
- Cyprus National Commission for Unesco—“Arkatena” Artisanal Rusks. Available online: http://www.unesco.org.cy/Programmes-Arkatena_artisanal_rusks,EN-PROGRAMMES-04-02-03-10,EN (accessed on 13 March 2021).
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Del Rio, B.; Alvarez-Sieiro, P.; Redruello, B.; Martin, M.C.; Fernandez, M.; Ladero, V.; Alvarez, M.A. Lactobacillus rossiae strain isolated from sourdough produces putrescine from arginine article. Sci. Rep. 2018, 8, 3989. [Google Scholar] [CrossRef] [Green Version]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Bartkevics, V.; Rusko, J.; Starkute, V.; Zadeike, D.; Juodeikiene, G. Changes in the free amino acids and the biogenic amine contents during lactic acid fermentation of different lupin species. Int. J. Food Sci. Technol. 2016, 51, 2049–2056. [Google Scholar] [CrossRef]
- Bartkiene, E.; Juodeikiene, G.; Vidmantiene, D. Nutritional quality of fermented defatted soya and flaxseed flours and their effect on texture and sensory characteristics of wheat sourdough bread. Int. J. Food Sci. Nutr. 2012, 63, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Rusko, J.; Starkute, V.; Bendoraitiene, E.; Zadeike, D.; Juodeikiene, G. The effect of Pediococcus acidilactici and Lactobacillus sakei on biogenic amines formation and free amino acid profile in different lupin during fermentation. LWT—Food Sci. Technol. 2016, 74, 40–47. [Google Scholar] [CrossRef]
- Kenten, R.H.; Mann, P.J.G. The oxidation of amines by extracts of pea seedlings. Biochem. J. 1952, 50, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- Kralj Cigić, I.; Rupnik, S.; Rijavec, T.; Poklar Ulrih, N.; Cigić, B. Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish. Foods 2020, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Özdestan, Ö.; Alpözen, E.; Güven, G.; Üren, A. Monitoring of Biogenic Amines in Kumru: A Traditional Fermented Cereal Food. Int. J. Food Prop. 2012, 15, 972–981. [Google Scholar] [CrossRef]
- Diana, M.; Rafecas, M.; Quílez, J. Free amino acids, acrylamide and biogenic amines in gamma-aminobutyric acid enriched sourdough and commercial breads. J. Cereal Sci. 2014, 60, 639–644. [Google Scholar] [CrossRef]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-Applications Acid (GABA): Biosynthesis, Role, Commercial Production, and Applications. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 57, pp. 413–452. ISBN 9780444640574. [Google Scholar]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant phenols in barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef] [Green Version]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Statist. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.Ô.M.; Jarvis, B. Observations on the use of statistical methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Rabell-González, J.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [Green Version]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Okamoto, A.; Sugi, E.; Koizumi, Y.; Yanagida, F.; Udaka, S. Polyamine Content of Ordinary Foodstuffs and Various Fermented Foods. Biosci. Biotechnol. Biochem. 1997, 61, 1582–1584. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, C.; Gómez-Tamayo, J.C.; Nebel, J.-C.; Pardo, L.; Gonzalez, A. Identifying human diamine sensors for death related putrescine and cadaverine molecules. PLoS Comput. Biol. 2018, 14, e1005945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Bulushi, I.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation-A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and other biogenic amines in food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012, 60, 3026–3032. [Google Scholar] [CrossRef]
- Linares, D.M.; Del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Blob, L.F.; Sharoky, M.; Campbell, B.J.; Kemper, E.M.; Gilmor, M.; VanDenBerg, C.M.; Azzaro, A.J. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007, 12, 25–34. [Google Scholar] [CrossRef]
- Daniel Collins, J.; Noerrung, B.; Budka, H.; Andreoletti, O.; Buncic, S.; Griffin, J.; Hald, T.; Havelaar, A.; Hope, J.; Klein, G.; et al. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. In Proceedings of the Joint Fao/Who Expert Meeting, Rome, Italy, 23–27 July 2012; ISBN 9789251078495. [Google Scholar]
- Ali, M.A.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2011, 55. [Google Scholar] [CrossRef] [Green Version]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sagara, T.; Bhandari, D.R.; Spengler, B.; Vollmann, J. Spermidine and other functional phytochemicals in soybean seeds: Spatial distribution as visualized by mass spectrometry imaging. Food Sci. Nutr. 2020, 8, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kubo, Y.; Horii, Y.; Nishiwaki, T.; Kamiyama, S.; Sone, H.; Watanabe, S. Bacterial degradation of spermine and expression of spermidine/spermine acetyltransferase in Bacillus subtilis (natto) under liquid cultivation. J. Gen. Appl. Microbiol. 2017, 63, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Horie, Y.; Goto, A.; Tsubuku, S.; Itoh, M.; Ikegawa, S.; Ogawa, S.; Higashi, T. Changes in Polyamine Content in Rice Bran due to Fermentation with Aspergillus oryzae Analyzed by LC/ESI-MS/MS Combined with Derivatization. Anal. Sci. 2019, 35, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Horii, Y.; Watanabe, S.; Kubo, Y.; Koguchi, K.; Hoshi, Y.; Matsumoto, K.; Soda, K. Comparison of soybean cultivars for enhancement of the polyamine contents in the fermented soybean natto using Bacillus subtilis (natto). Biosci. Biotechnol. Biochem. 2017, 81, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Granchi, L. Use of Selected Lactobacilli to Increase γ-Aminobutyric Acid (GABA) Content in Sourdough Bread Enriched with Amaranth Flour. Foods 2019, 8, 218. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiansawang, K.; Luangpituksa, P.; Varanyanond, W.; Hansawasdi, C. GABA (γ-aminobutyric acid) production, antioxidant activity in some germinated dietary seeds and the effect of cooking on their GABA content. Food Sci. Technol. 2016, 36, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Arispuro, D.M.; Cuevas-Rodríguez, E.O.; Milán-Carrillo, J.; León-López, L.; Gutiérrez-Dorado, R.; Reyes-Moreno, C. Optimal germination condition impacts on the antioxidant activity and phenolic acids profile in pigmented desi chickpea (Cicer arietinum L.) seeds. J. Food Sci. Technol. 2018, 55, 638–647. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira Raphaelli, C.; de Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin−Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumarie, C.; Séïde, M.; Marcocci, L.; Pietrangeli, P.; Mateescu, M.A. Diamine Oxidase from White Pea (Lathyrus sativus) Combined with Catalase Protects the Human Intestinal Caco-2 Cell Line from Histamine Damage. Appl. Biochem. Biotechnol. 2017, 182, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, H.; Han, Y.; Gu, Z. Purification of diamine oxidase and its properties in germinated fava bean (Vicia faba L.). J. Sci. Food Agric. 2012, 92, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Longu, S.; Mura, A.; Padiglia, A.; Medda, R.; Floris, G. Mechanism-based inactivators of plant copper/quinone containing amine oxidases. Phytochemistry 2005, 66, 1751–1758. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Ieri, F.; Campo, M.; Paolino, D.; Restuccia, D.; Romani, A. Biogenic amines, phenolic, and aroma-related compounds of unroasted and roasted cocoa beans with different origin. Foods 2019, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Min, J.; Han, X.Y.; Li, X.Y.; Hu, J.W.; Liu, H.; Cao, M.J.; Liu, G.M. Reduction of the histamine content and immunoreactivity of parvalbumin in Decapterus maruadsi by a Maillard reaction combined with pressure treatment. Food Funct. 2018, 9, 4897–4905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, Y.; He, X.; Hu, S.; Li, S.; Liu, Y. A study of the tyramine/glucose Maillard reaction: Variables, characterization, cytotoxicity and preliminary application. Food Chem. 2018, 239, 377–384. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, T.; Mejaš, R.; Jamnik, P.; Kralj Cigić, I.; Poklar Ulrih, N.; Cigić, B. Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough. Foods 2021, 10, 2840. https://doi.org/10.3390/foods10112840
Polak T, Mejaš R, Jamnik P, Kralj Cigić I, Poklar Ulrih N, Cigić B. Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough. Foods. 2021; 10(11):2840. https://doi.org/10.3390/foods10112840
Chicago/Turabian StylePolak, Tomaž, Rok Mejaš, Polona Jamnik, Irena Kralj Cigić, Nataša Poklar Ulrih, and Blaž Cigić. 2021. "Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough" Foods 10, no. 11: 2840. https://doi.org/10.3390/foods10112840
APA StylePolak, T., Mejaš, R., Jamnik, P., Kralj Cigić, I., Poklar Ulrih, N., & Cigić, B. (2021). Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough. Foods, 10(11), 2840. https://doi.org/10.3390/foods10112840








