Mapping Aspergillus niger Metabolite Biomarkers for In Situ and Early Evaluation of Table Grapes Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Growth Conditions
2.2. Grapes Contamination Protocol
2.3. Profiling Headspace Volatile Metabolites by HS-SPME-GC×GC-ToFMS
2.3.1. Fungal Cultures
2.3.2. Instrumentation
2.3.3. Contaminated Table Grapes
2.4. Statistical Analysis
3. Results and Discussion
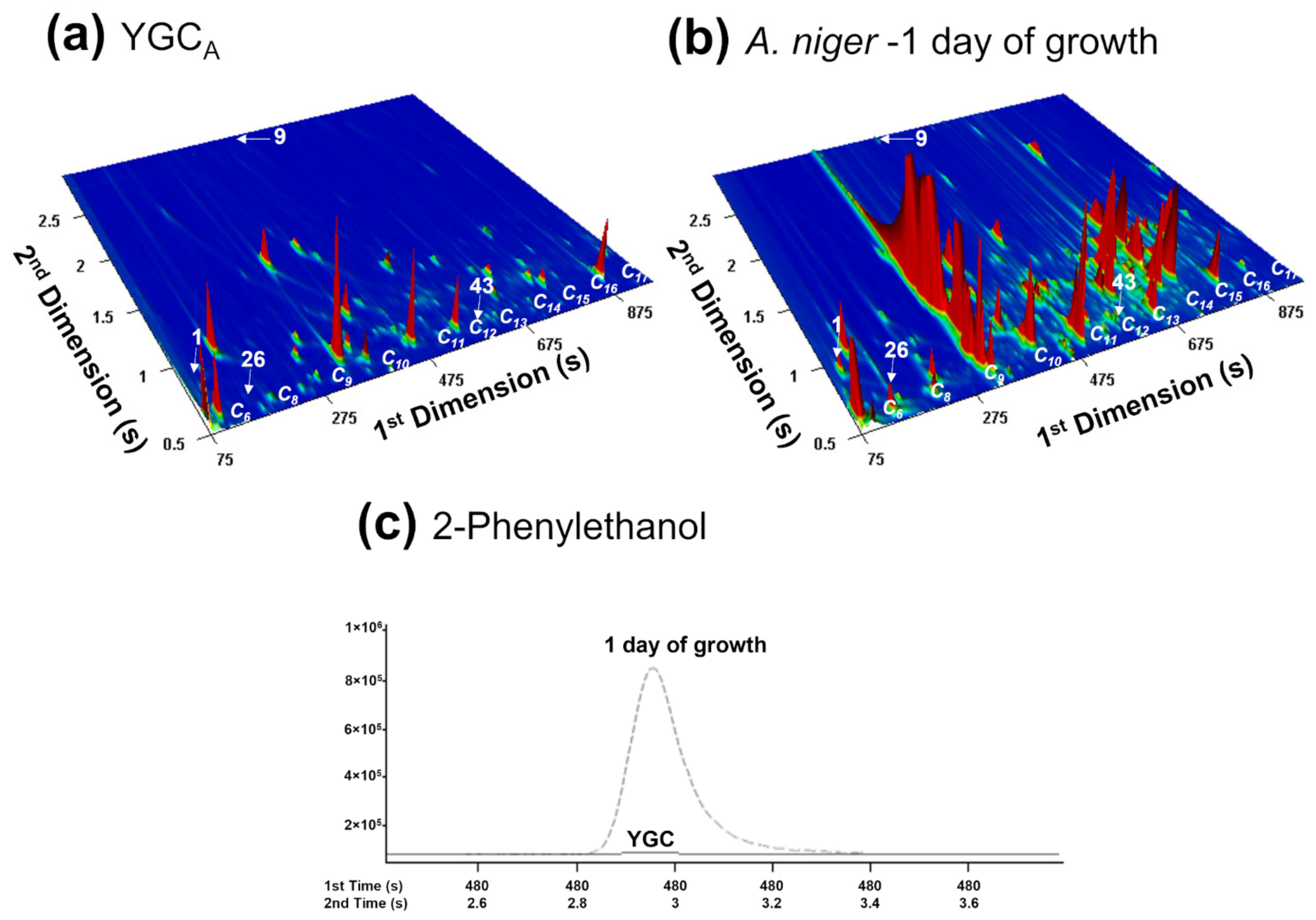
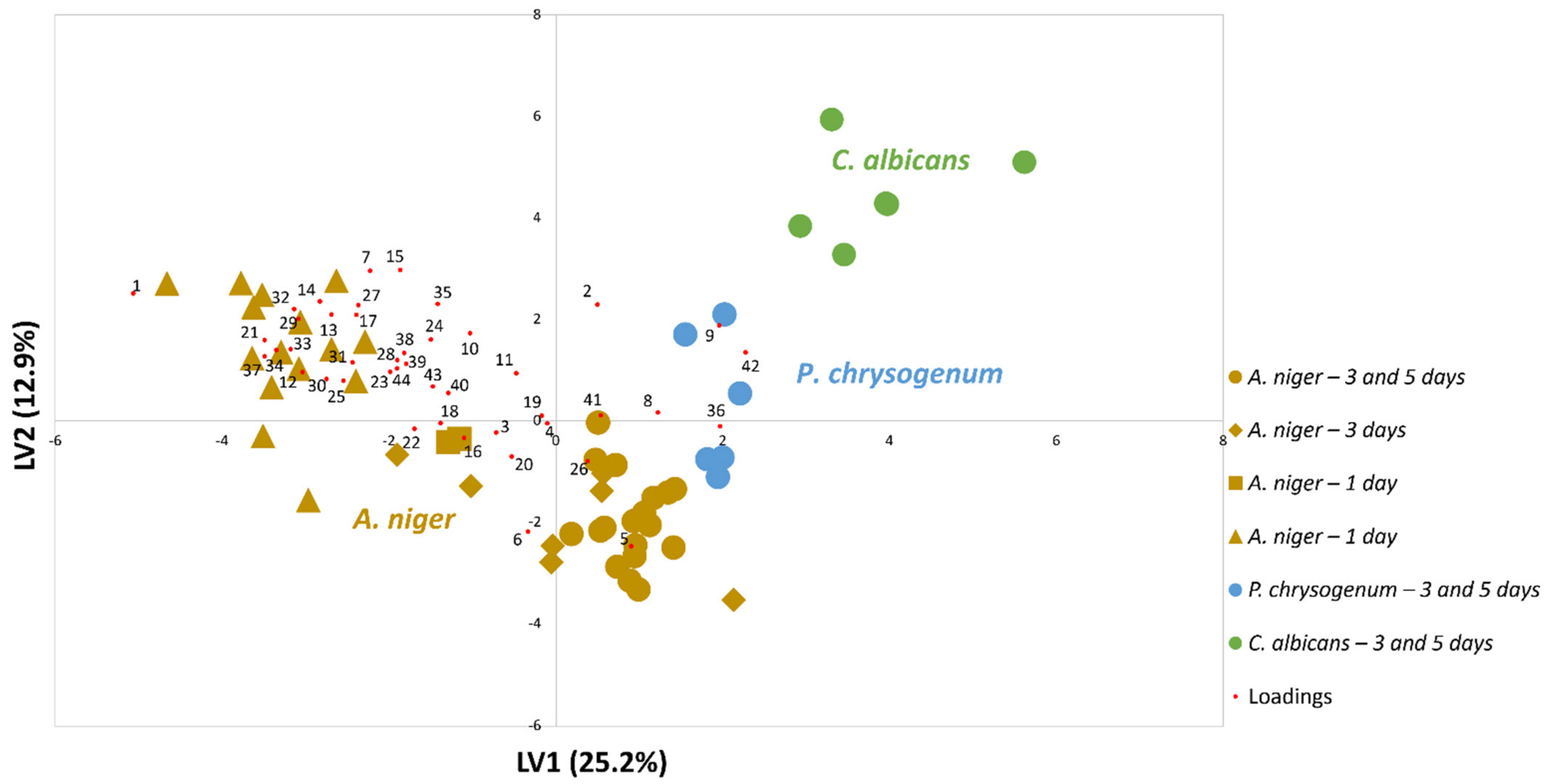
3.1. Evaluating the Potential of A. niger Metabolite Biomarkers Pattern for Strain Distinction at 1-Day of Culture Growth
3.2. Following the A. niger Metabolites Biomarkers Pattern over 7 Days
3.2.1. By Analysis of A. niger Cultures Obtained from Contaminated Grapes
3.2.2. By Direct Analysis of Contaminated Table Grapes—In Situ SPME
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finger, J.A.F.F.; Baroni, W.S.G.V.; Maffei, D.F.; Bastos, D.H.M.; Pinto, U.M. Overview of foodborne disease outbreaks in Brazil from 2000 to 2018. Foods 2019, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, J.V.; Tarazona, A.; Mateo, F.; Jiménez, M.; Mateo, E.M. Potential impact of engineered silver nanoparticles in the control of aflatoxins, ochratoxin A and the main aflatoxigenic and ochratoxigenic species affecting foods. Food Control 2019, 101, 58–68. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.; Kupper, K.C.; Augusto, F.; Fill, T.P. Biological Control of Citrus Postharvest Phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Freire, L.; Guerreiro, T.M.; Pia, A.K.R.; Lima, E.O.; Oliveira, D.N.; Melo, C.F.O.R.; Catharino, R.R.; Sant’Ana, A.S. A quantitative study on growth variability and production of ochratoxin A and its derivatives by A. carbonarius and A. niger in grape-based medium. Sci. Rep. 2018, 8, 14573. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, C.; Li, P.; Zhang, H.; Zhang, X.; Zheng, X.; Yang, Q.; Apaliya, M.T.; Boateng, N.A.S.; Sun, Y. The biocontrol effect of Sporidiobolus pararoseus Y16 against postharvest diseases in table grapes caused by Aspergillus niger and the possible mechanisms involved. Biol. Control 2017, 113, 18–25. [Google Scholar] [CrossRef]
- Freire, L.; Braga, P.A.C.; Furtado, M.M.; Delafiori, J.; Dias-Audibert, F.L.; Pereira, G.E.; Reyes, F.G.; Catharino, R.R.; Sant’Ana, A.S. From grape to wine: Fate of ochratoxin A during red, rose, and white winemaking process and the presence of ochratoxin derivatives in the final products. Food Control 2020, 113, 107167. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-PVA Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Impact of some environmental factors on growth and ochratoxin A production by Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2019, 291, 10–16. [Google Scholar] [CrossRef] [Green Version]
- dos Santos-Ciscon, B.A.; van Diepeningen, A.; da Cruz Machado, J.; Dias, I.E.; Waalwijk, C. Aspergillus species from Brazilian dry beans and their toxigenic potential. Int. J. Food Microbiol. 2019, 292, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Dachery, B.; Hernandes, K.C.; Veras, F.F.; Schmidt, L.; Augusti, P.R.; Manfroi, V.; Zini, C.A.; Welke, J.E. Effect of Aspergillus carbonarius on ochratoxin a levels, volatile profile and antioxidant activity of the grapes and respective wines. Food Res. Int. 2019, 126, 108687. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; García-Díaz, M.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Significance of Aspergillus niger aggregate species as contaminants of food products in Spain regarding their occurrence and their ability to produce mycotoxins. Food Microbiol. 2019, 82, 240–248. [Google Scholar] [CrossRef]
- Schueuermann, C.; Steel, C.C.; Blackman, J.W.; Clark, A.C.; Schwarz, L.J.; Moraga, J.; Collado, I.G.; Schmidtke, L.M. A GC–MS untargeted metabolomics approach for the classification of chemical differences in grape juices based on fungal pathogen. Food Chem. 2019, 270, 375–384. [Google Scholar] [CrossRef]
- Costa, C.P.; Gonçalves Silva, D.; Rudnitskaya, A.; Almeida, A.; Rocha, S.M. Shedding light on Aspergillus niger volatile exometabolome. Sci. Rep. 2016, 6, 27441. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Dandapani, H.; Thiel, K.; Jones, P.R. Microbial production of 1-octanol: A naturally excreted biofuel with diesel-like properties. Metab. Eng. Commun. 2015, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Erban, A.; Fehrle, I.; Martinez-Seidel, F.; Brigante, F.; Más, A.L.; Baroni, V.; Wunderlin, D.; Kopka, J. Discovery of food identity markers by metabolomics and machine learning technology. Sci. Rep. 2019, 9, 9697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.W.; Li, Q.H.; Xu, Z.D.; Dou, J.J. Mass spectrometry-based metabolomics in health and medical science: A systematic review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Meeus, I.; Rombouts, C.; Van Meulebroek, L.; Vanhaecke, L.; Smagghe, G. Metabolomics-based biomarker discovery for bee health monitoring: A proof of concept study concerning nutritional stress in Bombus terrestris. Sci. Rep. 2019, 9, 11423. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, A.; Ganopoulos, I.; Tryfinopoulou, P.; Panagou, E.Z.; Osanthanunkul, M.; Madesis, P.; Kizis, D. Rapid and accurate identification of black aspergilli from grapes using high-resolution melting (HRM) analysis. J. Sci. Food Agric. 2019, 99, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järvinen, A.K.; Laakso, S.; Piiparinen, P.; Aittakorpi, A.; Lindfors, M.; Huopaniemi, L.; Piiparinen, H.; Mäki, M. Rapid identification of bacterial pathogens using a PCR- and microarray-based assay. BMC Microbiol. 2009, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Schloter, M.; Abmus, B.; Hartmann, A. The Use of Immunological Methods To Detect and Identify Bacteria in the Environment. Biotech. Adv. 1995, 13, 75–90. [Google Scholar] [CrossRef]
- Fang, S.; Liu, S.; Song, J.; Huang, Q.; Xiang, Z. Recognition of pathogens in food matrixes based on the untargeted in vivo microbial metabolite profiling via a novel SPME/GC × GC-QTOFMS approach. Food Res. Int. 2021, 142, 110213. [Google Scholar] [CrossRef]
- Baptista, I.; Santos, M.; Rudnitskaya, A.; Saraiva, J.A.; Almeida, A. A comprehensive look into the volatile exometabolome of enteroxic and non-enterotoxic Staphylococcus aureus strains. Int. J. Biochem. Cell Biol. 2019, 108, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Metabolomics strategy for the mapping of volatile exometabolome from Saccharomyces spp. widely used in the food industry based on comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2017, 40, 2228–2237. [Google Scholar] [CrossRef]
- Fialho, M.B.; Toffano, L.; Pedroso, M.P.; Augusto, F.; Pascholati, S.F. Volatile organic compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J. Microbiol. Biotechnol. 2010, 26, 925–932. [Google Scholar] [CrossRef]
- Belinato, J.R.; Dias, F.F.G.; Caliman, J.D.; Augusto, F.; Hantao, L.W. Opportunities for green microextractions in comprehensive two-dimensional gas chromatography / mass spectrometry-based metabolomics—A review. Anal. Chim. Acta 2018, 1040, 1–18. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.R.B.; Kupper, K.C.; Augusto, F. In vivo investigation of the volatile metabolome of antiphytopathogenic yeast strains active against Penicillium digitatum using comprehensive two-dimensional gas chromatography and multivariate data analysis. Microchem. J. 2018, 141, 362–368. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Metabolomic profiling of the host response of tomato (Solanum lycopersicum) following infection by Ralstonia solanacearum. Int. J. Mol. Sci. 2019, 20, 3945. [Google Scholar] [CrossRef] [Green Version]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Wang, L.; Qu, L.; Zhang, L.; Hu, J.; Tang, F.; Lu, M. Metabolic responses of poplar to Apripona germari (Hope) as revealed by metabolite profiling. Int. J. Mol. Sci. 2016, 17, 923. [Google Scholar] [CrossRef] [Green Version]
- Alves, Z.; Melo, A.; Figueiredo, A.R.; Coimbra, M.A.; Gomes, A.C.; Rocha, S.M. Exploring the saccharomyces cerevisiae volatile metabolome: Indigenous versus commercial strains. PLoS ONE 2015, 10, e0143641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, P.; Santos, M.; Freitas, R.; Rocha, S.M.; Figueira, E. Response of Rhizobium to Cd exposure: A volatile perspective. Environ. Pollut. 2017, 231, 802–811. [Google Scholar] [CrossRef]
- Fonseca, A.M.A.; Dias, C.; Amaro, A.L.; Isidoro, N.; Pintado, M.; Silvestre, A.J.D.; Rocha, S.M. The impact of plant-based coatings in “ROCHA” pear preservation during cold storage: A metabolomic approach. Foods 2020, 9, 1299. [Google Scholar] [CrossRef]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Enlarging knowledge on lager beer volatile metabolites using multidimensional gas chromatography. Foods 2020, 9, 1276. [Google Scholar] [CrossRef]
- Matos, D.; Sá, C.; Cardoso, P.; Pires, A.; Rocha, S.M.; Figueira, E. The role of volatiles in Rhizobium tolerance to cadmium: Effects of aldehydes and alcohols on growth and biochemical endpoints. Ecotoxicol. Environ. Saf. 2019, 186, 109759. [Google Scholar] [CrossRef]
- Mousavi, F.; Bojko, B.; Bessonneau, V.; Pawliszyn, J. Cinnamaldehyde Characterization as an Antibacterial Agent toward E. coli Metabolic Profile Using 96-Blade Solid-Phase Microextraction Coupled to Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2016, 15, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Rees, C.A.; Franchina, F.A.; Nordick, K.V.; Kim, P.J.; Hill, J.E. Expanding the Klebsiella pneumoniae volatile metabolome using advanced analytical instrumentation for the detection of novel metabolites. J. Appl. Microbiol. 2017, 122, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Parastar, H.; Garreta-Lara, E.; Campos, B.; Barata, C.; Lacorte, S.; Tauler, R. Chemometrics comparison of gas chromatography with mass spectrometry and comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry Daphnia magna metabolic profiles exposed to salinity. J. Sep. Sci. 2018, 41, 2368–2379. [Google Scholar] [CrossRef]
- Carriço, Í.R.; Marques, J.; Trujillo-Rodriguez, M.J.; Anderson, J.L.; Rocha, S.M. Sorbent coatings for solid-phase microextraction targeted towards the analysis of death-related polar analytes coupled to comprehensive two-dimensional gas chromatography: Comparison of zwitterionic polymeric ionic liquids versus commercial coatings. Microchem. J. 2020, 158, 105243. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Xu, X.; Van Stee, L.L.P.; Williams, J.; Beens, J.; Adahchour, M.; Vreuls, R.J.J.; Brinkman, U.A.T.; Lelieveld, J. Comprehensive two-dimensional gas chromatography (GC×GC) measurements of volatile organic compounds in the atmosphere. Atmos. Chem. Phys. 2003, 3, 665–682. [Google Scholar] [CrossRef] [Green Version]
- Rocha, S.M.; Freitas, R.; Cardoso, P.; Santos, M.; Martins, R.; Figueira, E. Exploring the potentialities of comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry to distinguish bivalve species: Comparison of two clam species (Venerupis decussata and Venerupis philippinarum). J. Chromatogr. A 2013, 1315, 152–161. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Baptista, I.; Barros, A.S.; Gomes, N.C.M.; Cunha, Â.; Almeida, A.; Rocha, S.M. Can Volatile Organic Metabolites Be Used to Simultaneously Assess Microbial and Mite Contamination Level in Cereal Grains and Coffee Beans? PLoS ONE 2013, 8, e59338. [Google Scholar] [CrossRef]
- Silva, I.; Rocha, S.M.; Coimbra, M.A.; Marriott, P.J. Headspace solid-phase microextraction combined with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry for the determination of volatile compounds from marine salt. J. Chromatogr. A 2010, 1217, 5511–5521. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.R.; Leitão, G.G.; Santos, S.S.; Bizzo, H.R.; Lopes, D.; Alviano, C.S.; Alviano, D.S.; Leitão, S.G. Ethnopharmacological study of two Lippia species from Oriximiná, Brazil. J. Ethnopharmacol. 2006, 108, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Weldegergis, B.T.; Crouch, A.M.; Górecki, T.; de Villiers, A. Solid phase extraction in combination with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry for the detailed investigation of volatiles in South African red wines. Anal. Chim. Acta 2011, 701, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, X.; Liang, Y.; Fang, H.; Huang, L.-F.; Guo, F. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemom. Intell. Lab. Syst. 2006, 82, 218–228. [Google Scholar] [CrossRef]
- Loureiro, C.C.; Duarte, I.F.; Gomes, J.; Carrola, J.; Barros, A.S.; Gil, A.M.; Bousquet, J.; Bom, A.T.; Rocha, S.M. Urinary metabolomic changes as a predictive biomarker of asthma exacerbation. J. Allergy Clin. Immunol. 2014, 133, 261–263.e5. [Google Scholar] [CrossRef]
- Rocha, S.M.; Caldeira, M.; Carrola, J.; Santos, M.; Cruz, N.; Duarte, I.F. Exploring the human urine metabolomic potentialities by comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry. J. Chromatogr. A 2012, 1252, 155–163. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.L.; Zhou, G.H. Comparative study of volatile compounds in traditional Chinese Nanjing marinated duck by different extraction techniques. Int. J. Food Sci. Technol. 2007, 42, 543–550. [Google Scholar] [CrossRef]
- Caldeira, M.; Barros, A.S.; Bilelo, M.J.; Parada, A.; Câmara, J.S.; Rocha, S.M. Profiling allergic asthma volatile metabolic patterns using a headspace-solid phase microextraction/gas chromatography based methodology. J. Chromatogr. A 2011, 1218, 3771–3780. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiak, J.; Bonifay, V.; Otlewska, A.; Sunner, J.A.; Beech, I.B.; Stryszewska, T.; Kanka, S.; Oracz, J.; Zyzelewicz, D.; Gutarowska, B. Untargeted metabolomics approach in halophiles: Understanding the biodeterioration process of building materials. Front. Microbiol. 2017, 8, 2448. [Google Scholar] [CrossRef]
- Pan, X.; Liu, H.; Liu, J.; Wang, C.; Wen, J. Omics-based approaches reveal phospholipids remodeling of Rhizopus oryzae responding to furfural stress for fumaric acid-production from xylose. Bioresour. Technol. 2016, 222, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Liu, W.; Chen, Y.; Wang, F.; Lu, X.; Zheng, W. Untargeted volatile metabolomics using comprehensive two-dimensional gas chromatography-mass spectrometry—A solution for orange juice authentication. Talanta 2020, 217, 121038. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Shahid, M.; Jin, P.; Asher, A.; Kim, J. Identification of Metabolic Alterations in Breast Cancer Using Mass Spectrometry-Based Metabolomic Analysis. Metabolites 2020, 10, 170. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, G.; Inamdar, A.A.; Luo, J.; Zhang, N.; Yang, I.; Buckley, B.; Bennett, J.W. Volatile organic compounds emitted by filamentous fungi isolated from flooded homes after Hurricane Sandy show toxicity in a Drosophila bioassay. Indoor Air 2017, 27, 518–528. [Google Scholar] [CrossRef]
- Rees, C.A.; Burklund, A.; Stefanuto, P.H.; Schwartzman, J.D.; Hill, J.E. Comprehensive volatile metabolic fingerprinting of bacterial and fungal pathogen groups. J. Breath Res. 2018, 12, 026001. [Google Scholar] [CrossRef]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop. Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Pantoja, L.D.M.; do Nascimento, R.F.; de Araujo Nunes, A.B. Investigation of fungal volatile organic compounds in hospital air. Atmos. Pollut. Res. 2016, 7, 659–663. [Google Scholar] [CrossRef]
- Tong, H.; Wang, Y.; Li, Y.; Liu, S.; Chi, C.; Liu, D.; Guo, L.; Li, E.; Wang, C. Volatile organic metabolites identify patients with gastric carcinoma, gastric ulcer, or gastritis and control patients. Cancer Cell Int. 2017, 17, 108. [Google Scholar] [CrossRef]
- Aquino, S.; de Lima, J.E.A.; do Nascimento, A.P.B.; Reis, F.C. Analysis of fungal contamination in vehicle air filters and their impact as a bioaccumulator on indoor air quality. Air Qual. Atmos. Heal. 2018, 11, 1143–1153. [Google Scholar] [CrossRef]
- Cumeras, R.; Aksenov, A.A.; Pasamontes, A.; Fung, A.G.; Cianchetta, A.N.; Doan, H.; Davis, R.M.; Davis, C.E. Identification of fungal metabolites from inside Gallus gallus domesticus eggshells by non-invasively detecting volatile organic compounds (VOCs). Anal. Bioanal. Chem. 2016, 408, 6649–6658. [Google Scholar] [CrossRef]
- Jedidi, I.; Cruz, A.; González-Jaén, M.T.; Said, S. Aflatoxins and ochratoxin A and their Aspergillus causal species in Tunisian cereals. Food Addit. Contam. Part B Surveill. 2017, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Zhou, L.D.; Chen, H.; Wang, C.Z.; Xia, Z.N.; Yuan, C.S. Solid-phase microextraction technology for in vitro and in vivo metabolite analysis. TrAC Trends Anal. Chem. 2016, 80, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Souza-Silva, É.A.; Reyes-Garcés, N.; Gómez-Ríos, G.A.; Boyaci, E.; Bojko, B.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices III. Bioanalytical and clinical applications. TrAC Trends Anal. Chem. 2015, 71, 249–264. [Google Scholar] [CrossRef]
- Risticevic, S.; Souza-Silva, E.A.; Gionfriddo, E.; DeEll, J.R.; Cochran, J.; Hopkins, W.S.; Pawliszyn, J. Application of in vivo solid phase microextraction (SPME) in capturing metabolome of apple (Malus × domestica Borkh) fruit. Sci. Rep. 2020, 10, 6724. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Mishra, S.; Kumar, A.; Kumar, S.; Prasad, C.S. In vivo and in vitro control activity of plant essential oils against three strains of Aspergillus niger. Environ. Sci. Pollut. Res. 2017, 24, 21948–21959. [Google Scholar] [CrossRef]
- Li, F.-x.; Li, F.-h.; Yang, Y.-x.; Yin, R.; Ming, J. Comparison of phenolic profiles and antioxidant activities in skins and pulps of eleven grape cultivars (Vitis vinifera L.). J. Integr. Agric. 2019, 18, 1148–1158. [Google Scholar] [CrossRef]
- Gajera, H.P.; Katakpara, Z.A.; Patel, S.V.; Golakiya, B.A. Antioxidant defense response induced by Trichoderma viride against Aspergillus niger Van Tieghem causing collar rot in groundnut (Arachis hypogaea L.). Microb. Pathog. 2016, 91, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Masuo, S.; Osada, L.; Zhou, S.; Fujita, T.; Takaya, N. Aspergillus oryzae pathways that convert phenylalanine into the flavor volatile 2-phenylethanol. Fungal Genet. Biol. 2015, 77, 22–30. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Poole, P. Shining a light on the dark world of plant root–microbe interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 4281–4283. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Prosser, R.; Hasani, M.; Chen, H.; Skanes, B.; Lubitz, W.D.; Warriner, K. Degradation of chlorpyrifos and inactivation of Escherichia coli O157:H7 and Aspergillus niger on apples using an advanced oxidation process. Food Control 2020, 109, 106920. [Google Scholar] [CrossRef]



| Peak Number | 1tRa (s) | 2tRa (s) | Metabolite | CAS Number | Formula | MSI Level b | RICalc c | RILit d |
|---|---|---|---|---|---|---|---|---|
| 1 | 115 | 0.910 | 1-Butanol | 71-36-3 | C4H10O | 1 | 644 | 655 [41] |
| 2 | 150 | 1.160 | 3-Methyl-1-butanol | 123-51-3 | C5H12O | 1 | 718 | 706 [42] |
| 3 | 255 | 1.140 | 1-Hexanol | 111-27-3 | C6H14O | 1 | 878 | 877 [42] |
| 4 | 345 | 1.100 | 1-Heptanol | 111-70-6 | C7H16O | 2 | 975 | 974 [43] |
| 5 | 350 | 1.050 | 1-Octen-3-ol | 3391-86-4 | C8H16O | 1 | 980 | 992 [44] |
| 6 | 365 | 1.270 | 3-Octanol | 589-98-0 | C8H18O | 1 | 996 | 996 [45] |
| 7 | 395 | 0.990 | 2-Ethyl-1-hexanol | 104-76-7 | C8H18O | 2 | 1029 | 1038 [44] |
| 8 | 440 | 1.030 | 1-Octanol | 111-87-5 | C9H18O2 | 1 | 1079 | 1079 [44] |
| 9 | 475 | 3.030 | 2-Phenylethanol | 60-12-8 | C8H10O | 1 | 1120 | 1107 [46] |
| 10 | 805 | 2.060 | 2,4-bis(1,1-Dimethylethyl)phenol | 96-76-4 | C14H22O | 2 | 1514 | 1513 [47] |
| 11 | 110 | 0.460 | 3-Methylbutanal | 590-86-3 | C5H10O | 2 | 633 | 628 [48] |
| 12 | 190 | 0.590 | Hexanal | 66-25-1 | C6H12O | 1 | 801 | 800 [49] |
| 13 | 275 | 0.620 | Heptanal | 111-71-7 | C7H14O | 1 | 901 | 903 [49] |
| 14 | 465 | 0.630 | Nonanal | 124-19-6 | C9H18O | 1 | 1106 | 1106 [42] |
| 15 | 555 | 0.630 | Decanal | 112-31-2 | C10H20O | 2 | 1207 | 1206 [49] |
| 16 | 685 | 0.770 | 2-Undecenal | 2463-77-6 | C11H20O | 2 | 1364 | 1369 [50] |
| 17 | 720 | 0.650 | Dodecanal | 112-54-9 | C12H24O | 2 | 1407 | 1406 [49] |
| 18 | 335 | 1.550 | Benzaldehyde | 100-52-7 | C7H6O | 1 | 965 | 954 [51] |
| 19 | 410 | 1.620 | Benzeneacetaldehyde | 122-78-1 | C8H8O | 1 | 1046 | 1049 [41] |
| 20 | 135 | 0.530 | Methyl 2-methylpropenoate | 80-62-6 | C5H8O2 | 2 | 685 | 710 [41] |
| 21 | 695 | 0.920 | 3-Hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate | 74367-34-3 | C12H24O3 | 2 | 1376 | 1376 [13] |
| 22 | 545 | 1.050 | 2-Phenylethylacetate | 103-45-7 | C10H12O2 | 2 | 1196 | 1196 [13] |
| 23 | 880 | 0.490 | Hexadecane | 544-76-3 | C16H34 | 1 | 1601 | 1600 [49] |
| 24 | 965 | 0.430 | Heptadecane | 629-78-7 | C17H36 | 1 | 1701 | 1700 [49] |
| 25 | 115 | 0.460 | Benzene | 71-43-2 | C6H6 | 1 | 643 | 643 [13] |
| 26 | 170 | 0.540 | Toluene | 108-88-3 | C7H8 | 1 | 759 | 771 [41] |
| 27 | 250 | 0.590 | 1,3-Dimethylbenzene | 108-38-3 | C8H10 | 2 | 871 | 871 [13] |
| 28 | 270 | 0.640 | 1,2-Dimethylbenzene | 95-47-6 | C8H10 | 2 | 901 | 900 [42] |
| 29 | 325 | 0.580 | Propylbenzene | 103-65-1 | C9H12 | 2 | 953 | 959 [41] |
| 30 | 335 | 0.590 | 1-Ethyl-4-methylbenzene | 622-96-8 | C9H12 | 2 | 964 | 970 [41] |
| 31 | 365 | 0.640 | 1,3,5-Trimethylbenzene | 108-67-8 | C9H12 | 2 | 995 | 974 [41] |
| 32 | 390 | 0.580 | 1-Methyl-2-(1-methylethyl)benzene | 527-84-4 | C10H14 | 2 | 1023 | 1023 [13] |
| 33 | 390 | 0.690 | 1,2,3-Trimethylbenzene | 526-73-8 | C9H12 | 1 | 1023 | 1023 [13] |
| 34 | 425 | 0.610 | 2-Ethyl-1,4-dimethylbenzene | 1758-88-9 | C10H14 | 2 | 1062 | 1062 [13] |
| 35 | 700 | 1.270 | Biphenyl | 92-52-4 | C12H10 | 2 | 1383 | 1383 [13] |
| 36 | 880 | 1.020 | 2-Methyl-6-phenyl-1,6-heptadiene | 51708-97-5 | C14H18 | 2 | 1601 | 1601 [13] |
| 37 | 75 | 0.390 | 2-Propanone | 67-64-1 | C3H6O | 1 | 559 | 559 [13] |
| 38 | 265 | 0.580 | 3-Heptanone | 106-35-4 | C7H14O | 1 | 889 | 884 [41] |
| 39 | 355 | 0.740 | 6-Methyl-5-hepten-2-one | 110-93-0 | C8H14O | 1 | 985 | 985 [41] |
| 40 | 495 | 0.760 | 3-Nonen-2-one | 18402-83-0 | C9H16O | 2 | 1140 | 1140 [13] |
| 41 | 755 | 0.800 | 6,10-Dimethyl-5,9-undecadien-2-one | 3796-70-1 | C13H22O | 2 | 1451 | 1451 [13] |
| 42 | 435 | 0.790 | 2,6-Dimethyl-7-octen-2-ol | 18479-58-8 | C10H20O | 2 | 1073 | 1073 [13] |
| 43 | 625 | 0.630 | Endobornyl acetate | 76-49-3 | C12H20O2 | 2 | 1289 | 1289 [13] |
| 44 | 780 | 0.750 | α-Methylionone | 127-51-5 | C14H22O | 2 | 1482 | 1482 [13] |
| Parameters | Latent Variable 1 | Latent Variable 2 | Latent Variable 3 | Latent Variable 4 | Latent Variable 5 |
|---|---|---|---|---|---|
| Accuracy | 0.7778 | 0.7778 | 0.9815 | 1.0 | 1.0 |
| R2 | 0.4548 | 0.8821 | 0.9152 | 0.9312 | 0.9557 |
| Q2 | 0.2957 | 0.7888 | 0.8252 | 0.81084 | 0.8128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belinato, J.R.; Costa, C.P.; Almeida, A.; Rocha, S.M.; Augusto, F. Mapping Aspergillus niger Metabolite Biomarkers for In Situ and Early Evaluation of Table Grapes Contamination. Foods 2021, 10, 2870. https://doi.org/10.3390/foods10112870
Belinato JR, Costa CP, Almeida A, Rocha SM, Augusto F. Mapping Aspergillus niger Metabolite Biomarkers for In Situ and Early Evaluation of Table Grapes Contamination. Foods. 2021; 10(11):2870. https://doi.org/10.3390/foods10112870
Chicago/Turabian StyleBelinato, Joao Raul, Carina Pedrosa Costa, Adelaide Almeida, Silvia M. Rocha, and Fabio Augusto. 2021. "Mapping Aspergillus niger Metabolite Biomarkers for In Situ and Early Evaluation of Table Grapes Contamination" Foods 10, no. 11: 2870. https://doi.org/10.3390/foods10112870