Cooking African Pumpkin Leaves (Momordica balsamina L.) by Stir-Frying Improved Bioactivity and Bioaccessibility of Metabolites—Metabolomic and Chemometric Approaches
Abstract
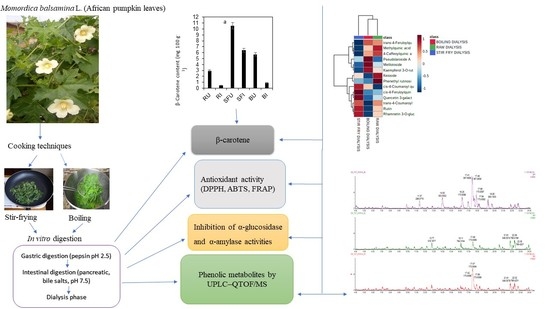
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Equipment
2.2. Household Cooking Techniques
2.3. Simulated Gastrointestinal Digestion
2.4. Extraction of Phenolic Metabolites
2.5. β-Carotenoid Content
2.6. Antioxidant Capacity
2.7. In Vitro α-Glucosidase Inhibitory Activity
2.8. α-Amylase Inhibitory Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Multivariate Analysis
3.2. Inhibition of Carbohydrate Hydrolysing Enzymes
3.3. The Relationship between the Bioactive Metabolites Identified and the Tested Biological Activities
3.4. Impact of Stir-Frying or Boiling on the Release and In Vitro Bioaccessibility of β-Carotene Compared to the Raw Whole Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jibril, A.H.; Musa, U.; Saidu, B.; Ajape, A.B.; Maina, I.H.; Jimoh, A.A.; Sani, A.; Yabo, Y.A.; Joafar, A.I. Effects of powdered Balsam Apple (Momordica balsamina L.) leafy supplement on blood glucose level and the haematological parameters of Japanese Quails. J. Anim. Res. 2018, 8, 99–105. [Google Scholar] [CrossRef]
- Souda, S.; George, S.; Mannathoko, N.; Goercke, I.; Chabaesele, K. Antioxidant and Antibacterial Activity of Methanol Extract of Momordica Balsamina. IRA Int. J. Appl. 2018, 10, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Goñi, I.; Saura-Calixto, F. Determination of β-carotene and lutein available from green leafy vegetables by an in vitro digestion and colonic fermentation method. J. Agric. Food Chem. 2005, 53, 2936–2940. [Google Scholar] [CrossRef]
- Madala, N.E.; Piater, L.; Dubery, I.; Steenkamp, P. Distribution patterns of flavonoids from Momordica species by ultra-high performance liquid chromatography quadrupole time of flight mass spectrometry: A metabolic profiling approach. Rev. Bras. Farmacogn. 2016, 26, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Changes in phenolics, carotenoids and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Bvenura, C.; Afolayon, A.J. The role of wild vegetables in household food security in South Africa: A review. Food Res. Int. 2015, 76, 1001–1011. [Google Scholar] [CrossRef]
- Mashiane, P.; Mashitoa, F.M.; Slabbert, R.M.; Sivakumar, D. Impact of household cooking techniques on colour, antioxidant and sensory properties of African pumpkin and pumpkin leaves. Int. J. Gastron. Food Sci. 2021, 23, 100307. [Google Scholar] [CrossRef]
- Subramaniam, S.; Rosdi, M.H.B.; Kuppusamy, U.R. Customized cooking methods enhance antioxidant, antiglycemic, and insulin-like properties of Momordica charantia and Moringa oleifera. J. Food Qual. 2017, 2017, 9561325. [Google Scholar] [CrossRef] [Green Version]
- Mashitoa, F.M.; Shoko, T.; Shai, J.L.; Slabbert, R.M.; Sivakumar, D. Changes in phenolic metabolites and biological activities of Pumpkin leaves (Cucurbita moschata Duchesne ex Poir. ) during blanching. Front. Nutr. 2021, 8, 86. [Google Scholar] [CrossRef]
- Managa, M.G.; Shai, J.; Phan, A.D.T.; Sultanbawa, Y.; Sivakumar, D. Impact of household cooking techniques on African nightshade and Chinese cabbage on phenolic compounds, antinutrients, in vitro antioxidants and β-glucosidase activity. Front. Nutr. 2020, 7, 292. [Google Scholar] [CrossRef]
- Akdaş, Z.Z.; Bakkalbaşi, E. Influence of different cooking methods on color, bioactive compounds, and antioxidant activity of kale. Int. J. Food Prop. 2017, 20, 877–887. [Google Scholar] [CrossRef]
- Moyo, S.M.; Serem, J.C.; Bester, M.J.; Mavumengwana, V.; Kayitesi, E. Hydrothermal Processing and In Vitro Simulated Human Digestion Affects the Bioaccessibility and Bioactivity of Phenolic Compounds in African Pumpkin (Momordica balsamina) Leaves. Molecules 2021, 26, 5201. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Arnal, M.; Barat, J.M.; Talens, P. Effect of cooking on protein digestion and antioxidant activity of different legume pastes. Foods 2021, 10, 47. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Seke, F.; Manhivi, V.E.; Shoko, T.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Effect of freeze drying and simulated gastrointestinal digestion of phenolic metabolites and antioxidant property of Natal plum (Carissa macrocarpa). Foods 2021, 10, 1420. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Panfili, G.; Frantiani, A.; Irano, M. Improved normal-phase high performance liquid chromatography procedure for the determination of carotenoids in cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef]
- Moloto, R.M.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of phenolic compounds, carotenoids, amino acid composition, in vitro antioxidants and anti-diabetic activities in the leaves of seven Cowpea (Vigna unguiculata) cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef]
- Carvalho, F.V.; Santana, L.F.; Diogenes, V.; da Silva, A.; Costa, L.; Zambotti-Villelae, L.; Colepicolo, P.; Ferraz, C.P.; Ribeiro, P.R. Combination of a multiplatform metabolite profiling approach and chemometrics as a powerful strategy to identify bioactive metabolites in Lepidium meyenii (Peruvian maca). Food Chem. 2021, 364, 130453. [Google Scholar] [CrossRef]
- Ferracane, R.; Pellegrini., N.; Visconti., A.; Graziani., G.; Chiavaro., E.; Miglio., C.; Fogliano, V. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J. Agric. Food Chem. 2008, 56, 8601–8608. [Google Scholar] [CrossRef]
- Xie, C.; Yu, K.; Zhong, D.; Yuan, T.; Ye, F.; Jarrel, J.A.; Millar, A.; Chen, X. Investigation of isomeric transformations of chlorogenic acid in buffers and biological matrixes by ultraperformance liquid chromatography coupled with hybrid quadrupole/ion mobility/orthogonal acceleration time-of-flight mass spectrometry. J. Agric. Food Chem. 2011, 59, 11078–11087. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Polyphenolic chemistry of tea and coffee: A century of progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef] [PubMed]
- Asamenew, G.; Kim, H.; Lee, M.; Lee, S.; Lee, S.; Cha, Y.; Lee, S.; Yoo, S.; Kim, J. Comprehensive characterization of hydroxycinnamoyl derivatives in green and roasted coffee beans: A new group of methyl hydroxycinnamoyl quinate. Food Chem. 2019, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Effect of some phenolic compounds and beverages on pepsin activity during simulated gastric digestion. J. Agric. Food Chem. 2005, 53, 8706–8713. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2015, 197, 466–473. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J. Agric. Food Chem. 2006, 54, 374–381. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Manhivi, V.; Slabbert, R.M.; Shai, J.L.; Sivakumar, D. Changes in antinutrients, phenolics, antioxidant activities and in vitro α-glucosidase inhibitory activity in pumpkin leaves (Cucurbita moschata) during different domestic cooking methods. Food Sci. Biotechnol. 2021, 30, 793–800. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Liu, Y. Inhibitory effects against α glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Van Elslande, A. Effect of In Vitro Digestion of Polyphenolic and Organic Acid Profile and Antioxidant Capacity of Raw and Cooked Momordica charantia. Master’s Thesis, School of Dietetics and Human Nutrition, McGill University, Montréal, QC, Canada, 2012. [Google Scholar]
- Ng, Z.X.; Rosman, N.F. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J. Food Sci. Technol. 2019, 56, 865–877. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. Spec. Publ. 2009, 37, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; de Castro, R.D.; Fernandez, L.G. Metabolite profiling, antioxidant and antibacterial activities of Brazilian propolis: Use of correlation and multivariate analyses to identify potential bioactive compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assefa, S.T.; Yang, E.; Chae, S.; Song, M.; Lee, J.; Cho, M.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, J.N.; Luu, A.Y.; Dragsted, L.O.; Arrigoni, E. Adaptation on an in vitro digestion method to screen carotenoid liberation and in vitro accessibility from different processed spinach preparations. Food Chem. 2017, 224, 407–413. [Google Scholar] [CrossRef] [PubMed]











| Raw Whole Leaves | Stir-Fried Leaves | Boiled Leaves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactive Metabolites (mg 100 g −1) | RU | RG | RI | RD | SFU | SFG | SFI | SFD | BU | BG | BI | BD |
| Methylquinic acid | 0.72 ± 0.13 *,i | 578.24 ± 0.6 a | 554.45 ± 0.4 b | 2.34 ± 0.57 g | 0.06 ± 0.01 k | 265.43 ± 0.97 c | 223.25 ± 0.7 d | 0.74 ± 0.13 i | 0.26 ± 0.05 j | 62.56 ± 0.97 f | 74.82 ± 3.3 e | 1.20 ± 0.1 h |
| Pseudolaroside A | 44.90 ± 0.2 f | 210.70 ± 0.9 d | 797.65 ± 0.7 a | 127.15 ± 0.87 e | 123.43 ± 0.4 e | 453.06 ± 0.42 c | 592.41 ± 0.1 b | 34.79 ± 0.43 g | 28.20 ± 0.04 h | 20.38 ± 0.64 i | 23.17 ± 1.4 i | 4.59 ± 0.6 j |
| β-D-glucosyl-2-coumarate (Melilotoside) | 2.75 ± 0.4 j | 11.48 ± 0.3 g | 13.94 ± 0.8 g | 15.54 ± 0.8 e | 5.77 ± 1.03 i | 29.38 ± 0.94 b | 35.11 ± 0.34 a | 22.40 ± 0.36 c | 1.11 ± 0.20 k | 7.07 ± 2.21 h | 17.21 ± 0.4 d | 0.40 ± 0.1 l |
| 4 caffeoylquinic acid (Cryptochlorogenic acid) | 1.20 ± 0.21 k | 68.48 ± 0.23 e | 92.68 ± 0.35 d | 2.94 ± 0.20 j | 92.19 ± 0.4 d | 835.73 ± 0.26 b | 925.81 ± 0.41 a | 20.05 ± 1.40 g | 13.82 ± 0.4 h | 40.03 ± 0.83 f | 108.32 ± 0.6 c | 9.00 ± 1.35 i |
| Cis 4-coumaroylquinic acid | 6.01 ± 1.0 h | 113.05 ± 0.1 c | 105.45 ± 0.8 d | 2.89 ± 0.20 j | 18.15 ± 0.2 g | 286.20 ± 0.73 b | 338.27 ± 0.06 a | 7.11 ± 0.50 h | 2.21 ± 0.40 j | 32.60 ± 0.28 f | 53.25 ± 0.15 e | 3.34 ± 0.5 i |
| Trans-4-coumaroylquinic acid | 3.18 ± 0.5 h | 14.90 ± 04 f | 73.47 ± 0.28 c | 2.98 ± 0.21 i | 21.20 ± 0.7 d | 112.37 ± 0.09 b | 275.00 ± 0.11 a | 4.16 ± 0.74 g | 2.47 ± 0.44 i | 15.00 ± 1.41 f | 17.26 ± 0.18 e | 0.80 ± 0.1 j |
| Cis-4-feruloylquinic acid | 273.6 ± 0.8 f | 2351.59 ± 0.6 b | 2593.32 ± 0.5 a | 162.92 ± 0.37 h | 178.7 ± 0.9 g | 2044.65 ± 0.2 d | 2164.70 ± 0.72 c | 75.05 ± 0.24 i | 47.49 ± 0.48 j | 250.15 ± 0.34 f | 375.42 ± 0.63 e | 19.38 ± 0.9 k |
| Quercetin-3-rutinoside (Rutin) | 31.86 ± 0.9 g | 230.82 ± 0.31 d | 277.95 ± 0.06 c | 5.07 ± 0.22 j | 73.66 ± 0.15 e | 665.89 ± 0.08 b | 746.56 ± 0.48 a | 38.68 ± 0.70 g | 38.12 ± 0.8 g | 50.95 ± 0.93 f | 21.71 ± 0.84 i | 1.86 ± 0.2 k |
| Trans-4-feruloylquinic acid | 44.52 ± 095 f | 156.33 ± 0.31 d | 308.26 ± 08.78 b | 61.59 ± 0.30 e | 47.27 ± 0.44 f | 287.93 ± 0.63 c | 341.59 ± 0.64 a | 17.20 ± 0.20 h | 3.55 ± 0.63 k | 13.59 ± 0.75 i | 27.23 ± 0.8 g | 6.41 ± 0.9 j |
| Quercetin 3-galactoside | 4.05 ± 0.72 f | 41.35 ± 0.96 c | 84.21 ± 0.44 b | 3.10 ± 0.75 g | 37.47 ± 0.6 d | 322.22 ± 0.97 a | 337.18 ± 0.09 a | 8.18 ± 0.57 e | 2.83 ± 0.51 h | 3.48 ± 0.72 g | 8.61 ± 0.74 e | 1.57 ± 0.23 i |
| Kaempferol-O-rutinoside (Nicotiflorin) | 42.05 ± 0.51 f | 455.43 ± 0.42 d | 715.34 ± 0.55 a | 13.83 ± 0.97 g | 77.42 ± 0.82 e | 629.04 ± 0.15 b | 513.64 ± 0.76 c | 12.76 ± 0.89 h | 9.23 ± 1.65 i | 6.07 ± 1.37 j | 9.44 ± 0.54 i | 1.57 ± 0.23 k |
| Isorhamnetin 3-O-robinoside (Keioside) | 7.59 ± 1.36 g | 112.35 ± 0.01 d | 155.23 ± 0.43 c | 1.59 ± 0.38 i | 45.32 ± 0.09 e | 330.99 ± 01.00 b | 352.25 ± 87.35 a | 9.89 ± 0.77 f | 2.27 ± 0.41 h | 1.91 ± 0.87 i | 1.53 ± 0.58 i | 0.24 ± 0.04 j |
| Rhamnetin-3-O-glucoside | 1.05 ± 0.19 f | 12.61 ± 0.08 d | 14.54 ± 0.24 c | 0.75 ± 0.18 h | 10.08 ± 1.80 e | 64.42 ± 0.91 b | 74.36 ± 38.68 a | 1.27 ± 0.23 f | 0.47 ± 0.08 i | 0.86 ± 0.35 g | 0.38 ± 0.37 j | nd |
| Phenethyl rutinoside | 17.41 ± 3.11 c | 2.49 ± 0.29 g | 14.38 ± 0.07 d | 3.60 ± 0.25 f | 11.07 ± 0.98 e | 22.83 ± 0.50 b | 35.23 ± 4.75 a | 1.64± 0.29 i | 1.82 ± 0.33 h | 3.05 ± 2.07 f | 2.84 ± 0.89 g | 1.00 ± 0.15 j |
| Antioxidant activity | ||||||||||||
| FRAP mmol TEAC g −1 | 3.65 ± 0.01 h | 6.41 ± 0.01 f | 11.69 ± 0.02 c | 1.20 ± 0.02 j | 9.73 ± 0.02 d | 12.92 ± 0.01 b | 16.40 ± 3.10 a | 2.60 ± 0.05 i | 1.38 ± 0.01 j | 5.57 ± 0.07 g | 7.75 ± 0.01 e | 1.18 ± 0.06 j |
| DPPH IC50 (mg mL−1) | 0.53 ± 0.01 b | 0.30 ± 0.00 d | 0.18 ± 0.05 f | 0.68 ± 0.02 a | 0.22 ± 0.01 e | 0.16 ± 0.02 f | 0.13 ± 0.00 g | 0.54 ± 0.01 b | 0.40 ± 0.01 c | 0.32 ± 0.00 d | 0.28 ± 0.00 e | 0.64 ± 0.05 a |
| ABTS IC50 (mg mL−1) | 0.58 ± 0.01 d | 0.31 ± 0.03 f | 0.15 ± 0.01 h | 0.80 ± 0.01 a | 0.21 ± 0.02 g | 0.14 ± 0.00 h | 0.08 ± 0.06 i | 0.64 ± 0.02 c | 0.48 ± 0.02 e | 0.34 ± 0.00 f | 0.28 ± 0.01 g | 0.71 ± 0.01 b |
| Treatment | α-Amylase IC50 mg mL−1 | α-Glucosidase IC50 mg mL−1 |
|---|---|---|
| Raw whole leaves | 0.24 ± 0.04 g | 0.41 ± 0.04 e |
| Raw leaves at gastric phase | 0.36 ± 0.50 e | 0.43 ± 0.10 e |
| Raw leaves at intestinal phase | 0.28 ± 0.40 f | 0.15 ± 0.20 h |
| Raw leaves at dialysis phase | 1.15 ± 0.15 b | 0.89 ± 0.04 b |
| Stir-fried leaves | 0.14 ± 0.03 h | 0.25 ± 0.02 g |
| Stir-fried leaves at gastric phase | 0.13 ± 0.20 h | 0.31 ± 0.00 f |
| Stir-fried leaves at intestinal phase | 0.08 ± 0.30 i | 0.06 ± 0.20 i |
| Stir-fried leaves at dialysis phase | 0.43 ± 0.04 d | 0.32 ± 0.10 f |
| Boiled leaves | 0.69 ± 0.02 c | 0.75 ± 0.30 c |
| Boiled leaves at gastric phase | 0.32 ± 0.10 e | 0.29 ± 0.20 g |
| Boiled leaves at intestinal phase | 0.27 ± 0.20 g | 0.16 ± 0.20 h |
| Boiled leaves at dialysis phase | 0.63 ± 0.05 c | 0.50 ± 0.05 d |
| Acarbose | 3.14 ± 0.13 a | 6.87 ±0.22 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashiane, P.; Manhivi, V.E.; Shoko, T.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Cooking African Pumpkin Leaves (Momordica balsamina L.) by Stir-Frying Improved Bioactivity and Bioaccessibility of Metabolites—Metabolomic and Chemometric Approaches. Foods 2021, 10, 2890. https://doi.org/10.3390/foods10112890
Mashiane P, Manhivi VE, Shoko T, Slabbert RM, Sultanbawa Y, Sivakumar D. Cooking African Pumpkin Leaves (Momordica balsamina L.) by Stir-Frying Improved Bioactivity and Bioaccessibility of Metabolites—Metabolomic and Chemometric Approaches. Foods. 2021; 10(11):2890. https://doi.org/10.3390/foods10112890
Chicago/Turabian StyleMashiane, Petunia, Vimbainashe E. Manhivi, Tinotenda Shoko, Retha M. Slabbert, Yasmina Sultanbawa, and Dharini Sivakumar. 2021. "Cooking African Pumpkin Leaves (Momordica balsamina L.) by Stir-Frying Improved Bioactivity and Bioaccessibility of Metabolites—Metabolomic and Chemometric Approaches" Foods 10, no. 11: 2890. https://doi.org/10.3390/foods10112890
APA StyleMashiane, P., Manhivi, V. E., Shoko, T., Slabbert, R. M., Sultanbawa, Y., & Sivakumar, D. (2021). Cooking African Pumpkin Leaves (Momordica balsamina L.) by Stir-Frying Improved Bioactivity and Bioaccessibility of Metabolites—Metabolomic and Chemometric Approaches. Foods, 10(11), 2890. https://doi.org/10.3390/foods10112890