Non-Thermal Technologies as Tools to Increase the Content of Health-Promoting Compounds in Whole Fruits and Vegetables While Retaining Quality Attributes
Abstract
1. Introduction
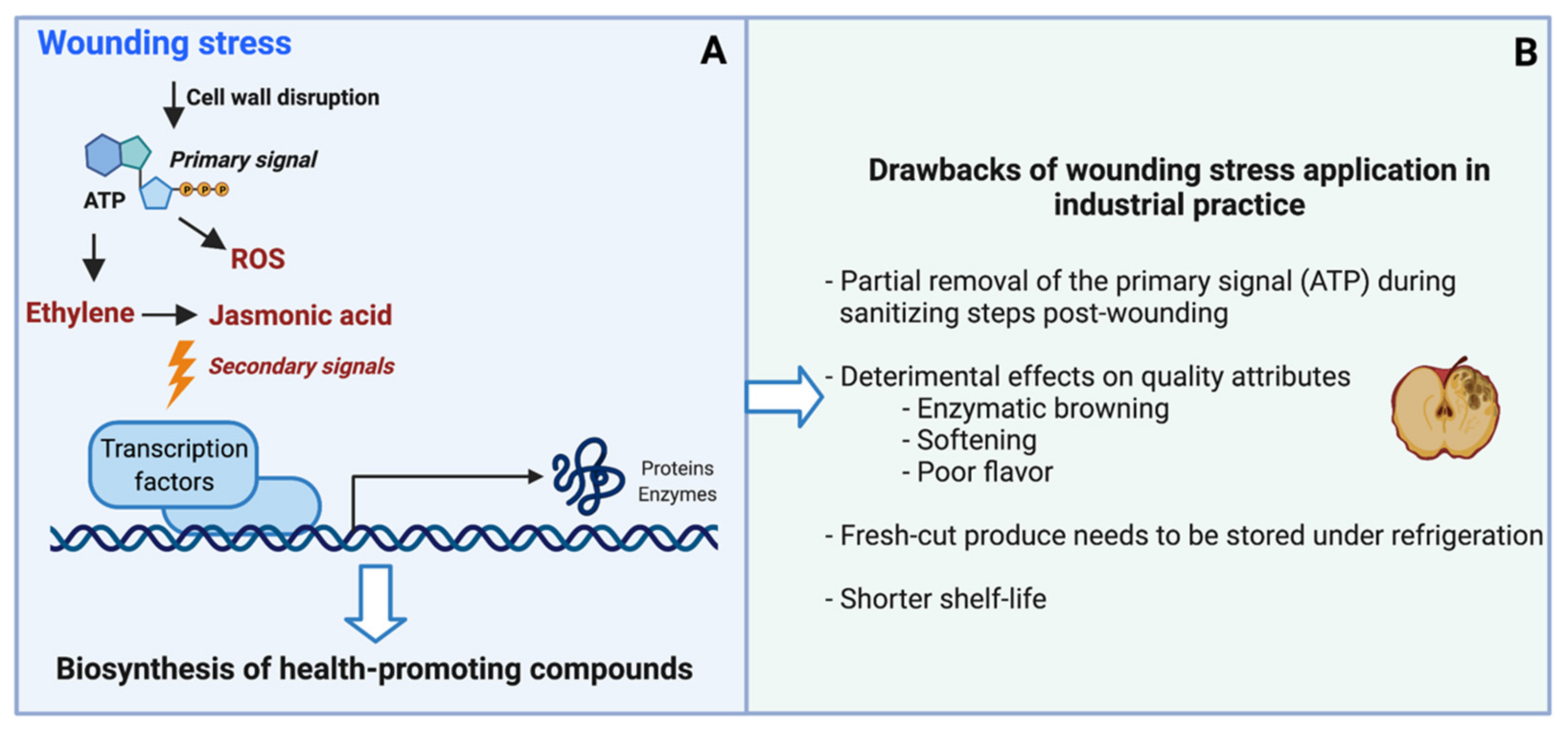
2. Wounding Stress as a Tool to Induce the Biosynthesis of Health-Promoting Compounds in Horticultural Crops: Mechanism and Drawbacks in Industrial Practice
3. Scientific Evidence for Non-Thermal Technologies (NTTs) That Emulate Wound-Induced Biosynthesis of Bioactive Compounds in Whole Fruits and Vegetables
3.1. High Hydrostatic Pressure (HHP)
3.1.1. Mango (Mangifera indica)
| Horticultural Crop | HHP Processing and Storage Conditions Evaluated | Main Findings | References | |
|---|---|---|---|---|
| Effects on the Biosynthesis of Health-Promoting Compounds | Effects on Quality and Physiological Attributes | |||
| Mango (Mangifera indica) | Whole mangoes (cv. Tainong) were subjected to 20, 40, 60, and 80 MPa for 10 min. Samples were stored for 16 days at 13 °C and ~85% relative humidity (RH). | HHP increased carotenoid biosynthesis at the transcriptional level. Samples treated at 20 MPa showed 43.7% higher total carotene content after storage. HHP increased ascorbic acid retention during storage. Samples treated with 40 MPa showed higher ascorbic acid retention. Except for the 40 MPa treatment, HHP-treated samples showed higher flavonoid content. In general, 20 MPa treatment resulted in the highest accumulation of total phenolics and carotenoids, while 40 MPa treated mangoes showed higher levels of ascorbic acid. | HHP treatment reduced respiration rate by 26.62, 20.25, 32.72, and 41.81%, for mangoes treated at 20, 40, 60, and 80 MPa, respectively, compared with the control. HHP treated samples showed higher a * (redness) values during ripening compared with the control. HHP treatment decreased firmness at the initial storage time (1 day). However, after 7 days, no significant difference was observed in firmness values between HHP-treated samples and the control. HHP-treated mangoes showed higher titratable acidity (from days 7 to 16), higher reducing sugar content and higher moisture loss. | [24] |
| Whole mangoes (cv. Ataulfo) were treated with HHP at 15, 30, or 60 MPa for 10 or 20 min. Non-treated fruit was used as the control. Samples were stored for 14 days at 25 °C and 85–90% of RH. | HHP treatments at 30 and 60 MPa for 20 min increased ascorbic acid content by 30.7–46.1% compared to the control before storage. HHP treatment at 15 MPa for 10 min induced a higher accumulation of total carotenoids (100%) and phenolics (41.2%) compared with the control at 14 days of storage. Results indicated that low-pressure treatments induced the biosynthesis of nutraceuticals in mango. | HHP did not inhibit the ripening process of mango. At the climacteric peak (9 days), the HHP-treated samples showed a lower respiration rate. Ethylene production was lower in samples treated at 30 and 60 MPa, regardless of the pressurization time (10 or 20 min). HHP-treated mangoes showed lower firmness values during storage, whereas at 14 days, no significant difference was detected between HHP samples and the control. HHP treatment increased moisture loss. At 9 days of storage, the pulp of HHP-treated mangoes showed a more intense orange color than non-treated samples. | [25,26] | |
| Carrot (Daucus carota) | Whole carrots were treated at 60 or 100 MPa for the come-up time (CUT). Samples were stored for 3 days at 15 °C. | Immediately after HHP application, carrots treated at 100 MPa showed an increase of free (5-O-caffeoylquinic acid, 63.9% and 3,4-di-O-feruloylquinic acid, 228.6%) and bound (p-coumaric acid, 82.6%) phenolics. On day 1, the 60 MPa samples showed accumulation of 4,5-di-O-caffeoylquinic acid (60.2%) and isocoumarin (98.9%), whereas the 100 MPa samples presented increases of chlorogenic acid (291.2%) and 3,4-di-O-feruloylquinic acid (466.1%). On day 2, an increase in bound phenolics (rutin, 85.5% and p-coumaric acid, 214.7%) was observed in samples treated at 60 MPa. On day 3, the 100 MPa samples presented higher quercetin (371.2%) content. | On day 2, the 60 MPa and 100 MPa samples showed 380.2% and 139.7% higher phenylalanine-ammonia lyase (PAL) activity, respectively, than the control. On day 3, the 60 MPa samples presented 212% higher PAL activity than the control, whereas no significant difference was observed between the 100 MPa samples and the control. During storage, higher ethylene production and respiration rate were detected in the 60 and 100 MPa samples compared with the control. | [27] |
| HHP treatments were applied for the CUT as a single pulse or multi-pulse (2P, 3P, and 4P). In addition, a single sustained treatment (5 min) was applied at 60 or 100 MPa. Samples were stored for 48 h at 15 °C. | Immediately after HHP treatment, the extractability of phenolics increased by 66.65% and 80.77% in 3P 100 MPa and 4P 60 MPa samples, respectively. After storage, CUT 60 MPa samples accumulated free (163.05%) and bound (36.95%) phenolics. Total xanthophylls increased by 27.16% after CUT 60 MPa treatment, whereas no changes were observed after storage. | The authors did not report quality characteristics and physiological measurements of the samples. | [28] | |
3.1.2. Carrot (Daucus carota)
3.2. Ultrasound (US)
3.2.1. Lettuce (Lactuca sativa)
3.2.2. Strawberry (Fragaria x ananassa)
| Horticultural Crop | US Processing and Storage Conditions Evaluated | Main Findings | References | |
|---|---|---|---|---|
| Effects on the Biosynthesis of Health-Promoting Compounds | Effects on Quality and Physiological Attributes | |||
| Romaine lettuce (Lactuca sativa, var. longifolia) | Whole leaf lettuce was treated with US (25 kHz) at an acoustic power density of 26 W/L for 1–3 min. Samples were stored at room temperature for 150 h. | Immediately after treatment, no significant difference in total phenolics was detected between the control and US-treated lettuce. At 60 h of storage, samples treated for 1 min of US showed 22.50% higher phenolics than the control. Lettuce treated with US for 2 and 3 min did not show significant increases in phenolics compared with the control. After 90 h, no significant difference in phenolic content was detected between the control and US-treated samples. | In the first 30 h, no phenylalanine ammonia-lyase (PAL) activation was detected due to US treatment. At 60 h, samples treated with 2 and 3 min of US showed the highest PAL activity. US-treated lettuce showed higher firmness (maximum force, N) than the control (water washed) immediately after treatment and during storage. No significant difference was detected in the color characteristics of lettuce between treatments. | [30] |
| Strawberry (Fragaria x ananassa, cv. chandler) | US treatment (33 kHz, 60 W, 25 °C) was applied to strawberries for different times (0, 10, 20, 30, 40, and 60 min). Samples were stored for 15 days at 4 °C. | US treatments (10–30 min) increased ascorbic acid retention in the tissue, where decreases of 54.68, 36.68, 35.57, and 32.20% were observed for treatment times of 0, 10, 20, and 30 min, respectively. US treatments of 40 and 60 min resulted in a decrease in the retention of ascorbic acid. US increased total phenolics on day 1. As the US time increased from 0 to 40 min, a significant increase (7.91%) in total phenolics was quantified. However, 60 min US treatment decreased phenolic content by 7.91%. Except for 60 min US treatment, total phenolics increased in US-treated samples as well as in the control. | On day 15, an increase of 13.84, 4.32, 3.20, 3.50, 2.93, and 13.88% in pH value was detected in samples subjected to US for 0, 10, 20, 30, 40, and 60 min. respectively. At day 15, total soluble solids increased by 24.66, 21.43, 18.23, 17.07, 18.84, and 30.05% for US treatment of 0, 10, 20, 30, 40, and 60 min, respectively. Immediately after treatment, the value of ΔE* increased by 4.61, 5.25, 6.16, 7.94, and 11.29% for 10, 20, 30, 40, and 60 min US treatments, respectively. US-treated strawberries had better color retention during storage. Fruit firmness was better retained in US (20–30 min)-treated samples. Microbial load also decreased with US treatment. | [31] |
| Carrot (Daucus carota) | Whole carrots were treated with US (frequency 24 kHz, amplitude 100 mm) for 5 min at 20 °C. Samples were stored at 20 °C for 3 days. | As an immediate response to US, carrots showed 21.1% higher levels of total carotenoids as compared with the control. After storage, carotenoid content decreased. However, US-treated samples showed a lower decrease in carotenoid (−7.6%) than the control (−16.4%). US treatment induced an immediate decrease in total phenolic content (−62.5%) compared with the control. After 3 days of storage, phenolic content in US-treated samples increased by 129.2%, whereas for control samples the phenolic content did not change. Chlorogenic acid was the main phenolic compound that increased in US-treated whole carrots, which showed 41.8% higher content at 1 day of storage than the control. | On day 3 of storage, US-treated whole carrots showed the highest isocoumarin content, 164.0% higher than the control and 240.8% higher than the control before storage. Isocoumarin exerts a bitter flavor in carrots at concentrations of 200 mg/kg [LaFuente et al. 1996]. However, the levels detected in sonicated carrots were below the threshold of sensory perception (40 mg/kg). US-treated samples showed a 2.04-fold increase in expression of the PAL gene immediately after treatment. During the first 0.5 days of storage, PAL activity increased in US-treated carrots, showing 64.8% higher activity than the control before storage. US induced an immediate increase in respiration rate. US-treated whole carrots showed 27.9%, 66.0%, and 162.0%, higher levels of volatile organic compounds (indicators of ethylene production) at 0.5, 1.5, and 2 days of storage, respectively. | [32] |
| Broccoli (Brassica oleracea L. var. Italica, cv. Tlaloc®) | Broccoli florets were treated with US (20 min, frequency 24 kHz, amplitude 100 μm). US-treated broccoli florets were subjected to exogenous methyl jasmonate (MJ, 250 ppm) and/or ethylene (ET, 1000 ppm) during storage. Samples were stored for 3 days at 15 °C. | As an immediate response to US treatment, the extractability of glucosinolates (glucoraphanin (795%), glucobrassicin (78.6%), and 4-hydroxy glucobrassicin (153%)) and phenolics (1-sinapoyl-2-feruloylgentiobiose (57.23%)) was increased. At 3 days, samples treated with US and MJ showed the highest accumulation of gluconasturtiin (755.9%), neoglucobrassicin (232.8%), 4-hydroxy glucobrassicin (187.1%), glucoerucin (111.92%), 1,2,2-trisinapoylgentiobiose (136.7%), 3-O-caffeoylquinic acid (73.4%), and 1-sinapoyl-2-ferulolylgentiobiose (56.0%) as compared with the control. Ascorbic acid content decreased during storage. However, US and exogenous phytohormones (MJ and ET) in combination reduced ascorbic acid degradation. | The authors did not report quality characteristics and physiological measurements of the samples. | [33] |
| Common bean (Phaseolus vulgaris, cv. Kabulengeti) sprouts | Seeds were treated with US at different power (0, 180, and 360 W) and time (0, 30, 45, and 60 min) before sprouting. Seeds were sprouted in darkness (at 25 °C) for 96 h. | At 96 h of sprouting, the accumulation of total phenolic acids, flavonoids, and anthocyanins increased with the intensity of the US treatment. Total phenolic acids, flavonoids, and anthocyanins were 1065.27%, 559.8%, and 1052.9%, higher at 96 h of sprouting, respectively, in seeds treated with 360 W (60 min) as compared with the control. | US treatments decreased radicle emergence time, increased radicle length (72.37%), and induced the maximum hypocotyl growth (60.44%) when comparing 360 W (60 min) with the control at 96 h of sprouting time. US treatment enhanced the sprouting percentage, sprouting index and vigor of the samples, improving the quality characteristics of common bean sprouts. Hydrogen peroxide production and the activity of catalase, glutathione peroxidase, PAL, and tyrosine ammonia-lyase were also higher during sprouting of seeds treated with 360 W for 60 min compared with the control. | [34] |
| Soybean (Glycine max L. cv. Dongnong 48) sprouts | Soybean seeds were treated with US at different power levels (0, 100, 200, and 300 W, 40 kHz, for 30 min). The seeds were germinated at 30 °C for 5 days in darkness. | Gamma-aminobutyric acid (GABA) accumulated in higher concentrations in soybean sprouts as the US power level increased, with sprouts from seeds treated at 300 W for 30 min showing 43.5% higher GABA content compared with the control. US pretreatment in seeds (300 W, 30 min) generated soybean sprouts with lower content of daidzin (−79.62%) and genistin (−70.95%), and higher content of daidzein (39.13%) and genistein (94.91%) as compared with the control sprouts. | The germination rate and average length of sprouts increased by 18.07% and 20.41%, respectively, after US (300 W, 30 min) as compared with the control. US pretreatment (300 W, 30 min) reduced lipoxygenase (LOX) activity by 36.22 to 55.57% (depending on the LOX isomer), resulting on sprouts with improved odor and flavor. Sprouts obtained from seeds treated at 300 W for 30 min showed the highest decrease in IgE-binding potency (51.39%), and resulted in soybean sprouts with 98.78% less trypsin inhibitor. | [35] |
| Peanut (Arachis hypogaea L.) sprouts. Three cultivars were evaluated (Fuhua12, Fuhua 18, and Baisha 1016) | Peanut seeds were treated with US at 28, 45, and 100 kHz for 15, 20, and 30 min. Seeds were germinated under dark at 28 °C and 90% relative humidity for 5 days. | Resveratrol content of sprouts increased with prolonged US and soaking times, showing optimum conditions at 20 min and 6 h, respectively. Fuhua cultivar treated with US (100 kHz, 20 min) presented an increase in resveratrol content (980.1%) after sprouting compared with the control. | Germination rate was increased for seeds treated with 100 kHz ultrasonic waves. The allergic protein content completely decreased at 3 days of germination when treating the seeds with 100 kHz for 20 min before germination. | [36] |
3.2.3. Carrot (Daucus carota)
3.2.4. Broccoli (Brassica oleracea)
3.2.5. Common Bean (Phaseolus vulgaris) Sprouts
3.2.6. Soybean (Glycine max) Sprouts
3.2.7. Peanut (Glycine max) Sprouts
3.3. Pulsed Electric Fields (PEF)
3.3.1. Apple (Malus domestica)
3.3.2. Tomato (Lycopersicon esculentum)
3.3.3. Carrot (Daucus carota)
4. Industrial Implementation and Economic Feasibility of Using Non-Thermal Technologies as Tools to Enhance the Content of Health-Promoting Compounds in Whole Fruits and Vegetables
- Energy cost: 230 kWh ∗ USD 0.067/kWh ∗ 20 h/day = USD 308.20/day
- Water cost: 1059 ft3/day ∗ USD 0.011/ft3 = USD 11.65/day
- Maintenance cost: USD 500,000/year ∗ 0.0027 years/day = USD 1350/day
- Estimated HHP processing cost for 30 tons of carrots = USD 1669.85/day
5. Further Research Needs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cisneros-Zevallos, L. The power of plants: How fruit and vegetables work as source of nutraceuticals and supplements. Int. J. Food Sci. Nutr. 2021, 72, 660–664. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme Stresses and the impact on nutraceuticals and quality during pre- and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Reyes, L.F.; Villarreal, J.E.; Cisneros-Zevallos, L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Gastélum-Estrada, A.; Hurtado-Romero, A.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Sanitizing after fresh-cutting carrots reduces the wound-induced accumulation of phenolic antioxidants compared to sanitizing before fresh-cutting. J. Sci. Food Agric. 2020, 100, 4995–4998. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A.; Pech, J.C.; Koiwa, H. Signaling Molecules Involved in the Postharvest Stress Response of Plants. In Handbook of Plant and Crop Physiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 259–276. [Google Scholar]
- Jacobo-Velázquez, D.A.; Cuéllar-Villarreal, M.D.R.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Ramos-Parra, P.A.; Hernández-Brenes, C. Nonthermal processing technologies as elicitors to induce the biosynthesis and accumulation of nutraceuticals in plant foods. Trends Food Sci. Technol. 2017, 60, 80–87. [Google Scholar] [CrossRef]
- Schreck, S.; Dörnenburg, H.; Knorr, D. Research report: Evaluation of hydrogen peroxide production in tomato (Lycopersicum esculentum) suspension cultures as a stress reaction to high pressure treatment. Food Biotechnol. 1996, 10, 163–171. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Monitoring the impact of high-pressure processing on the biosynthesis of plant metabolites using plant cell cultures. Trends Food Sci. Technol. 1998, 9, 355–361. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Ho, K.-P.; Qi, S. Ultrasound-induced physiological effects and secondary metabolite (saponin) production in Panax ginseng cell cultures. Ultrasound Med. Biol. 2001, 27, 1147–1152. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Enhancing carotenoid and phenolic contents in plant food matrices by applying non-thermal technologies: Bioproduction vs improved extractability. Trends Food Sci. Technol. 2021, 112, 622–630. [Google Scholar] [CrossRef]
- Denoya, G.; Colletti, A.C.; Vaudagna, S.R.; Polenta, G. Application of non-thermal technologies as a stress factor to increase the content of health-promoting compounds of minimally processed fruits and vegetables. Curr. Opin. Food Sci. 2021, 42, 224–236. [Google Scholar] [CrossRef]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; Rodríguez, S.D.C.; Cao, C.-M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.K.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Viacava, F.; Santana-Gálvez, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Sequential application of postharvest wounding stress and extrusion as an innovative tool to increase the concentration of free and bound phenolics in carrots. Food Chem. 2020, 307, 125551. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Jacobo-Velázquez, D.A. Effects of wounding stress and storage temperature on the accumulation of chlorogenic acid isomers in potatoes (Solanum tuberosum). Appl. Sci. 2021, 11, 8891. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Serment-Moreno, V.; Jacobo-Velázquez, D.A.; Torres, J.A.; Welti-Chanes, J. Microstructural and physiological changes in plant cell induced by pressure: Their role on the availability and pressure-temperature stability of phytochemicals. Food Eng. Rev. 2017, 9, 314–334. [Google Scholar] [CrossRef]
- Hu, K.; Peng, D.; Wang, L.; Liu, H.; Xie, B.; Sun, Z. Effect of mild high hydrostatic pressure treatments on physiological and physicochemical characteristics and carotenoid biosynthesis in postharvest mango. Postharvest Biol. Technol. 2021, 172, 111381. [Google Scholar] [CrossRef]
- Álvarez-Virrueta, D.; García-López, E.; Montalvo-González, E.; Ramírez, J.; Mata-Montes-De-Oca, M.; Tovar-Gómez, B. Efecto de las altas presiones hidrostáticas en la fisiología postcosecha del mango ‘Ataulfo’. CyTA-J. Food 2012, 10, 173–181. [Google Scholar] [CrossRef][Green Version]
- Ortega, V.G.; Ramirez, J.; Velazquez, G.; Tovar, B.; Mata, M.; Montalvo-González, E. Effect of high hydrostatic pressure on antioxidant content of ’Ataulfo’ mango during postharvest maturation. Food Sci. Technol. 2013, 33, 561–568. [Google Scholar] [CrossRef]
- Viacava, F.; Ortega, E.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Using high hydrostatic pressure processing come-up time as an innovative tool to induce the biosynthesis of free and bound phenolics in whole carrots. Food Bioprocess Technol. 2020, 13, 1717–1727. [Google Scholar] [CrossRef]
- Viacava, F.; Ramos-Parra, P.; Welti-Chanes, J.; Jacobo-Velázquez, D. High hydrostatic pressure processing of whole carrots: Effect of static and multi-pulsed mild intensity hydrostatic pressure treatments on bioactive compounds. Foods 2021, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Garza, I.P.; Ramos-Parra, P.A.; Hernández-Brenes, C.; Jacobo-Velázquez, D.A. Effects of postharvest ripening on the nutraceutical and physicochemical properties of mango (Mangifera indica L. cv Keitt). Postharvest Biol. Technol. 2015, 103, 45–54. [Google Scholar] [CrossRef]
- Yu, J.; Engeseth, N.J.; Feng, H. High intensity ultrasound as an abiotic elicitor—Effects on antioxidant capacity and overall quality of romaine lettuce. Food Bioprocess Technol. 2016, 9, 262–273. [Google Scholar] [CrossRef]
- Gani, A.; Baba, W.N.; Ahmad, M.; Shah, U.; Khan, A.A.; Wani, I.A.; Masoodi, F.; Gani, A. Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry. LWT-Food Sci. Technol. 2016, 66, 496–502. [Google Scholar] [CrossRef]
- Cuéllar-Villarreal, M.D.R.; Ortega, E.; Becerra-Moreno, A.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Effects of ultrasound treatment and storage time on the extractability and biosynthesis of nutraceuticals in carrot (Daucus carota). Postharvest Biol. Technol. 2016, 119, 18–26. [Google Scholar] [CrossRef]
- Aguilar-Camacho, M.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets. Ultrason. Sonochem. 2019, 50, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.O.; Ngadi, M. Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrason. Sonochem. 2020, 64, 104974. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, J.; Yang, A.; Chen, H. The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Res. Int. 2015, 77, 704–710. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Shi, A.; Liu, L.; Wang, Q. Preparation of resveratrol-enriched and poor allergic protein peanut sprout from ultrasound treated peanut seeds. Ultrason. Sonochem. 2016, 28, 334–340. [Google Scholar] [CrossRef]
- Lafuente, M.T.; López-Gálvez, G.; Cantwell, M.; Yang, S.F. Factors influencing ethylene-induced isocoumarin formation and increased respiration in carrots. J. Am. Soc. Hortic. Sci. 1996, 121, 537–542. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Vendrell-Pacheco, M.; Martín-Belloso, O.; Elez-Martínez, P. Effect of pulsed electric fields on the antioxidant potential of apples stored at different temperatures. Postharvest Biol. Technol. 2017, 132, 195–201. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Oliu, G.O.; Odriozola-Serrano, I.; Lamuela-Raventos, R.M.; Martín-Belloso, O.; Elez-Martinez, P. Effects of pulsed electric fields on the bioactive compound content and antioxidant capacity of tomato fruit. J. Agric. Food Chem. 2012, 60, 3126–3134. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Oliu, G.O.; Odriozola-Serrano, I.; Lamuela-Raventos, R.M.; Martín-Belloso, O.; Elez-Martinez, P. Metabolite profiling of phenolic and carotenoid contents in tomatoes after moderate-intensity pulsed electric field treatments. Food Chem. 2013, 136, 199–205. [Google Scholar] [CrossRef]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martinez, P.; Soliva-Fortuny, R. Enhancing the carotenoid content of tomato fruit with pulsed electric field treatments: Effects on respiratory activity and quality attributes. Postharvest Biol. Technol. 2018, 137, 113–118. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martinez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Enhancing phenolic content in carrots by pulsed electric fields during post-treatment time: Effects on cell viability and quality attributes. Innov. Food Sci. Emerg. Technol. 2020, 59, 102252. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martinez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed electric fields affect endogenous enzyme activities, respiration and biosynthesis of phenolic compounds in carrots. Postharvest Biol. Technol. 2020, 168, 111284. [Google Scholar] [CrossRef]
- Khouryieh, H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2020, 67, 102559. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal technologies for food processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, V.M.; Pataro, G.; Tiwari, B.; Gozzi, M.; Meireles, M.Á.A.; Wang, S.; Guamis, B.; Pan, Z.; Ramaswamy, H.; Sastry, S.; et al. Guidelines on reporting treatment conditions for emerging technologies in food processing. Crit. Rev. Food Sci. Nutr. 2021, 1–25. [Google Scholar] [CrossRef]
- Hiperbaric Website. Available online: https://www.hiperbaric.com/en/hpp-technology/equipment/ (accessed on 4 September 2021).

| Horticultural Crop | MIPEF Processing and Storage Conditions Evaluated | Main Findings | References | |
|---|---|---|---|---|
| Effects on the Biosynthesis of Health-Promoting Compounds | Effects on Quality and Physiological Attributes | |||
| Apple (Malus domestica, var. Golden delicious) | Whole apple fruits were treated at 0.4–2 kV cm−1 using 5–35 monopolar pulses of 4 μs at a frequency of 0.1 Hz (energy input of 0.008–1.3 kJ kg−1). Samples were stored at 4 and 22 °C for 48 h. | MIPEF treatment (0.008 kJ kg−1) induced an increase in total phenolic (13%) and flavan-3-ol (92%) contents in fruits stored at 22 °C for 24 h, and in flavonoids (58%) in samples stored at 4 °C for 24 h. | The authors did not report quality characteristics and physiological measurements of the samples. | [38] |
| Tomato (Lycopersicon esculentum Mill. cv. Daniella) | Tomato fruits were subjected to different electric field strengths (from 0.4 to 2.0 kV cm−1) and number of pulses (from 5 to 30). Samples were stored at 4 °C for 24 h. | Except for 2 kV/cm treatment, MIPEF-treated tomatoes showed higher phenolic content after 24 h of treatment than the control. The increases in phenolic content of tomatoes ranged from 6.6% (five pulses at 0.4 kV cm−1) to 44.6% (30 pulses at 1.2 kV cm−1). Accumulation of lycopene was detected after storage of MIPEF-treated tomato fruit, which ranged from 0.6% (18 pulses at 2 kV cm−1) to 31.8% (five pulses at 1.2 kV cm−1) as compared with non-treated tomato. | The authors did not report quality characteristics and physiological measurements of the samples. | [39] |
| MIPEF treatment at 1.2 kV cm−1 and 30 pulses resulted in the highest accumulation of caffeic acid-O-glucoside (170%), caffeic acid (140%), and chlorogenic acid (152%). MIPEF treatments at 1.2 kV cm−1 and five pulses presented the highest accumulation of α-carotene (93%), 9-cis-lycopene (94%) and 13-cis-lycopene (140%), respectively. | The authors did not report quality characteristics and physiological measurements of the samples. | [40] | ||
| Tomato (Licopersicon esculentum Mill. cv. Raf) | Tomato fruits were subjected to different electric field strengths (40, 120, and 200 kV m−1) and number of pulses (5, 18 and 30 pulses). Specific energy input ranged from 0.02 to 2.31 kJ kg−1. Samples were stored at 4 °C for 24 h. | The highest increase in carotenoid content (50%) was achieved in tomato fruits treated with an energy input of 2.31 kJ kg−1 (200 kV m−1−30 pulses). | MIPEF-treated tomatoes showed a significant increase in the respiration rate of tomato fruit. Ethylene concentration was higher (53%) in fruits subjected to the lowest electric field strength. Further increase in PEF intensity inhibited ethylene production. Acetaldehyde synthesis was induced when tomatoes were treated at energy inputs higher than 0.38 kJ kg−1. The higher the treatment intensity, the greater the softening effect. Total soluble solids and pH values increased with the treatment intensity. | [41] |
| Carrot (Daucus carota cv. Nantes) | Whole carrots were subjected to different electric field strengths (0.8, 2, and 3.5 kV cm−1) and number of pulses (5, 12, and 30). Samples were stored for 48 h at 4 °C. | The largest increase in phenolic content was observed at 24 h, after applying five pulses of 3.5 kV cm−1 (39.5%) and 30 pulses of 0.8 kV cm−1 (40.1%). | A correlation between the specific energy input and cell viability was found. After applying 3.5 kV cm−1, viability decreased by 87.5–79.4%. At 24 h, whole carrots treated with five pulses of 3.5 kV cm−1 and 30 pulses of 0.8 kV cm−1 showed texture softening while preserved the color. | [42] |
| Whole carrots were processed in a PEF batch system. Samples were treated with five pulses of 350 kV m−1 (580 ± 80 J kg−1). Samples were stored for 36 h at 4 °C. | Immediately after PEF treatment, whole carrots showed a decrease in p-coumaric acid (−42.3%), protocatechuic acid (−78.1%), and ferulic acid (−56.3%). The maximum accumulation of phenolics in whole carrots was reached after 24 h of PEF treatment, where whole carrots presented a significant increase in total phenolics (80.2%) and chlorogenic (74.9%), ferulic (52.2%), and p-OH-benzoic (94.7%) acid compared to the control. At 36 h of storage a decrease in phenolic content was observed. | PEF induced an immediate increase in respiration. From 12 to 36 h, PEF-treated carrots presented a 123–164% higher respiration rate than untreated carrots. PEF did not induce an immediate increase in volatile organic compound production in whole carrots. However, at 12 h of storage, samples treated with PEF generated higher amounts of acetaldehyde (7 pg kg−1 s−1), ethanol (68 ng kg−1 s−1), and ethylene (50 ng kg−1 s−1), whereas these volatiles were not detected in untreated carrots. PEF application delayed the peak of maximum peroxidase enzymatic activity for 12 h. Pectin methylesterase (PME) activity increased during the first 12 h of storage in the control. In contrast, PEF induced an immediate increase (164%) in enzyme activity, which remained stable for the following storage time. Polygalacturonase (PG) activity immediately decreased by 31%–32% after treatment. Phenylalanine ammonia-lyase (PAL) activity in untreated carrots remained stable during storage, whereas PEF-treated samples showed a constant increase in PAL activity during storage, showing the highest increase (153%) at the end of the study. | [43] | |
| Non-Thermal Technology | General Principle of Action | Scaling-Up Feasibility | Cost of Operation/Maintenance |
|---|---|---|---|
| High Hydrostatic Pressure (HHP) | A high-pressure pump injects liquid into a treatment chamber where the product of interest is located. High pressure generates structural changes in tissues, cells, and molecules depending on process conditions. | High There is industrial equipment that can process tens of tons per hour. | High Due to the mechanical complexity of the equipment and the constant wearing of sealing components, the cost of maintenance is relatively high. |
| Pulsed Electric Fields (PEF) | Electrodes generate repetitive short electric pulses in a treatment chamber designed for solids, suspensions, or liquids. The pulsed electric field generates changes in the permeability of the cell wall and cell membrane, causing changes in metabolic flux and cell viability depending on process conditions. | Medium There is equipment available at industrial scales, although processing capability is somewhat limited compared to HHP. | Medium The equipment is robust in general terms, and the configuration of the electrodes may enhance energy efficiency. |
| Ultrasound (US) | Ultrasound waves are generated, propagating through a transmitting medium. As the sound wave propagates, the fluctuations in pressure generate cavitation (formation and collapse of microbubbles), promoting shear stress that affects the integrity of cells and molecules depending on process conditions. | Low-Medium Most of the equipment is still at pilot-plant scale. Although there are efforts for application at larger scales, the reach of sound wave propagation seems to be a limiting factor. | Medium The equipment is robust in general terms. The sound source elements erode constantly and must be periodically replaced. A fraction of the energy dissipates as heat, decreasing efficiency. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobo-Velázquez, D.A.; Benavides, J. Non-Thermal Technologies as Tools to Increase the Content of Health-Promoting Compounds in Whole Fruits and Vegetables While Retaining Quality Attributes. Foods 2021, 10, 2904. https://doi.org/10.3390/foods10122904
Jacobo-Velázquez DA, Benavides J. Non-Thermal Technologies as Tools to Increase the Content of Health-Promoting Compounds in Whole Fruits and Vegetables While Retaining Quality Attributes. Foods. 2021; 10(12):2904. https://doi.org/10.3390/foods10122904
Chicago/Turabian StyleJacobo-Velázquez, Daniel A., and Jorge Benavides. 2021. "Non-Thermal Technologies as Tools to Increase the Content of Health-Promoting Compounds in Whole Fruits and Vegetables While Retaining Quality Attributes" Foods 10, no. 12: 2904. https://doi.org/10.3390/foods10122904
APA StyleJacobo-Velázquez, D. A., & Benavides, J. (2021). Non-Thermal Technologies as Tools to Increase the Content of Health-Promoting Compounds in Whole Fruits and Vegetables While Retaining Quality Attributes. Foods, 10(12), 2904. https://doi.org/10.3390/foods10122904







