Abstract
This study aimed at examining the effects of curcumin supplementation on growth performance, antioxidant capacity, and meat quality of ducks. To investigate these effects, 600 healthy ducks were randomly assigned to four treatment groups with 10 replicates pens, and each pen contained 15 ducks. Ducks were fed a diet containing curcumin at levels of 0, 300, 400, and 500 mg kg−1 in different groups. The results demonstrated that curcumin supplementation is beneficial to the growth performance (p < 0.05) of ducks and antioxidant capacity (p < 0.05) of duck meat. In addition, dietary curcumin raised the meat quality of ducks, improving the meat color, increasing water-holding capacity, and inhibiting lipid and protein oxidation. In conclusion, the present study provides important insights into both the nutrient and qualities of ducks, finding that a dietary inclusion of 400–500 mg/kg of curcumin (kg−1) has the greatest effect.
1. Introduction
Duck meat is a popular product and is widely consumed throughout the world particularly in Asia [1]. In addition, duck meat is highly susceptible to oxidation because it has a high content of polyunsaturated fatty acids and essential amino acids. Lipid oxidation causes rancidity in meat [2] and has a positive relationship with protein oxidation [3]. Jin et al. (2020) reported that adding curcumin to the diet of ducks increased their antioxidant capacity, showing inhibited lipid and protein oxidation in their meat [4].
Curcumin, derived from turmeric rhizomes (Curcuma Longa Linn), was used as a coloring and flavoring agent and an antioxidation additive and is used in livestock as a feed additive [5,6,7]. Curcumin supplementation enhanced the meat quality of broilers by their increasing antioxidant capacity, maintaining cell membrane integrity, and increasing water-holding capacity [8]. Sahin et al. (2012) reported that curcumin supplementation activated the Nrf2/HO-1 pathway, upregulated antioxidation capacity, and increased the feed intake and growth performance of quail [9]. Similar studies reported that supplementing the diet with antioxidants improved growth performance and meat quality, and increased animals antioxidant capacity [10,11,12,13]. Khan et al. (2012) reported that curcumin protected the integrity of cell membranes against lipid and protein oxidation and decreased the cross-linking and particle size of proteins [14]. Daneshyar (2012) reported that supplementing the diet with turmeric (5 mg/kg−1) increased the meat quality of chicken by increasing their antioxidation capacity, suppressing lipid oxidation, and maintaining the contents of volatile compounds [15].
The aim of this study was to examine and evaluate the effects of curcumin supplementation on growth performance, antioxidant capacity, and meat quality. This study offers insights into the application of curcumin in livestock and poultry farming and the food industry.
2. Materials and Methods
2.1. Chemicals
Curcumin was obtained from Nanjing Nutri-herb Biotech Co., Ltd. (Nanjing, China, CAS: 458-37-7) with 99% purity.
2.2. Birds and Husbandry
One-day-old male ducks (Anas platyrhynchos, 600) were assigned to four groups: ducks fed with diet containing curcumin at different levels 0 (T0, control group), 300 (T300), 400 (T400), and 500 mg kg−1 (T500). Ducks were fed in cages (150 cm × 60 cm × 70 cm), and randomly assigned to 10 cages per treatment (15 ducks per cage). Their daily diet intake was monitored, and their weight was measured at regular intervals throughout the research.
2.3. Sample Collection
At 70 days of age, all ducks fasted for 12 h, ten ducks from each group were randomly selected and slaughtered. In order to evaluate the changes in meat quality, the entire pectoralis major muscle of each duck was taken, and then stored for 24 h at 4 °C.
2.4. Antioxidant Enzyme Assay
The duck meat was chipped and mixed in PBS (the mass ratio of sample to PBS was 1:9; 4 °C, 0.01 M, pH = 7.2–7.4). An analysis of the total superoxide dismutase (T-SOD), glutathione peroxidase (GPx), and catalase (CAT) activity in the liver was conducted using commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Determination of pH and Color
The duck meat pH value was measured using a pH metre electrode (HI9125; Hanna Instruments, Padova, Italy). The duck meat color was determined using a colorimeter (Minolta CR-400; Konica Minolta, Tokyo, Japan). Ducks breast muscles were evaluated for changes in colour that three times in different locations of each sample.
2.6. Shear Force Assay
The shear forces (N) of samples were measured according to the process used by Jin et al. (2021) [16]. Two strips (1 ×1.27 × 10 cm) were cut parallel to the duck breast muscle fibres. Shear forces were evaluated using a texture analyser (Stable Micro System; TA: XT2i, Surrey, UKcity, England) with HDP/BSW (Warner-Bratzler Blade, Stable Micro System; TA: XT2i, Surrey, UK), which was attached to a load cell with a weight of 25 kg, the greatest force value of the sample was recorded.
2.7. Determination of Drip Loss
Drip loss was calculated following the process used by Jin et al. (2021) [16]. Duck samples were trimmed and weighed (W1, the original weight), then placed into a box for 24 h at 4 °C. The sample was removed, blotted dry, and weighed (W2, final weight) after 24 h at 4 °C. The drip loss was calculated as follows:
2.8. Determination of Cooking Loss
Cooking loss was calculated following the process used by Jin et al. (2021) [16]. The meat sample (2 × 5 × 4 cm) was carefully weighed, then placed into a thin-walled plastic bag and maintained in a water bath (85 °C); it was taken out when the internal temperature of the sample was 75 °C. The duck meat was blotted with filter paper and weighed again when the internal meat temperature cooled to 25 °C.
2.9. Determination of Water Mobility and Distribution
The water in samples was measured at room temperature (25 °C). The duck breast muscle sample (1 × 1 × 2 cm3) was placed in an 18 mm nuclear magnetic resonance tube and determined using the Minispecmq 20 1H low-field nuclear magnetic resonance (LF-NMR) analyser (Bruker Optik GmbH, Ettlingen, Germany) at a static magnetic field strength of 0.47 T. One-dimensional (1H) spectra of the relaxation times (T2b, T21, and T22) were acquired using the Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence. The raw data were normalized, analysed by the CONTIN algorithm, and represented by a continuous distribution of exponentials in a decay curve.
2.10. Thiobarbituric Acid Reactive Substance (TBARS) Assay
The TBARS value was calculated following the process used by Han et al. (2019) [17] with small alterations. Duck breast muscles were individually chopped using a micro-waring blender for 30 s. The chopped sample (4.00 g) was placed into a 25 mL screw-cap test tube; and mixed with three drops of antioxidant solution (BHA), 3 mL of TBA solution, and 17 mL of TCA-HCl solution. The mixture was vortexed and then heated in a 100 °C boiling water bath for 30 min. After the reaction, the 5 mL solution (at room temperature) was mixed with 5 mL of chloroform for 1 min, and then centrifuged at 1800× g for 10 min. The supernatant was centrifuged again under the same conditions, and then the obtained absorbance of the supernatant was read at 532 nm.
2.11. Determination of Carbonyl Content
The carbonyl sample was calculated following the process used by Mercier et al. (1988) [18]. The absorbance of the solution at 370 nm was measured, and the carbonyl content was evaluated according to the absorption coefficient of protein hydrazones L M−1 cm−1 according to the following formulas:
where A532 is the solution measured at 532 nm, C is the sample protein concentration (mg prot L−1), and 1.25 × 106 was the absorption coefficient of protein hydrazones.
2.12. Myofibrillar Protein (MP) Sulfhydryl Content Assay
The total sulfhydryl (SH) and reactive sulfhydryl contents in MP were determined by 5,5-dithiobis (2-nitrobenzoic acid) (DNTB) reagent according to the process used by Wang et al. (2020) [19]. The values of total sulfhydryl and reactive sulfhydryl were calculated as follows:
where A412 and A540 are the solution absorbances of the sample at 540 nm.
2.13. Determination of Myofibrillar Protein Solubility
The MP solution (1 mg mL−1, 5 mL) (15 M 1,4-piperazinediethane sulfonic acid (PIPES), 0.6 M NaCl, pH = 6.25) was centrifuged (10,000× g, 20 min, 4 °C). The supernatant of the MP solution was added into 4 mL of Biuret reagent and remained at 25 °C for 30 min. The MP solution dispersion solubility was expressed as:
where S1 is the MP solubility after centrifugation at 10,000× g for 20 min, and S2 is the original MP solubility in the MP solution.
2.14. Determination of Volatile Compounds
The volatile compounds of samples were extracted and detected according to the process used by Jin et al. (2021) [16]. Meat (3.00 g) was mixed with internal standard substance (IS) and placed into a headspace vial with PTFE/silicone septum. The extraction was performed at 45 °C for 40 min in a solid-phase microextraction (SPME) with a 75 µm carboxen/polydimethylsiloxane (CAR/PDMS) fibre, after sample equilibration 25 min at 45 °C. Volatile compounds extraction in a GC/MS (gas chromatography/mass spectrometry) system was conducted, then desorbed at 230 °C for 3 min. Then, desorption was set to 40 °C for 2 min, raised to 100 °C at 10 °C/min, and ultimately raised for 240 °C at 18 °C min−1 and held for 6 min under helium as carrier gas (1.0 mL min−1). The ion source temperatures were set to 230 °C. The mass spectrometer was scanned in the range of 33 to 450 m/z.
2.15. Statistical Analysis
The data were collected three times to obtain the mean value in each sample (ten ducks (samples) in each group), then analysed statistically using one-way ANOVA test in SPSS (Version 24.0, SPSS Inc., Chicago, IL, USA) using Duncan’s multiple comparison test at a 5% probability level. Statistical significance was indicated by p < 0.05. Curve estimation including linear and quadratic responses was made by assessing the orthogonal polynomial contrasts for effects of curcumin supplementation on growth performance and meat quality in Anas platyrhynchos.
3. Results
3.1. Growth Performance of Ducks
The effects of dietary curcumin on the growth performance of ducks are shown in Table 1. Dietary curcumin significantly increased the final weight, weight gain, and feed intake of ducks in curcumin groups relative to those in the T0 group (p < 0.05); however, there were no significant differences between the curcumin-supplemented groups (p > 0.05). In addition, there was no significant difference in F/C between the curcumin groups and the control group (p = 0.828).

Table 1.
Effects of dietary curcumin on growth performance of ducks.
3.2. Antioxidant Enzyme
As shown in Figure 1, although there was a significant increase in GPx activity (Figure 1A, p = 0.002) in the duck samples among groups, there was no significant difference in GPx activity in ducks breast muscle among the curcumin-supplemented groups (p > 0.05). Curcumin supplementation had no effect on T-SOD activity (Figure 1B, p = 0.288) and CAT activity (Figure 1C, p = 0.088); it also had no significant impact on the activities of T-SOD (p = 0.771) and CAT (p = 0.201) in the breast muscle of ducks among curcumin-supplemented groups.
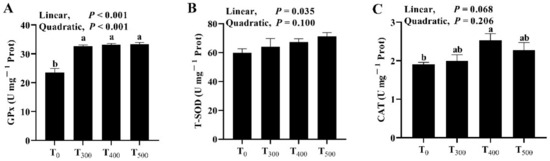
Figure 1.
Effect of dietary curcumin on the antioxidation capacity of the duck breast muscle. (A) Effect of dietary curcumin on the GPx activity of the duck breast muscle. (B) Effect of dietary curcumin on the T-SOD activity of the duck breast muscle. (C) Effect of dietary curcumin on the CAT activity of the duck breast muscle. T0: ducks fed with the basal diet; T300, T400, and T500: ducks fed with 300, 400, and 500 mg of curcumin in kg−1 of basal diet. GPx, glutathione peroxidase; T-SOD, total superoxide dismutase; CAT, catalase. a, b Values with different letter above the column mean significant difference (p < 0.05).
3.3. Meat Quality
3.3.1. Changes in Meat Colour
As shown in Table 2, dietary curcumin significantly increased a * values at 24 h (p = 0.002) and significantly decreased the L * value at 24 h (p = 0.002); however, there were no significant differences for the a * value (p = 0.775), L * value at 15 min (p = 0.147), nor b * values at 15 min (p = 0.254) and 24 h (p = 0.141) in the breast muscle of ducks.

Table 2.
Effects of dietary curcumin on color and pH of duck breast muscle.
3.3.2. pH Values Changes
As shown in Table 2, the pH value at 15 min (p = 0.002) significantly increased with curcumin supplementation, but there were no significant differences in pH values at 24 h (p = 0.084).
3.3.3. Changes in Shear Force, Drip Loss, Cooking Loss and Water Distribution
As shown in Figure 2A, curcumin supplementation significantly decreased shear force (p < 0.05). As shown in Figure 2B, C, drip loss (p = 0.049), and cooking losses (p = 0.006) significantly decreased with curcumin supplementation. As shown in Figure 2D, a decreasing T2b trend was observed in the samples with curcumin supplementation, but this was not a significant difference (p > 0.05). However, there were linear and quadratic decreases for T21 and T22 with curcumin supplementation (p < 0.01), and the T21 and T22 values were lowest in the T500 group.
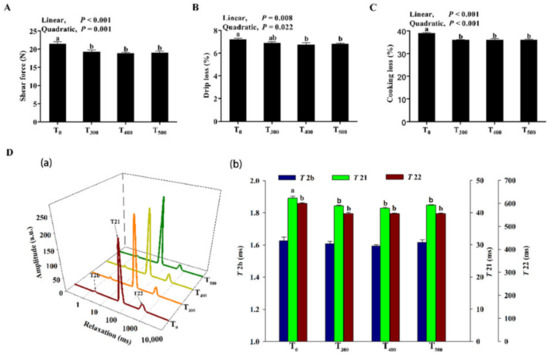
Figure 2.
Effect of curcumin supplementation on duck breast muscle. (A) Shear force at 24 h. (B) Drip loss at 24 h. (C) Cooking loss at 24 h. (D) Influence of curcumin supplementation on T2 relaxation times of duck breast muscle (a), and changes in the T2 relaxation times (b); T2b: minor component between 0 and 10 ms; T21: major component between 10 and 100 ms; T22: third component between 100 and 1000 ms. a, b Values with different letter above the column mean significant difference (p < 0.05).
3.4. Changes of TBARS, Carbonyl and Sulfhydryl Contents
As showcased in Figure 3A, there were significantly decreased TBARS values with curcumin supplementation (p < 0.05) after 24 h at 4 °C, but no significant difference in the TBARS value was observed among the curcumin-supplemented groups (p > 0.05). As depicted in Figure 3B, the carbonyl content in the sample decreased with a linear (p < 0.001) and quadratic (p < 0.001) response with curcumin supplementation, and a significant decrease in carbonyl content among curcumin-supplemented groups was observed (p < 0.05). As demonstrated in Figure 3C, the reactive and total sulfhydryl contents in MP significantly increased with curcumin supplementation and were the highest in the T500 group.
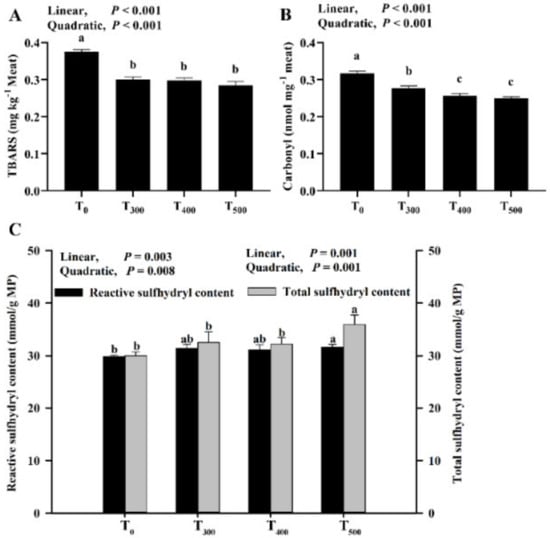
Figure 3.
Effect of curcumin supplementation on lipid and protein oxidation and sulfhydryl content in duck breast muscle. (A) TBARS (thiobarbituric acid reactive substance). (B) Carbonyl (carbonyl was to evaluate the protein oxidation). (C) Reactive and total sulfhydryl was to evaluate the deformation of the protein. a, b, c Values with different letter above the column mean significant difference (p < 0.05).
3.5. Solubility of Myofibrillar Protein
As depicted in Figure 4, MP solubility increased (linear, p < 0.01; quadratic, p < 0. 01) with curcumin supplementation, but there was no significant difference among the curcumin-supplemented groups (p > 0.05). MP solubility was the highest in the T400 group.
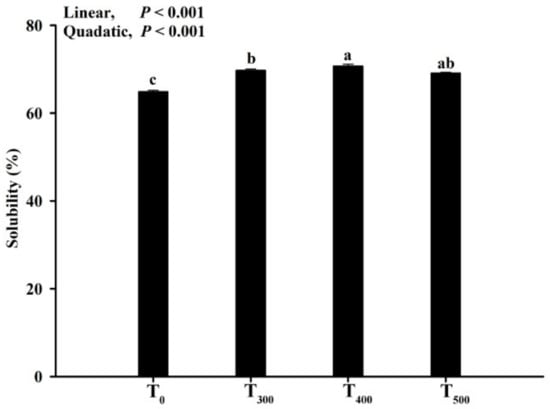
Figure 4.
Effect of curcumin supplementation on the protein solubility of the duck breast muscle. Solubility represents proteins dissolved in the solvent. a, b, c Values with different letter above the column mean significant difference (p < 0.05).
3.6. Particle Size of Myofibrillar Protein
As shown in Figure 5, the average particle diameter ranges of the myofibrillar protein were mainly concentrated in 1–1000 µm. The d43 (volume-mean diameter) and d32 (volume-surface mean diameter) decreased with curcumin supplementation, and a significant difference in the d43 and d32 of duck breast muscle in the curcumin-supplemented groups was observed (p < 0.05). The particle size of the myofibrillar protein was lowest in the T500 group.
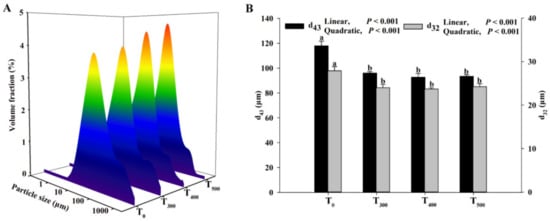
Figure 5.
Effect of curcumin supplementation on the protein particle size of the duck breast muscle. (A) The average particle size (μm) was used to evaluate the size of protein. (B) d43 (volume-mean diameter), d32: volume-surface mean diameter. a, b Values with different letter above the column mean significant difference (p < 0.05).
3.7. Volatile Compounds Content
As shown in Table S2 (Supplementary Materials), 56 volatile compounds—within the samples—were extracted from the samples using SPME. The concentrations of aldehydes significantly decreased with curcumin supplementation (p < 0.05). Significant decreases in ketone concentrations occurred with curcumin supplementation (p < 0.05). The concentrations of most alcohol types (1-Pentanol, 1-Octen-3-ol,1-Hexanol, cyclostyle alcohol, 3-Octanol, 2-Decen-1-ol, 2-methyl-cyclohexanol, 4-(1,1-dimethylethyl), (E)-trans-2-Undecen-1-ol, and 2,3-Butanediol) were enriched with dietary curcumin levels (p < 0.05), except for three alcohols (2-ethyl, 1-Octanol, and 1-Hexanol) (p < 0.05). Curcumin supplementation linearly and quadratically inhibited the generation of acids (p < 0.05), except for dodecanoic acid and octanoic acid. The ester concentration was enhanced with curcumin supplementation (p < 0.05). Silicide concentrations decreased significantly with curcumin supplementation (p < 0.05). The volatile compounds including furan, alkanes, alanine, alkenes, and sulfide, and allyl methyl increased significantly with curcumin supplementation (p < 0.05).
4. Discussion
In this study, adding curcumin into the diet significantly increased WG and FI, and had an insignificant effect on the F/C of ducks with curcumin supplementation. In comparison with ducks in ochratoxin A (2 mg/kg−1), dietary curcumin increased the growth performance of ducks fed corn contaminated with ochratoxin A [20]. Abd El-Hack et al. (2021) reported that biological curcumin can improve the growth performance of broilers increasing nutrient intake and antioxidant capacity [21]. In addition, similar studies demonstrated that supplementing the diet with curcumin enhanced growth performance by increasing antioxidation capacity and improving the intestinal integrity of poultry, such as in Arbor Acres broiler chickens [22], Ross broiler chickens [12], wenchang broiler chickens [8], and quail [9].
Antioxidation enzymes such as SOD, GPx and CAT are important components of the antioxidation system. GPx, SOD and CAT are the first line of cell defence against free radicals and reactive oxygen species (ROS) and are indispensable in the defence strategy of antioxidants in the body [23]. The results reveal curcumin-supplemented diet have the ability to enhance the body’s antioxidation capacity in this study. This finding corroborates the results of another study that found dietary curcumin enhanced the antioxidant ability of laying hens by increasing SOD, T-AOC, CAT and GPx activities under heat-stressed environmental conditions [24]. Similarly, Jin et al. (2021) reported curcumin supplementation in the diet increased the activity of antioxidant enzymes of ducks fed Aflatoxin B1 [25].
The meat quality of livestock and poultry is mainly reflected by indicators such as colour, tenderness and water-holding capacity. Meat colour is an important indicator of consumer acceptance. Changes in meat colour may be related to the antioxidant and water-holding capacities. The increases in the a * value and decreases in L * and b * values for finishing pigs and ducks were accompanied by antioxidation capacity upregulation [16,26]. In this study, the improvement in duck meat quality alongside the increase in the a * value and the decrease in L * and b * values may be related to the upregulation of antioxidant capacity induced by curcumin supplementation. The increase in meat quality of transport-stress-impaired meat may be related to the increased antioxidant properties caused by resveratrol supplementation in the diet of the broilers [27].
The pH value is another indicator that is used to evaluate meat quality. In this study, curcumin supplementation significantly inhibited the reduction in pH values among curcumin groups compared to the control group; this phenomenon may be related to changes in the antioxidant capacity. Supplementing the diet with antioxidants increased pH values in the meat of pigs and ducks—as the antioxidation capacity of animals was upregulated [16,28]. Shear force is an indicator of meat tenderness, reflecting the content and nature of connective tissue and the chemical structural state of the myofibrillar protein in muscle. In this study, increasing the levels of dietary curcumin in ducks significantly reduced shear force values. Meng et al. (2020) reported that supplementing the diet with antioxidants decreased the shear force levels of pork as the antioxidation capacity increased and improved meat quality [28]. Drip loss is an indicator used to evaluate the sample water-holding capacity. Consumers are reluctant to accept meat with a high drip loss percentage. In our present study, dietary curcumin significantly decreased drip and cooking losses in duck breast muscle. Zhang et al. (2015) demonstrated that dietary curcumin decreased cooking and drip losses in broilers breast muscle, in line with our results [29]. In this study, decreases in drip and cooking losses occurred as antioxidant capacity increased in duck breast muscle. A similar study showed that supplementing the diet with curcumin significantly decreased the drip loss, and increased meat redness values of pigs [30]. In addition, water mobility and distribution in meat can be evaluated using low field-nuclear magnetic resonance (LF-NMR), which reflects the water state in a sample. The alteration of the water protein was associated with an increase in the mechanical damage and denaturation of proteins [31]. Cheng et al. (2016) reported increased drip loss—correlated with lipid peroxidation—destroyed the cell membrane [32]. A longer value means that the water is more fluidic [33]. Curcumin supplementation significantly decreased the T21 and T22 in duck breast muscle in this study. Zhang et al. (2019) reported that an increase in the TBARS value occurred with an increase in T21 and T22 relaxation in the porcine longissimus muscles (lumborum) which was in line with the results in this study [34].
MDA is a biomarker and secondary product of lipid oxidation that can be evaluated using TBARS values. Lipid oxidation of meat and meat products is a common event during the post-mortem period. Jin et al. (2021) reported that a significant decrease in the protein carbonylation of duck breast muscle occurred during the post-mortem period when the animal’s diet was supplemented with resveratrol [35]. Our previous study demonstrated that supplementing the diet with antioxidants inhibited lipid and protein oxidation [4,36]. Similarly, Karami et al. (2011) reported that 0.5% dietary turmeric powder-fed (5 g turmeric powder/kg dry matter intake added instead to the roughage part of a diet) goat decreased the MDA content in goat meat, which was vacuum-packed and refrigerated under retail conditions for 0, 7 or 14 days at 4 °C [37]. Protein carbonyl accumulation in meat demonstrated that muscle protein underwent oxidative reactions during the post-mortem period. Therefore, protein carbonyls produced in meat and meat products is an indicator to assess the levels of protein oxidation [30]. The results in this research were consistent with another study reporting that supplementation dietary curcumin decreased broiler chicken meat carbonyl contents after storage [22]. In beef steaks, protein oxidation led to the aggregation of myosin owing to bisulfide bonds cross-linking the protein [38], in line with the results in this study. Protein oxidation and aggregation were attributed to a decrease in protein solubility. Li et al. (2019) reported that MP showed more solubility with decreasing protein oxidation [39]. The results in this study were supported by changes in the protein carbonyls and sulfhydryl contents in duck meat.
MP particle size is an important indicator in the evaluation of protein denaturation. A previous study used d32 to describe the protein surface, which was related to the conformational changes in proteins caused by protein folding [40]. An increase in the protein particle size was observed to occur alongside the oxidation of meat during storage [38]. Another similar study revealed that mulberry polyphenols in dried minced pork slices weakened the protein cross-linking and aggregation, inhibiting protein denaturation during heat processing and storage [41].
In this study, most of the volatile compounds were identified in Beijing roasted ducks [42,43]. As presented in Table S2, the concentration of 56 volatile compounds in meat indicated that lipid oxidation was effectively inhibited by dietary curcumin. The changes in volatile compounds can also be proven by TBARS and carbonyl values. During storage, the number of alcohol types generated from lipid oxidation was higher in curcumin groups relative to those in the control group. However, the ester and acid concentrations were lower in that curcumin inhibited lipid and protein oxidation. Aldehydes were secondary products in the lipid oxidation process. Significant decreases in nonanal and hexanal (p < 0.01) occurred in the curcumin groups. Furan, 2-pentyl, has an important role in meat aroma and contributes sweet and buttery flavours to meat [44]. The furan content in the curcumin group was increased compared with that in the control group. Changes in volatile compounds in the duck breast may be related to dietary curcumin, in that supplementing the diet with antioxidants delayed the oxidation process and inhibited the generation of volatile compounds in tissues at an early post-mortem stage for animals owing to the higher antioxidant capacity [45]. Cantharidin, as a potent anticancer small molecule, is used in traditional Chinese medicine [46,47]. Interestingly, cantharidin was identified in duck meat for the first time and only found in ducks fed a basal diet supplementation with 500 mg of curcumin kg−1. However, the mechanism of the generation of cantharidin in duck meat of 500 mg of curcumin kg−1 of the basal diet group requires further investigation.
5. Conclusions
Curcumin supplementation enhanced duck’s growth performance and meat quality, protecting the protein against cross-linking bisulfide bonds and maintaining a lower MP particle size for a longer post-mortem period in duck breast muscle. Most volatile compounds decreased as curcumin supplementation increased. The results of the present study showed curcumin supplementation at a level of 400–500 mg/kg feed can improve duck meat quality by limiting the extent of lipid oxidation during the post-mortem period.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10122981/s1, Table S1: Ingredient composition and nutrient content of the basal diet (%, as-fed basis), Table S2: Concentration (ng g−1) of volatile compounds identified and quantified by gas chromatography/mass spectrometry in the duck breast muscle with different dietary curcumin.
Author Contributions
S.J. and X.F. led experimental work; S.J. wrote the first draft of the manuscript; S.J., H.Y., F.L. and Q.P. performed the feeding ducks for 70 days and determined the main experiments assay; A.S. reviewed this manuscript; X.F. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (31772638, 32072768) and Natural Science Foundation of Heilongjiang Province (C2016022).
Institutional Review Board Statement
The experimental protocol was conducted in compliance with the Guide for the Care and Use of Agricultural Animals in Agriculture Research and Teaching of Northeast Agricultural University (Protocol number: NEAU-[2011]-9).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used and/or analysed in this study are available from the corresponding author on reasonable request.
Acknowledgments
Authors would also like to thank Bo Wang and Fangfei Li for their help in the successful completion of this research. Part of this current research had been published in https://www.researchsquare.com/article/rs-58095/v1 (accessed on 18 August 2020) (Jin et al.) [48] with a pre-print style.
Conflicts of Interest
The authors have no competing interest to declare.
References
- Khan, M.A.; Ali, S.; Yang, H.; Kamboh, A.A.; Ahmad, Z.; Tume, R.K.; Zhou, G. Improvement of color, texture and food safety of ready-to-eat high pressure-heat treated duck breast. Food Chem. 2019, 277, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.M.; Rangel, A.; Urbano, S.; Mitzi, G.; Moreno, G.M. Dxidação lipídica da carne ovina. Acta Vet. Bras. 2013, 7, 14–28. [Google Scholar]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.J.; Pang, Q.; Liu, R.Q.; Yang, H.; Liu, F.J.; Wang, M.; Shan, A.S.; Feng, X.J. Dietary curcumin decreased lipid oxidation and enhanced the myofibrillar protein structure of the duck (Anas platyrhynchos) breast muscle when subjected to storage. LWT—Food Sci. Technol. 2020, 133, 109986. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Gupta, R.K.; Srivastava, R. Curcumin-the yellow magic. Asian J. Appl. Sci. 2011, 4, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Huang, H.; Zhou, L.; Li, W.; Zhou, H.; Hou, G.; Liu, J.; Hu, L. Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of wenchang broiler chickens. Ital. J. Anim. Sci. 2016, 14, 345–349. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, Z.; Tuzcu, M.; Sahin, N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012, 50, 4035–4041. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Bachtiar, K.R.; Prayitno, C.H. The effects of mangosteen peel (Garcinia mangostana L.) and Turmeric (Curcuma domestica Val) flour dietary supplementation on the growth performance, lipid profile, and abdominal fat content in Cihateup ducks. Vet. World 2019, 12, 402. [Google Scholar] [CrossRef]
- Nasir, R.; Naeem, M.; Rui, Y.; Xiang, Z.; Tian, W. Effect of dietary supplementation of curcumin on growth performance, intestinal morphology and nutrients utilization of broiler chicks. J. Poult. Sci. 2013, 50, 44–52. [Google Scholar]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar] [CrossRef]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Javdani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V.; Laudadio, V. The use of turmeric (Curcuma longa) in poultry feed. Worlds Poult. Sci. J. 2012, 68, 97–103. [Google Scholar] [CrossRef]
- Daneshyar, M. The effect of dietary turmeric on antioxidant properties of thigh meat in broiler chickens after slaughter. Anim. Sci. J. 2012, 83, 599–604. [Google Scholar] [CrossRef]
- Jin, S.; Pang, Q.; Yang, H.; Diao, X.P.; Feng, X.J. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (Anas platyrhynchos). Food Chem. 2021, 363, 130263. [Google Scholar] [CrossRef]
- Han, G.; Zhang, L.; Li, Q.; Wang, Y.; Chen, Q.; Kong, B. Impacts of different altitudes and natural drying times on lipolysis, lipid oxidation and flavour profile of traditional Tibetan yak jerky. Meat Sci. 2019, 162, 108030. [Google Scholar] [CrossRef]
- Mercier, Y.; Gatellier, P.; Viau, M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998, 48, 301–318. [Google Scholar] [CrossRef]
- Wang, B.; Kong, B.; Li, F.; Liu, Q.; Zhang, H.; Xia, X. Changes in the thermal stability and structure of protein from porcine longissimus dorsi induced by different thawing methods. Food Chem. 2020, 316, 126375. [Google Scholar] [CrossRef]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal 2018, 13, 42–52. [Google Scholar] [CrossRef]
- El-Hack, A.; Mohamed, E.; Alaidaroos, B.A.; Farsi, R.M.; Abou-Kassem, D.E.; El-Saadony, M.T.; Ashour, E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals 2021, 11, 1878. [Google Scholar] [CrossRef]
- Rajput, N.; Ali, S.; Naeem, M.; Khan, M.A.; Wang, T. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on pigmentation, oxidative stability and quality of meat from broiler chickens affected by a coccidiosis challenge. Br. Poult. Sci. 2014, 55, 501–509. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2019, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Nawab, A.; Li, G.; Liu, W.; Lan, R.; An, L. Effect of dietary curcumin on the antioxidant status of laying hens under high- temperature condition. J. Therm. Biol. 2019, 86, 102449. [Google Scholar] [CrossRef]
- Jin, S.J.; Yang, H.; Jiao, Y.H.; Pang, Q.; Wang, Y.J.; Wang, M.; Shan, A.S.; Feng, X.J. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.Q.; Yu, B.; Zheng, P.; Huang, Z.Q.; Mao, X.B.; Chen, D.W. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Zhao, X.H.; Chen, X.Y.; Yang, L.; Geng, Z.Y. Dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poult. Sci. 2017, 96, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Sun, S.; Bai, Y.; Luo, Z.; Li, Z.; Shi, B.; Shan, A. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. 2020, 167, 108176. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Lu, C.; Bai, K.; Zhang, L.; Wang, T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 2015, 63, 3880–3886. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yan, E.; He, J.; Zhong, X.; Zhang, L.; Wang, C.; Wang, T. Dietary supplemented curcumin improves meat quality and antioxidant status of intrauterine growth retardation growing pigs via Nrf2 signal pathway. Animals 2020, 10, 539. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, M.; Bhandari, B.; Gao, Z. Effects of malondialdehyde-induced protein modification on water functionality and physicochemical state of fish myofibrillar protein gel. Food Res. Int. 2016, 86, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.; Niu, Y.; Zheng, X.C.; Zhang, H.; Chen, Y.P.; Zhang, M. A comparison of natural (D-α-tocopherol) and synthetic (DL-α-tocopherol acetate) vitamin E supplementation on the growth performance, meat quality and oxidative status of broilers. Asian-Australas. J. Anim. Sci. 2016, 29, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.; Xu, X.; Shan, H.; Wang, H.; Yang, J.; Zhou, G. Effects of high-intensity ultrasound, high-pressure processing, and high-pressure homogenization on the physicochemical and functional properties of myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2018, 45, 354–360. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, X.; Liu, Q.; Chen, Q.; Kong, B. Changes in microstructure, quality and water distribution of porcine longissimus muscles subjected to ultrasound-assisted immersion freezing during frozen storage. Meat Sci. 2019, 151, 24–32. [Google Scholar] [CrossRef]
- Jin, S.; Wang, M.; Yang, H.; Shan, A.; Feng, X. Dietary supplementation of resveratrol improved the oxidative stability and spatial conformation of myofibrillar protein in frozen-thawed duck breast meat. Food Biosci. 2021, 43, 101261. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Jin, S.; Pang, Q.; Shan, A.; Feng, X.J. Dietary resveratrol alleviated lipopolysaccharide-induced ileitis through Nrf2 and NF-kappaB signalling pathways in ducks (Anas platyrhynchos). J. Anim. Physiol. Anim. Nutr. 2021, 2. online ahead of print. [Google Scholar] [CrossRef]
- Karami, M.; Alimon, A.R.; Goh, Y.M. Effect of vitamin E, Andrographis paniculata and turmeric as dietary antioxidant supplementation on lipid and color stability of goat meat. Small Rumin. Res. 2011, 97, 67–71. [Google Scholar] [CrossRef]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; Zhou, Z.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S.; Zhu, L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason. Sonochem. 2013, 20, 187–195. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, L.; Xiang, R.; Liu, X.; Zhu, M. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, H.; Ma, C. Aroma-active compounds of beijing roast duck. Flavour Fragr. J. 2009, 24, 186–191. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of key aroma compounds in beijing roasted duck by gas chromatography-olfactometry-mass spectrometry, odor-activity values, and aroma-recombination experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Muriel, E.; Antequera, T.; Petron, M.J.; Andres, A.I.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391–400. [Google Scholar] [CrossRef]
- North, M.K.; Zotte, A.D.; Hoffman, L.C. The effects of dietary quercetin supplementation on the meat quality and volatile profile of rabbit meat during chilled storage. Meat Sci. 2019, 158, 107905. [Google Scholar] [CrossRef]
- Wang, G.S. Medical uses of mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, M.; Shan, A.S.; Feng, X.J. Avian host defense cathelicidins: Structure, expression, biological functions, and potential therapeutic applications. Poult. Sci. 2020, 99, 6434–6445. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Liu, F.; Diao, X.; Pang, Q.; Liu, R.; Wang, M.; Wang, Y.; Liu, M.; Zhou, X.; et al. Effect of dietary curcumin on the growth performance, serum antioxidation and meat quality of ducks (Anas platyrhynchos). Available online: https://www.researchsquare.com/article/rs-58095/v1 (accessed on 18 August 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
