Effect of Kernel Size and Its Potential Interaction with Genotype on Key Quality Traits of Durum Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wheat Samples
2.2. Wheat Physical Properties
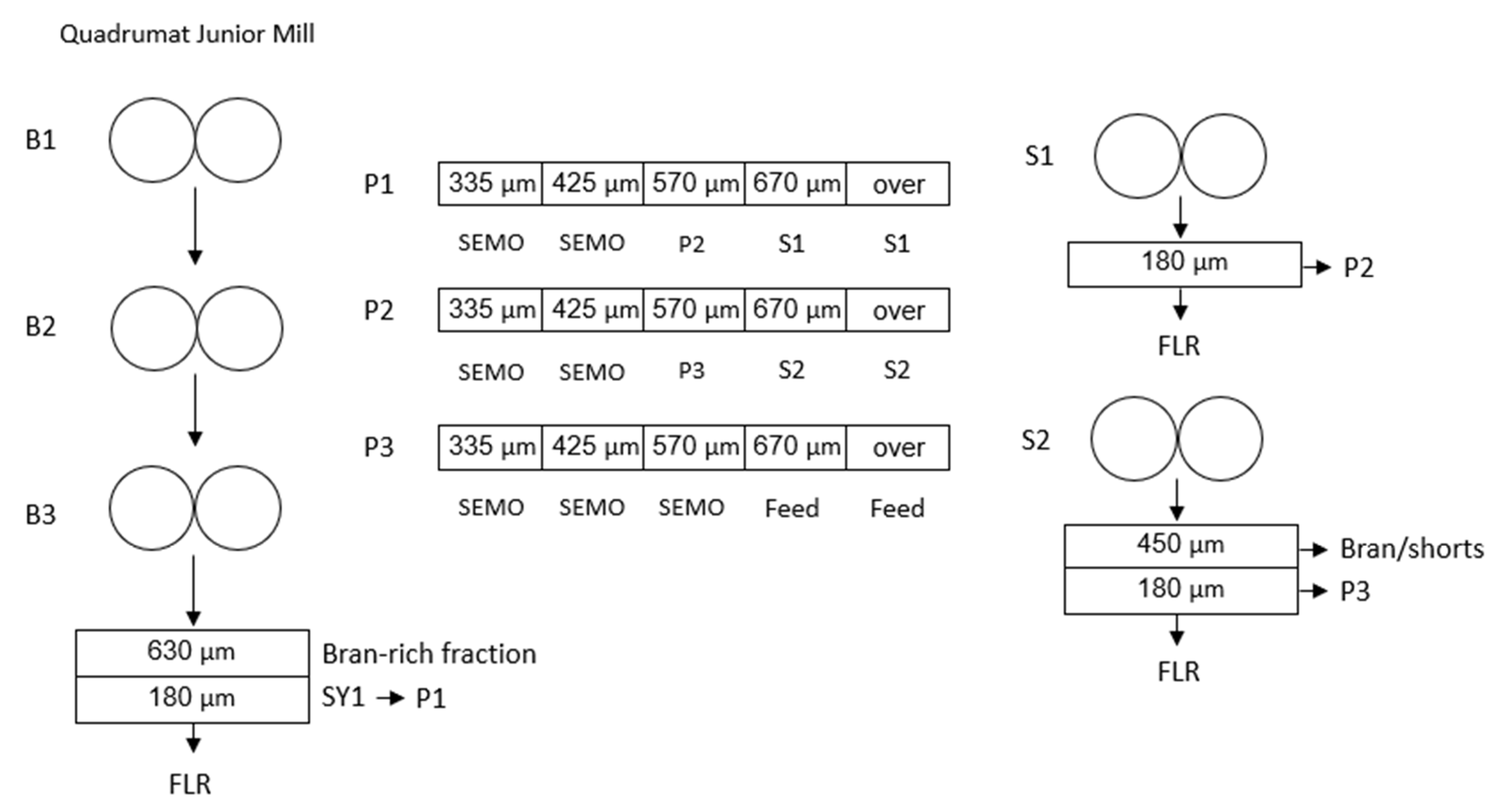
2.3. Standard Durum Milling Procedure
2.4. Micro-Milling and Purification Protocol
2.5. Semolina Quality Testing
2.6. Spaghetti Processing and Color Measurement
2.7. Statistical Analysis
3. Results and Discussion
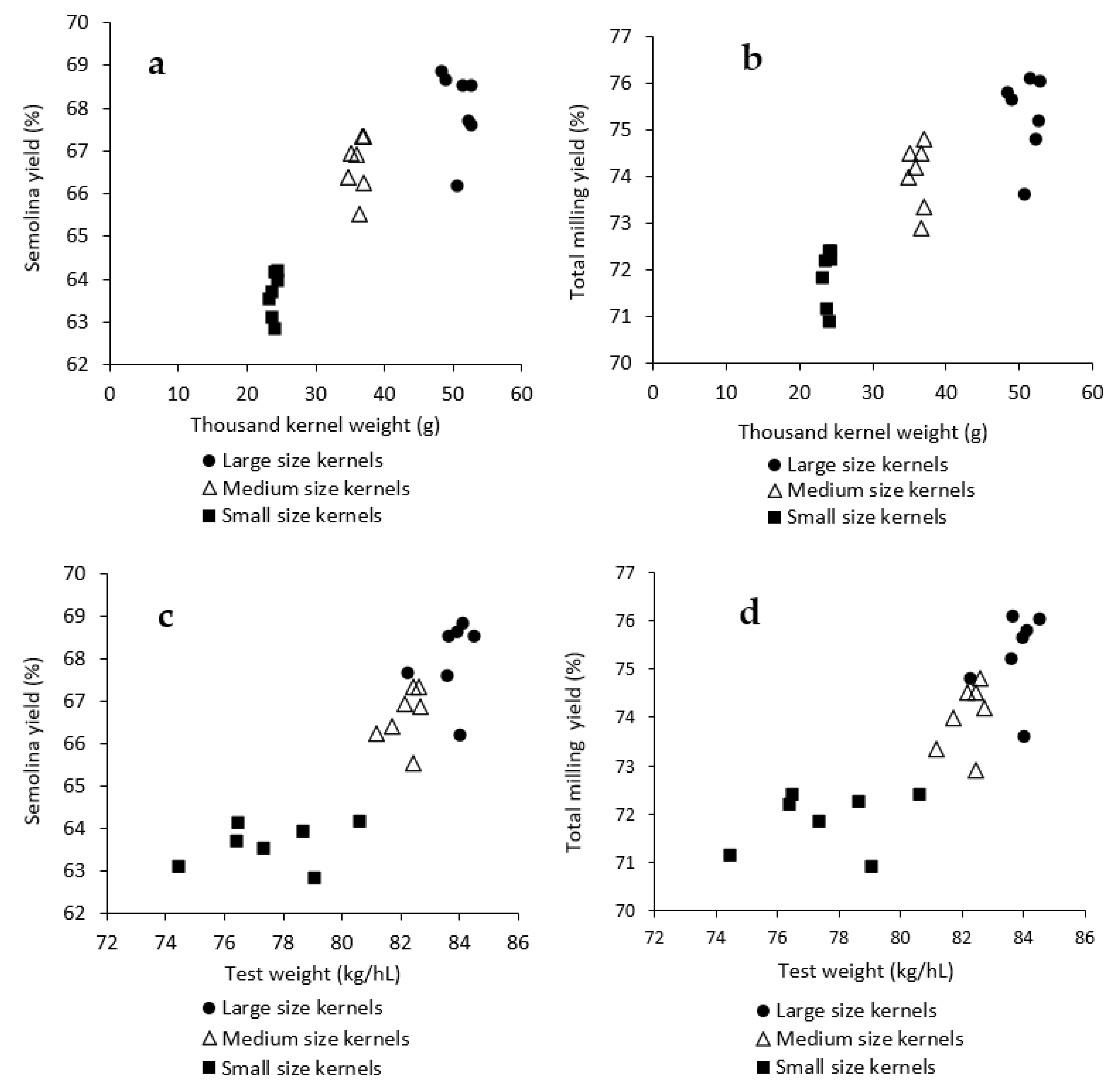
3.1. Influence of Kernel Size and Genotype on Physical Properties of Durum Wheat
3.2. Influence of Kernel Size and Genotype on Milling Quality of Durum Wheat
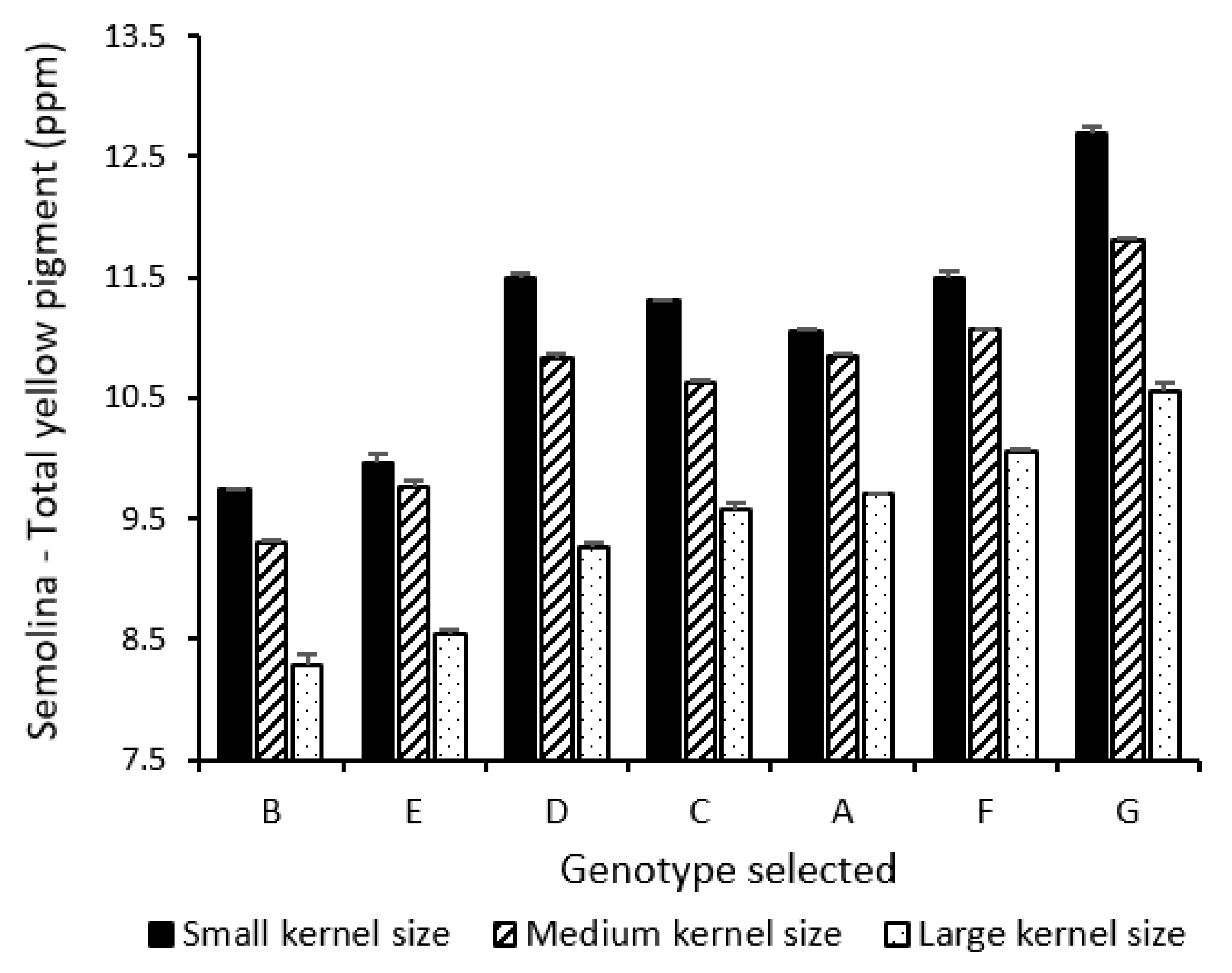
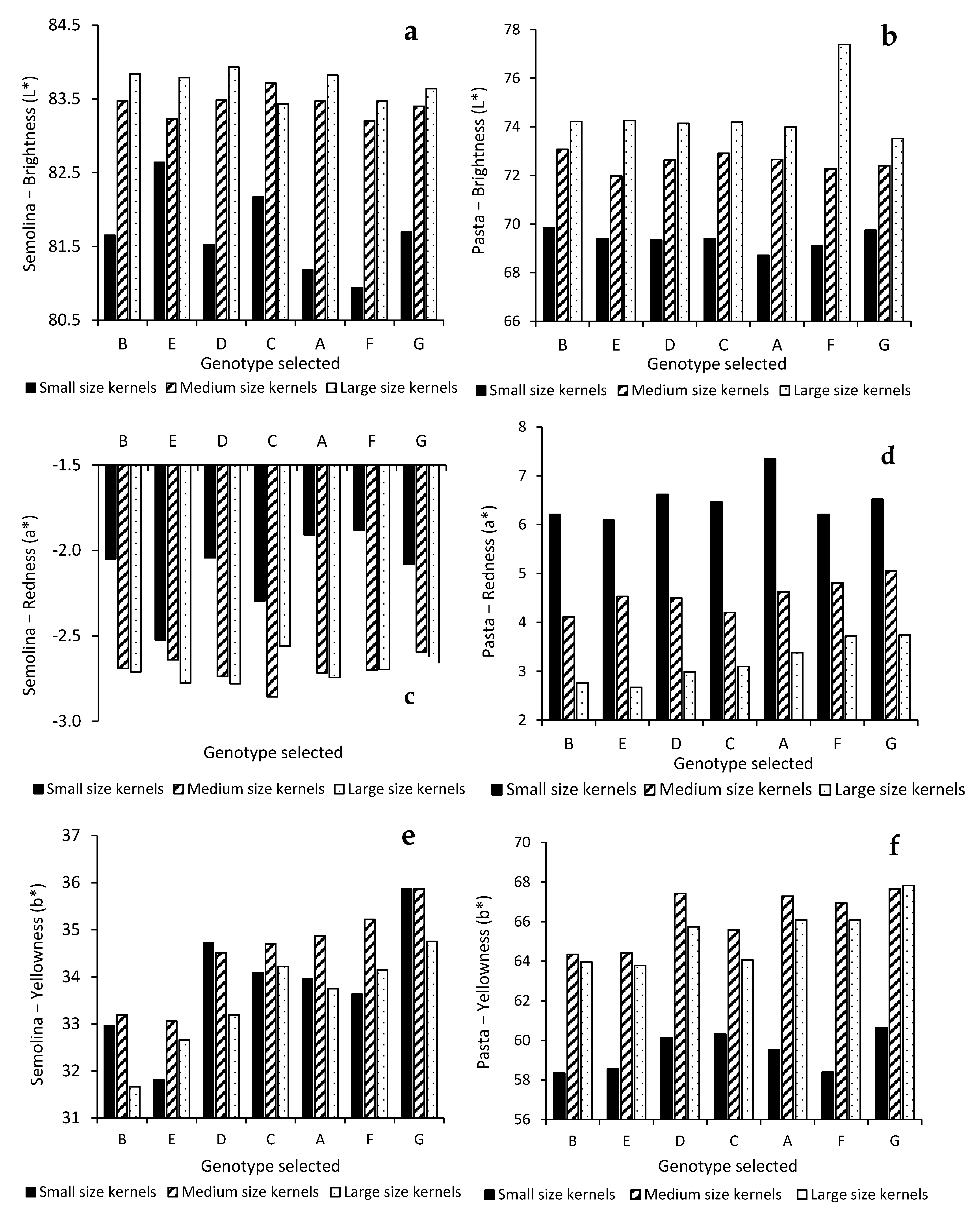
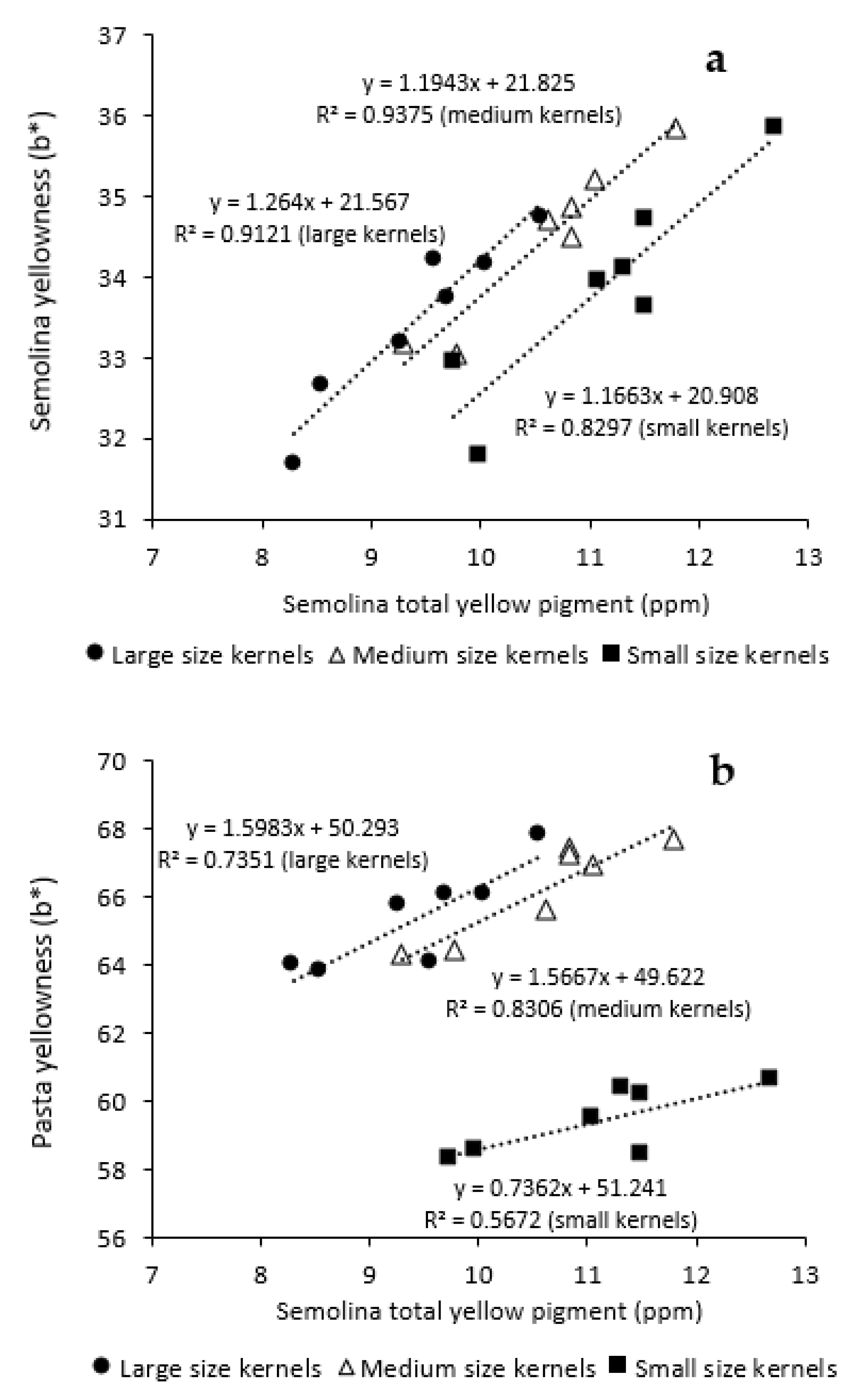
3.3. Influence of Kernel Size and Genotype on Semolina and Pasta Color Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Feillet, P.; Dexter, J.E. Quality requirements of durum wheat for semolina milling and pasta production. In Pasta and Noodle Technology, 1st ed.; Kruger, J.E., Matsuo, R.B., Dick, J.W., Eds.; AACC: St. Paul, MN, USA, 1996; pp. 95–131. [Google Scholar]
- Sissions, M.; Abecassis, J.; Marchylo, B. Methods used to access and predict quality of durum wheat, semolina, and pasta. In Durum Wheat Chemistry and Technology; Sissions, M., Abecassis, J., Marchylo, B., Carcea, M., Eds.; AACC International: St. Paul, MN, USA, 2012; pp. 213–234. [Google Scholar]
- Dexter, J.E.; Matsuo, R.R.; Martin, D.G. The relationship of durum wheat test weight to milling performance and spaghetti quality. CFW 1987, 32, 772–777. [Google Scholar]
- Dexter, J.E.; Symons, S.J. Impact of durum wheat test weight, kernel size, kernel weight and protein content on semolina milling quality. Int. Miller. 2007, 1, 27–33. [Google Scholar]
- Wang, K.; Fu, B.X. Inter-relationships between test weight, thousand kernel weight, kernel size distribution and their effects on durum wheat milling, semolina composition and pasta processing quality. Foods 2020, 9, 1308. [Google Scholar] [CrossRef]
- Fu, B.; Wang, K.; Dupuis, B.; Taylor, D.; Nam, S. Kernel vitreousness and protein content: Relationship, interaction and synergistic effects on durum wheat quality. J. Cereal Sci. 2017, 78, 2–9. [Google Scholar] [CrossRef]
- Baasandorj, T.; Ohm, J.; Manthey, F.; Simek, S. Effect of kernel size and mill type on protein, milling yield, and baking quality of hard red spring wheat. Cereal Chem. 2015, 92, 81–87. [Google Scholar] [CrossRef]
- Canadian Grain Commission: Procedure and Equipment for Determining Test Weight. Available online: https://www.grainscanada.gc.ca/en/grain-quality/grain-grading/grading-factors/grading-factors-wheat/test-weight.html (accessed on 12 July 2021).
- Dexter, J.E.; Matsuo, R.R.; Kruger, J.R. The spaghetti making quality of commercial durum wheat samples of variable amylase activity. Cereal Chem. 1990, 67, 405–412. [Google Scholar]
- Wang, K.; Taylor, D.; Pozniak, C.; Fu, B.X. Developing a high-throughput micromilling protocol for evaluating durum wheat milling performance and semolina quality. Cereal Chem. 2019, 96, 802–814. [Google Scholar] [CrossRef]
- Williams, P.; Sobering, D.; Antoniszyn, J. Protein testing methods. In Wheat Protein Production and Marketing; Fowler, D.B., Geddes, W.E., Johnston, A.M., Preston, K.R., Eds.; University Extension Press: Saskatoon, SK, Canada, 1998; pp. 37–47. [Google Scholar]
- AACC International. Approved Method of Analysis, 11th ed.; Methods 76.31.01 and Methods 38-12.02; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Fu, B.; Schlichting, L.; Pozniak, C.J.; Singh, A.K. Pigment loss from semolina to dough: Rapid measurement and relationship with pasta color. J. Cereal Sci. 2013, 57, 560–566. [Google Scholar] [CrossRef]
- Hook, S.C. Specific weight and wheat quality. J. Sci. Food Agric. 1984, 35, 1136–1141. [Google Scholar] [CrossRef]
- Tkachuk, R.; Kuzina, F.D. Wheat: Relationship between some physical and chemical properties. Can. J. Plant Sci. 1979, 59, 15–20. [Google Scholar] [CrossRef]
- Cabral, A.L.; Jordan, M.C.; Larson, G.; Somers, D.J.; Humphreys, D.G.; McCartney, C.A. Relationships between QTL for grain shape, grain weight, test weight, milling yield, and plant height in the spring wheat cross RL4452/’AC Domain’. PLoS ONE 2018, 13, e0190681. [Google Scholar] [CrossRef]
- Yabwalo, D.N.; Berzonsky, W.A.; Brabec, D.; Pearson, T.; Glover, K.D.; Kleinjan, J.L. Impact of grain morphology and genotype by environment interactions on test weight of spring and winter wheat (Triticum aestivum L.). Euphytica 2018, 2014, 125–141. [Google Scholar] [CrossRef]
- Samaan, J.; EI-Khayat, G.H.; Manthey, F.A.; Fuller, M.P.; Brennan, C.S. Durum wheat quality: II. The relationship of kernel physicochemical composition to semolina quality and end product utilization. Int. J. Food Sci. Technol. 2006, 41, 47–55. [Google Scholar] [CrossRef]
- Simmons, L.; Meredith, P. Width, weight, endosperm, and bran of the wheat grain as determinants of flour milling yield in normal and shrivelled wheats. N. Z. J. Sci. 1979, 22, 1–10. [Google Scholar]
- Troccoli, A.; di Fonzo, N. Relationship between kernel size features and test weight in Triticum durum. Cereal Chem. 1999, 76, 45–49. [Google Scholar] [CrossRef]
- Dziki, D.; Laskowski, J. Wheat kernel physical properties and milling process. Acta Agrophysica 2005, 6, 59–71. [Google Scholar]
- Lyford, C.P.; Kidd, W.; Rayas-Duarte, P.; Deyoe, C. Prediction of flour extraction rate in hard red winter wheat using the single kernel characterization. J. Food Qual. 2005, 28, 279–288. [Google Scholar] [CrossRef]
- Chaurand, M.; Lempereur, I.; Roulland, T.M.; Autran, J.C.; Abecassis, J. Genetic and agronomic effects on semolina milling value of durum wheat (Triticum durum Desf.). Crop. Sci. 1999, 2, 277. [Google Scholar]
- Peyron, S.; Surget, A.; Mabille, F.; Autran, J.C.; Rouau, X.; Abecassis, J. Evaluation of tissue dissociation of durum wheat grain (Triticum durum Desf.) generated by milling process. J. Cereal Sci. 2002, 36, 199–208. [Google Scholar] [CrossRef]
- Novaro, P.; Colucci, F.; Venora, G.; D’Egidio, M.G.D. Image analysis of whole grains: A noninvasive method to predict semolina yield in durum wheat. Cereal Chem. 2001, 78, 217–221. [Google Scholar] [CrossRef]
- Haraszi, R.; Sissons, M.; Juhasz, A.; Kadkol, G.; Tamas, L.; Anderssen, R. Using rheological phenotype phases to predict rheological features of wheat hardness and milling potential of durum wheat. Cereal Chem. 2016, 93, 369–376. [Google Scholar] [CrossRef]
- Alvarez, J.B.; Martin, L.M.; Martin, A. Genetic variation for carotenoid pigment content in the amphiploid Hordeum chilense x Triticum turgidum conv. durum. Plant Breed. 1999, 118, 187–189. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Troccoli, A.; Di Fonzo, N.; Fares, C. Durum wheat lipoxygenase activity and other quality parameters that affect pasta color. Cereal Chem. 1999, 76, 335–340. [Google Scholar] [CrossRef]
- Matsuo, R.R.; Dexter, J.E. Relationship between some durum wheat physical characteristics and semolina milling properties. Can. J. Plant Sci. 1980, 60, 49–53. [Google Scholar] [CrossRef]
- Acquistucci, R. Influence of Maillard reaction on protein modification and color development in pasta. Comparison of different drying conditions. LWT 2000, 33, 48–52. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C.; Massini, R.; Lerici, C.R. Effects of drying processing on the Maillard reaction in pasta. Food Res. Int. 1999, 32, 193–199. [Google Scholar] [CrossRef]
- Joubert, M.; Morel, M.; Lullien-Pellerin, V. Pasta color and viscoelastivity: Revisiting the role of particle size, ash, and protein content. Cereal Chem. 2018, 95, 386–398. [Google Scholar] [CrossRef]
- Lempereur, I.; Rouau, X.; Abecassis, J. Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum.) grain and its milling fractions. J. Cereal Sci. 1997, 25, 103–110. [Google Scholar] [CrossRef]
- Lintas, C.; D’Appolonia, B.L. Effect of spaghetti processing on semolina carbohydrates. Cereal Chem. 1973, 50, 563–570. [Google Scholar]
| Line | Wheat Properties | Standard Milling | Micro-Milling | Semolina Properties-Standard Milling | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | HVK (%) | TWT (kg/hL) | TKW (g) | KSD (%) | Protein (%) | Ash (%) | SY (%) | TMY (%) | SY (%) | TMY (%) | Protein (%) | Ash (%) | TYP (ppm) | Gluten index(%) | |||
| Small | Medium | Large | |||||||||||||||
| A | 1 | 94 | 83.6 | 48.0 | 5.8 | 22.3 | 71.9 | 14.3 | 1.40 | 68.1 | 76.2 | 68.1 | 75.7 | 13.3 | 0.65 | 10.3 | 62 |
| B | 1 | 92 | 83.1 | 44.8 | 5.8 | 29.3 | 64.9 | 14.5 | 1.37 | 68.0 | 76.0 | 68.0 | 75.1 | 13.4 | 0.65 | 9.0 | 70 |
| C | 1 | 92 | 82.8 | 45.7 | 6.0 | 24.6 | 69.4 | 15.2 | 1.38 | 67.2 | 75.3 | 67.8 | 75.6 | 13.1 | 0.61 | 10.7 | 85 |
| D | 1 | 91 | 82.8 | 43.2 | 7.6 | 33.2 | 59.2 | 14.3 | 1.47 | 66.3 | 74.7 | 67.8 | 75.0 | 14.2 | 0.62 | 10.3 | 77 |
| E | 1 | 95 | 83.0 | 46.6 | 4.9 | 26.3 | 68.9 | 14.9 | 1.39 | 66.9 | 75.6 | 67.0 | 74.7 | 13.9 | 0.62 | 9.4 | 66 |
| F | 1 | 93 | 81.5 | 47.7 | 3.5 | 22.4 | 74.2 | 15.5 | 1.45 | 66.1 | 73.4 | 67.0 | 74.2 | 14.4 | 0.63 | 10.8 | 57 |
| G | 1 | 96 | 83.0 | 44.7 | 7.4 | 32.3 | 60.3 | 14.7 | 1.44 | 65.2 | 73.4 | 65.7 | 73.4 | 13.7 | 0.69 | 11.5 | 56 |
| Mean | 93.3 | 82.8 | 45.8 | 5.9 | 27.2 | 67.0 | 14.8 | 1.42 | 66.8 | 74.9 | 67.3 | 74.8 | 13.8 | 0.64 | 10.3 | 69 | |
| Range | 5 | 2.1 | 4.8 | 4.1 | 10.9 | 15.0 | 1.2 | 0.10 | 2.9 | 2.8 | 2.4 | 2.3 | 1.3 | 0.08 | 2.5 | 29 | |
| F Values | Percentage of Variability Assignable to Each Factor | ||||||
|---|---|---|---|---|---|---|---|
| Kernel Size | Genotype | Interactions | Kernel Size | Genotype | Interactions | Error | |
| Wheat properties | |||||||
| TWT | 502 **** | 215.2 **** | 66.8 **** | 82.6 | 10.6 | 6.6 | 0.2 |
| TKW | 9365.2 **** | 8.3 *** | 11.2 **** | 98.9 | 0.3 | 0.7 | 0.1 |
| Protein | 555.5 **** | 2159.1 **** | 68.9 **** | 7.4 | 86.6 | 5.5 | 0.4 |
| Ash | 800.9 **** | 281.2 **** | 33.9 **** | 42.6 | 44.9 | 10.8 | 1.7 |
| Milling quality | |||||||
| Semolina yield | 13177.7 **** | 546.9 **** | 36.0 **** | 87.6 | 10.9 | 1.4 | 0.1 |
| Total milling yield | 7392.8 **** | 531.9 **** | 24.4 **** | 80.8 | 17.4 | 1.6 | 0.1 |
| Semolina ash | 2143.3 **** | 131.0 **** | 30.6 **** | 77.9 | 14.3 | 6.7 | 1.1 |
| Semolina color | |||||||
| Semolina TYP | 2897.9 **** | 1276.6 **** | 24.5 **** | 42.1 | 55.6 | 2.1 | 0.2 |
| L* | 608.8 **** | 10.4 **** | 9.6 **** | 84.7 | 4.3 | 8.0 | 2.9 |
| a* | 238.2 **** | 5.7 *** | 6.6 **** | 75.5 | 5.4 | 12.5 | 6.7 |
| b* | 96.0 **** | 177.8 **** | 13.5 **** | 13.1 | 73.0 | 11.0 | 2.9 |
| Pasta color | |||||||
| L* | 18998 **** | 184.9 **** | 293.1 **** | 89.1 | 2.6 | 8.1 | 0.1 |
| a* | 30824.8 **** | 452.5 **** | 150.0 **** | 93.1 | 4.0 | 2.7 | 0.1 |
| b* | 9625.5 **** | 406.4 **** | 59.0 **** | 85.9 | 10.9 | 3.2 | 0.1 |
| Wheat Properties | Milling Quality | Semolina Color | Pasta Color | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TWT (kg/hL) | TKW (g) | Protein (%) | Ash (%) | SY (%) | TMY (%) | Semolina Ash (%) | Semolina TYP | L* | a* | b* | L* | a* | b* | |
| Large kernels | ||||||||||||||
| Mean | 83.7 | 51.0 | 14.8 | 1.41 | 68.0 | 75.3 | 0.57 | 9.4 | 83.7 | −2.7 | 33.5 | 74.5 | 3.2 | 65.4 |
| SD | 0.7 | 1.8 | 0.5 | 0.04 | 0.9 | 0.9 | 0.02 | 0.8 | 0.2 | 0.1 | 1.1 | 1.3 | 0.4 | 1.5 |
| Range | 2.2 | 4.5 | 1.3 | 0.11 | 2.7 | 2.5 | 0.06 | 2.3 | 0.5 | 0.2 | 3.1 | 3.9 | 1.1 | 4.0 |
| F values | 130 **** | 23.81 *** | 1343.5 **** | 130.6 **** | 203.7 **** | 220.4 **** | 52. 7**** | 511 **** | 9.27 *** | 5.7 ** | 49.5 *** | 658 **** | 299 **** | 223 **** |
| Medium kernels | ||||||||||||||
| Average | 82.2 | 36.1 | 14.6 | 1.41 | 66.7 | 74.0 | 0.60 | 10.6 | 83.4 | −2.7 | 34.5 | 72.6 | 4.6 | 66.2 |
| SD | 0.6 | 0.9 | 0.5 | 0.04 | 0.7 | 0.7 | 0.02 | 0.8 | 0.2 | 0.1 | 1.0 | 0.4 | 0.3 | 1.4 |
| Range | 1.5 | 2.2 | 1.3 | 0.08 | 1.8 | 1.9 | 0.05 | 2.5 | 0.5 | 0.3 | 2.8 | 1.1 | 0.9 | 3.3 |
| F values | 73.3 **** | 4.5 * | 1100.1 **** | 96.0 **** | 170.6 **** | 160.8 **** | 73.2 **** | 1464 **** | 11.5 *** | 11.5 *** | 68 *** | 56 **** | 171 **** | 205 **** |
| Small kernels | ||||||||||||||
| Average | 77.6 | 23.9 | 14.9 | 1.49 | 63.6 | 71.9 | 0.67 | 11.1 | 81.7 | −2.1 | 33.9 | 69.4 | 6.5 | 59.4 |
| SD | 2.0 | 0.4 | 0.4 | 0.05 | 0.5 | 0.6 | 0.03 | 1.0 | 0.6 | 0.2 | 1.3 | 0.4 | 0.4 | 1.0 |
| Range | 6.1 | 1.2 | 1.2 | 0.15 | 1.3 | 1.5 | 0.07 | 3.0 | 1.7 | 0.6 | 4.1 | 1.1 | 1.3 | 2.3 |
| F values | 465.6 **** | 1.9 ns | 386.4 **** | 121.0 **** | 128.6 **** | 106.1 **** | 70.3 **** | 2149 **** | 17.3 **** | 9 *** | 92 *** | 57 **** | 282 **** | 96 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Taylor, D.; Chen, Y.; Suchy, J.; Fu, B.X. Effect of Kernel Size and Its Potential Interaction with Genotype on Key Quality Traits of Durum Wheat. Foods 2021, 10, 2992. https://doi.org/10.3390/foods10122992
Wang K, Taylor D, Chen Y, Suchy J, Fu BX. Effect of Kernel Size and Its Potential Interaction with Genotype on Key Quality Traits of Durum Wheat. Foods. 2021; 10(12):2992. https://doi.org/10.3390/foods10122992
Chicago/Turabian StyleWang, Kun, Dale Taylor, Yuming Chen, Jerry Suchy, and Bin Xiao Fu. 2021. "Effect of Kernel Size and Its Potential Interaction with Genotype on Key Quality Traits of Durum Wheat" Foods 10, no. 12: 2992. https://doi.org/10.3390/foods10122992
APA StyleWang, K., Taylor, D., Chen, Y., Suchy, J., & Fu, B. X. (2021). Effect of Kernel Size and Its Potential Interaction with Genotype on Key Quality Traits of Durum Wheat. Foods, 10(12), 2992. https://doi.org/10.3390/foods10122992






