Authentication of Transylvanian Spirits Based on Isotope and Elemental Signatures in Conjunction with Statistical Methods
Abstract
:1. Introduction
2. Experimental
2.1. Sample Description
2.2. Isotope Measurements
2.2.1. IRMS—Isotope-Ratio Mass Spectrometry
13C Measurements
2H and 18O Measurements
2.2.2. NMR Measurements
Samples Preparation for NMR Analysis
2.3. ICP-MS Determinations
2.4. Statistical Data Processing
3. Results
4. Discussion
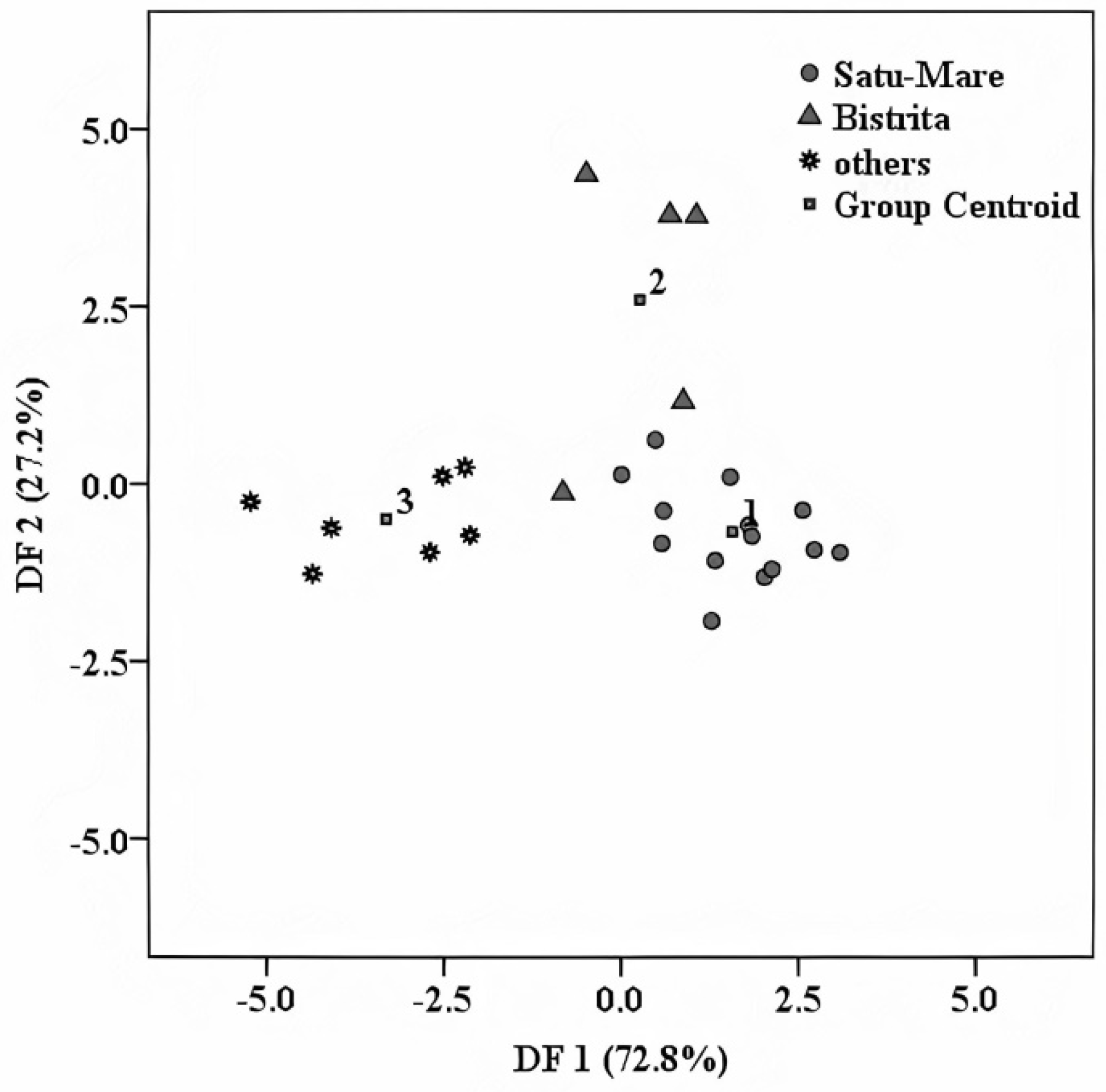
4.1. Geographical Differentiation
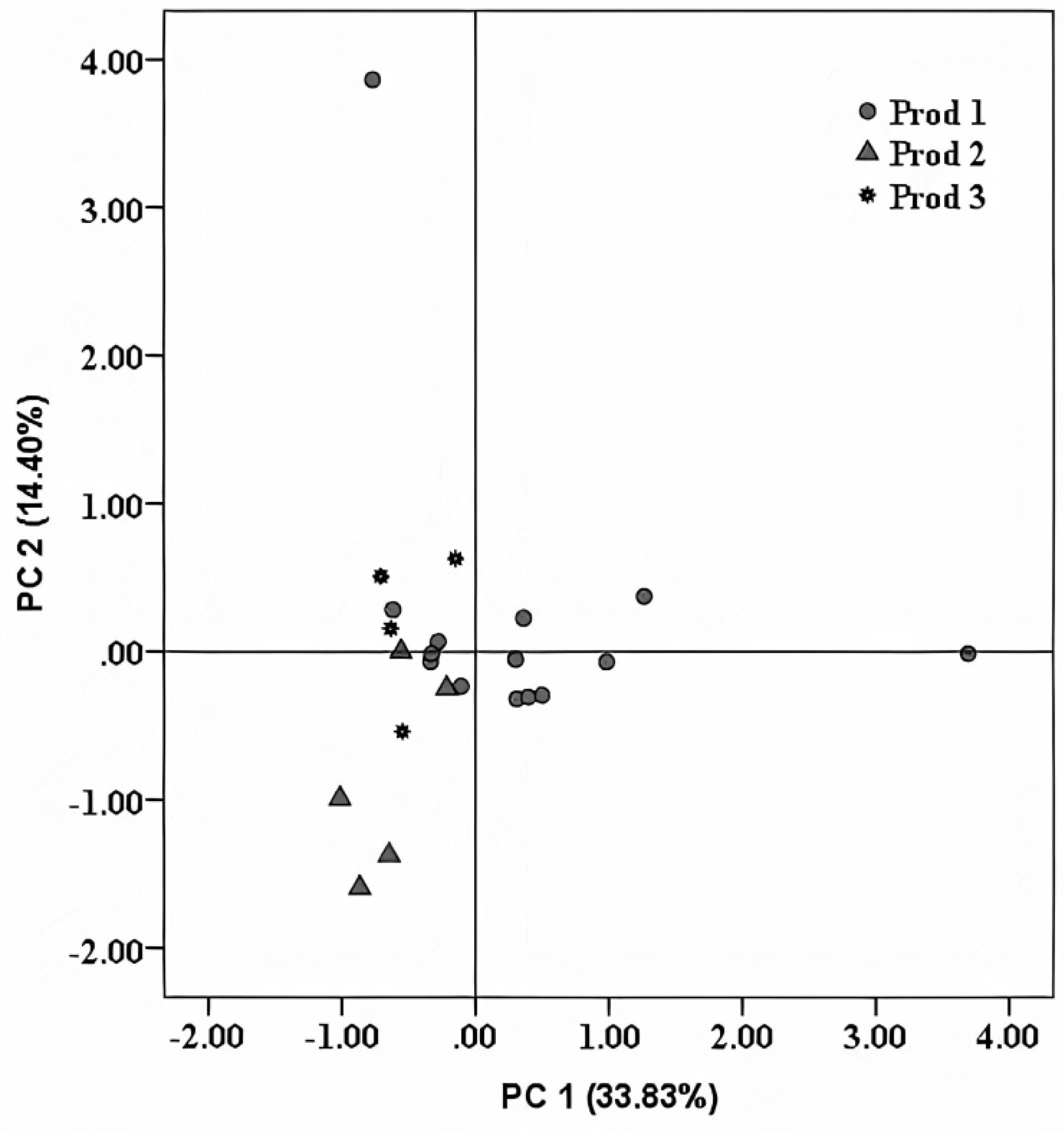
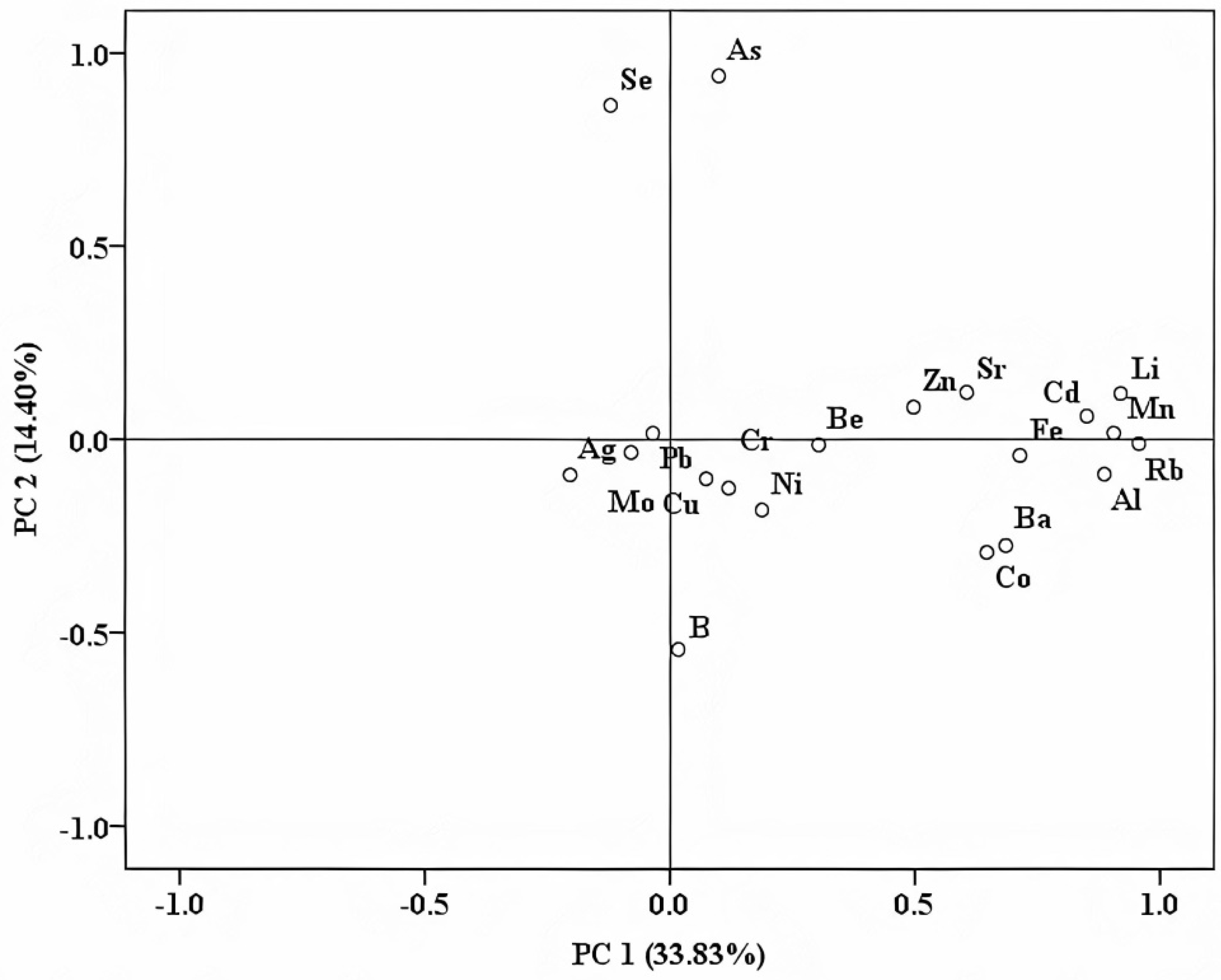
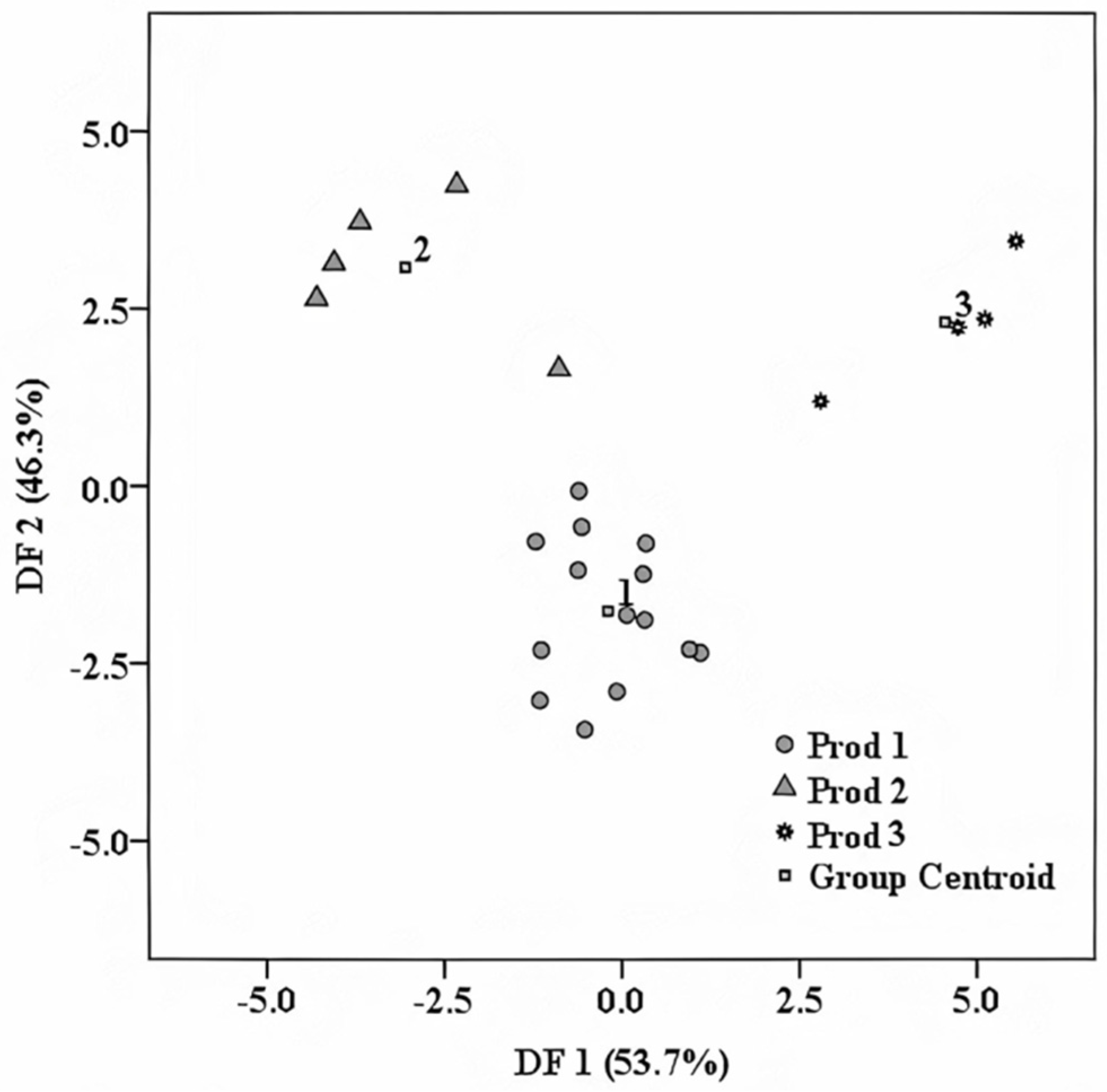
4.2. Trademark Specific Fingerprint
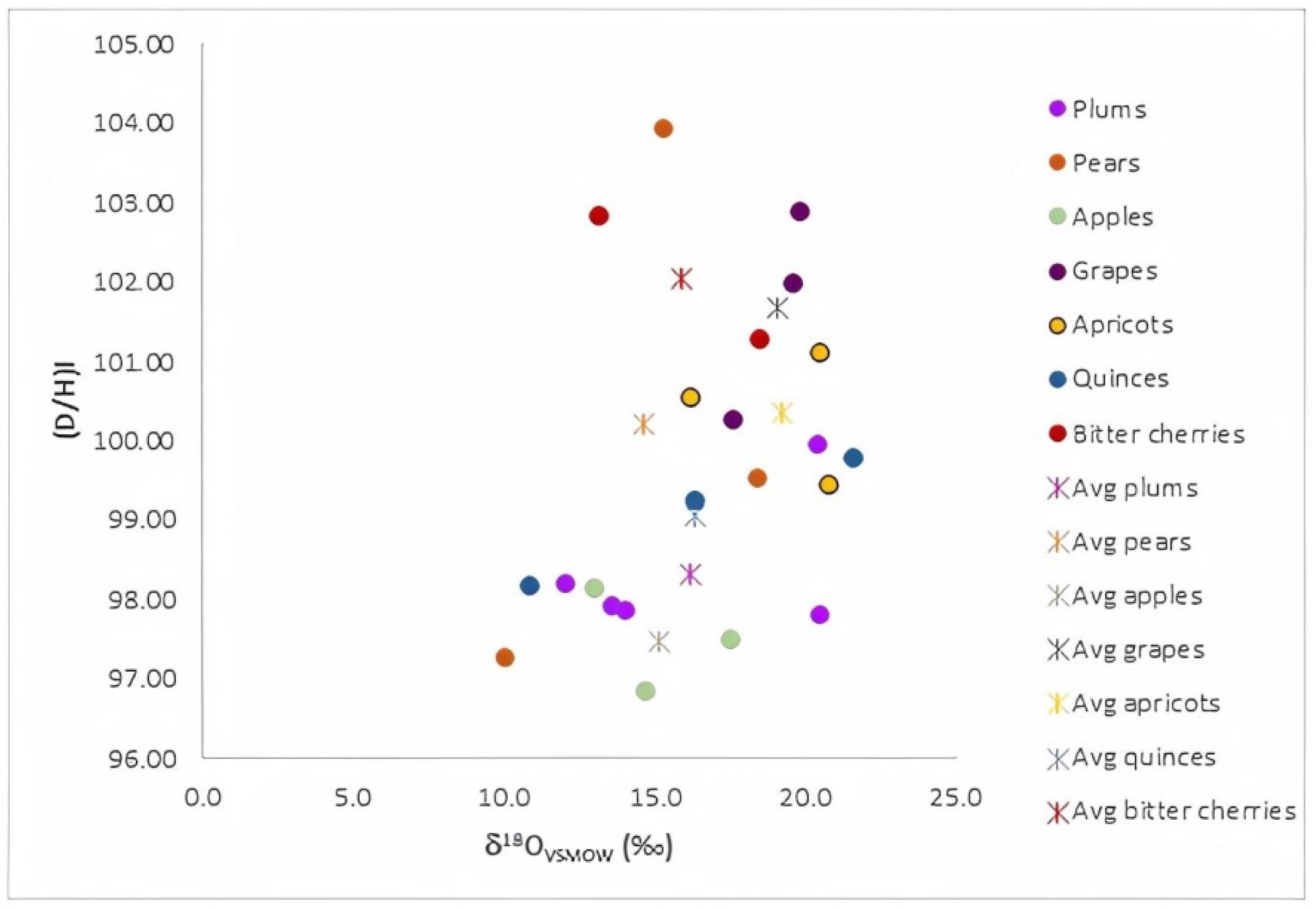
4.3. Fruit Variety Differentiation of Raw Materials Used for Fruit Distillates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| No. | Provider | Variety | County | Mg | Ca | Na | K | Cu | Fe | Zn | Al | B | Li | Mn | Co | Ni | Rb | Sr | Mo | Ag | Cd | Ba | Pb | Cr | As | Se | Be | Ce | Dy | Er | Eu | Gd | Ho | Lu | La | Nd | Pr | Sm | Tb | Th | Tm | Yb | Y | δ18O | δ2H | δ13C | (D/H)I ppm | (D/H)II ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | Concentration (µg/L) | ‰ | ||||||||||||||||||||||||||||||||||||||||||||||
| 1 | Producer 1 | Plum | SM | 2.5 | 3.1 | 2.4 | 6.7 | 1.3 | 0.9 | 1.5 | 0.2 | 0.2 | 2.5 | 71.3 | 1.0 | 137.5 | 38.5 | 30.5 | 2.7 | 0.1 | 0.4 | 15.5 | 14.3 | 109.8 | 16.4 | 0.1 | 0.3 | 2.2 | 0.13 | 0.05 | 0.05 | 1.15 | 0.03 | 0.01 | 1.10 | 0.88 | 0.55 | 0.38 | 0.03 | 0.30 | 0.03 | 0.08 | 0.88 | 12.1 | −209.2 | −25.6 | 98.19 | 125 |
| 2 | Pear | SM | 0.02 | 1.7 | 2.5 | 7.2 | 1.4 | 0.3 | 0.4 | 0.1 | 0.3 | 2.3 | 117.0 | 0.3 | 24.5 | 40.3 | 20.0 | 0.8 | 0.1 | 0.3 | 34.0 | 16.5 | 16.0 | 16.3 | 0.4 | 0.1 | 3.9 | 0.00 | 0.01 | 0.03 | 0.75 | 0.01 | 0.00 | 0.08 | 0.15 | 0.08 | 0.05 | 0.01 | 0.03 | 0.00 | 0.00 | 0.08 | 15.4 | −193.7 | −21.9 | 103.89 | 126.19 | |
| 3 | Quince | SM | 5.1 | 3.6 | 2.2 | 4.7 | 0.4 | 0.2 | 0.4 | 0.1 | 0.2 | 1.5 | 41.3 | 0.5 | 27.0 | 13.5 | 24.3 | 1.2 | 0.1 | 0.3 | 9.5 | 5.5 | 38.3 | 15.9 | 0.1 | 0.1 | 5.0 | 0.03 | 0.01 | 0.01 | 1.23 | 0.01 | 0.01 | 0.15 | 0.38 | 0.23 | 0.05 | 0.01 | 0.03 | 0.01 | 0.01 | 0.15 | 16.1 | −189.4 | −23.9 | 99.22 | 125.5 | |
| 4 | Sour cherry | SM | 28.7 | 0.5 | 2.0 | 8.1 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 2.4 | 74.8 | 0.8 | 43.0 | 45.5 | 13.5 | 1.1 | 0.1 | 0.2 | 21.0 | 4.5 | 44.5 | 15.3 | 0.8 | 0.2 | 3.5 | 0.03 | 0.03 | 0.03 | 0.95 | 0.01 | 0.01 | 0.25 | 0.30 | 0.15 | 0.05 | 0.01 | 0.05 | 0.01 | 0.01 | 0.23 | 16.7 | −177.8 | −25.2 | 99.56 | 126.38 | |
| 5 | Blackcurrant | SM | 2.7 | 2.7 | 2.3 | 7.5 | 1.3 | 0.5 | 0.3 | 0.2 | 0.3 | 4.0 | 104.3 | 0.5 | 47.3 | 56.5 | 29.0 | 2.5 | 0.1 | 0.3 | 16.8 | 5.8 | 14.8 | 17.2 | 0.1 | 0.1 | 4.4 | 0.03 | 0.01 | 0.03 | 3.45 | 0.01 | 0.01 | 0.15 | 0.60 | 0.20 | 0.05 | 0.00 | 0.03 | 0.01 | 0.05 | 0.13 | 15.4 | −192 | −21.1 | 101.21 | 126.02 | |
| 6 | Bitter cherry | SM | 2.5 | 1.6 | 2.1 | 7.6 | 1.5 | 0.5 | 0.6 | 0.2 | 0.3 | 2.3 | 116.3 | 0.3 | 43.5 | 63.8 | 14.5 | 2.4 | 1.4 | 0.2 | 5.5 | 5.3 | 8.0 | 16.6 | 0.2 | 0.2 | 3.7 | 0.00 | 0.01 | 0.01 | 1.80 | 0.01 | 0.01 | 0.05 | 0.18 | 0.08 | 0.03 | 0.01 | 0.03 | 0.00 | 0.00 | 0.08 | 18.6 | −184.5 | −25.1 | 101.23 | 125.85 | |
| 7 | Apple | SM | 2.4 | 3.8 | 2.0 | 2.5 | 0.1 | 0.05 | 0.05 | 0.1 | 0.1 | 2.8 | 21.8 | 0.4 | 10.5 | 5.8 | 34.5 | 0.2 | 0.5 | 0.1 | 7.8 | 0.2 | 13.8 | 16.3 | 0.1 | 0.0 | 3.3 | 0.00 | 0.01 | 0.01 | 0.53 | 0.01 | 0.00 | 0.08 | 0.18 | 0.08 | 0.05 | 0.01 | 0.01 | 0.00 | 0.00 | 0.05 | 13.1 | −214.5 | −25.6 | 98.13 | 123.65 | |
| 8 | Grape | SM | 9.4 | 2.6 | 2.6 | 6.1 | 1.3 | 0.3 | 0.5 | 0.1 | 0.2 | 4.2 | 71.8 | 0.6 | 25.2 | 26.6 | 22.4 | 0.1 | 0.1 | 0.3 | 24.2 | 5.0 | 4.4 | 14.0 | 0.5 | 0.1 | 4.9 | 0.01 | 0.01 | 0.02 | 1.68 | 0.01 | 0.01 | 0.08 | 0.10 | 0.06 | 0.04 | 0.01 | 0.02 | 0.00 | 0.01 | 0.06 | 19.9 | −176.5 | −26.2 | 102.86 | 129.83 | |
| 9 | Beer | SM | 0.7 | 2.1 | 1.4 | 0.3 | 0.02 | 0.001 | 0.001 | 0.02 | 0.1 | 2.5 | 4.4 | 0.0 | 1.1 | 0.6 | 13.4 | 0.1 | 0.1 | 0.1 | 0.8 | 1.0 | 0.9 | 56.9 | 10.1 | 0.04 | 1.6 | 0.01 | 0.00 | 0.01 | 1.02 | 0.01 | 0.01 | 0.02 | 0.12 | 0.04 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 16.2 | −203.6 | −25.6 | 99.98 | 125.4 | |
| 10 | Bitter cherry | SM | 2.0 | 5.4 | 3.1 | 7.9 | 1.2 | 0.6 | 9.7 | 0.2 | 0.6 | 5.3 | 676.3 | 1.4 | 43.8 | 122.3 | 60.0 | 0.1 | 0.1 | 0.6 | 5.8 | 11.3 | 11.5 | 15.8 | 0.9 | 0.2 | 4.9 | 0.08 | 0.08 | 0.03 | 4.13 | 0.03 | 0.01 | 0.25 | 0.43 | 0.15 | 0.08 | 0.03 | 0.03 | 0.01 | 0.08 | 0.65 | 13.2 | −195.1 | −18.6 | 102.81 | 121.94 | |
| 11 | Grape | SM | 1.6 | 3.9 | 2.5 | 7.8 | 4.8 | 1.0 | 0.8 | 0.3 | 0.3 | 4.0 | 181.0 | 0.8 | 29.2 | 83.8 | 30.4 | 0.4 | 0.1 | 0.5 | 17.6 | 7.0 | 11.6 | 17.9 | 0.8 | 0.1 | 5.9 | 0.01 | 0.01 | 0.01 | 2.30 | 0.01 | 0.00 | 0.08 | 0.14 | 0.08 | 0.06 | 0.01 | 0.02 | 0.01 | 0.02 | 0.08 | 19.7 | −174.9 | −24.7 | 101.96 | 125.79 | |
| 12 | Grape | SM | 2.0 | 7.2 | 5.8 | 7.6 | 5.3 | 0.7 | 2.7 | 0.5 | 0.5 | 18.0 | 928.2 | 1.6 | 50.4 | 217.0 | 91.2 | 0.6 | 0.1 | 1.1 | 40.4 | 28.8 | 35.0 | 18.3 | 0.9 | 0.1 | 7.0 | 0.02 | 0.02 | 0.04 | 1.36 | 0.01 | 0.00 | 0.14 | 0.18 | 0.08 | 0.06 | 0.01 | 0.04 | 0.01 | 0.01 | 0.16 | 17.7 | −193 | −24.7 | 100.23 | 127.15 | |
| 13 | Apple | SM | 0.3 | 0.2 | 1.0 | 2.7 | 0.1 | 0.01 | 0.4 | 0.1 | 0.1 | 1.7 | 18.8 | 0.5 | 8.1 | 5.0 | 4.2 | 0.2 | 0.1 | 0.4 | 1.9 | 2.0 | 2.5 | 14.5 | 0.2 | 0.1 | 3.6 | 0.00 | 0.02 | 0.02 | 3.19 | 0.02 | 0.00 | 0.04 | 0.46 | 0.15 | 0.04 | 0.02 | 0.04 | 0.02 | 0.04 | 0.08 | 17.6 | −188.9 | −26.2 | 97.46 | 122.43 | |
| 14 | Fruit mixture | SM | 0.7 | 0.8 | 1.2 | 0.6 | 0.03 | 0.002 | 0.3 | 0.01 | 0.1 | 2.1 | 16.1 | 0.2 | 9.6 | 1.4 | 8.2 | 0.1 | 0.2 | 0.3 | 1.4 | 0.9 | 12.5 | 16.9 | 0.7 | 0.1 | 0.9 | 0.00 | 0.02 | 0.02 | 1.46 | 0.02 | 0.02 | 0.02 | 0.29 | 0.11 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 | 0.07 | 13.3 | −202.3 | −26.1 | 97.06 | 123.24 | |
| 15 | Producer 2 | Pear | BN | 0.3 | 2.0 | 1.4 | 1.1 | 0.7 | 0.2 | 0.1 | 0.1 | 1.0 | 0.4 | 10.7 | 0.9 | 67.4 | 1.9 | 12.9 | 2.9 | 0.1 | 0.1 | 9.0 | 9.0 | 80.0 | 0.7 | 0.1 | 0.02 | 0.2 | 0.01 | 0.02 | 0.01 | 10.45 | 0.01 | 0.00 | 0.07 | 0.21 | 0.10 | 0.05 | 0.01 | 0.02 | 0.01 | 0.10 | 0.10 | 18.5 | −202.7 | −26.1 | 99.5 | 126.5 |
| 16 | Quince | BN | 0.5 | 5.1 | 1.3 | 2.2 | 1.9 | 0.1 | 0.05 | 0.1 | 1.0 | 0.5 | 31.2 | 1.4 | 58.8 | 4.3 | 18.3 | 0.4 | 0.1 | 0.1 | 12.2 | 0.8 | 22.4 | 1.2 | 0.2 | 0.02 | 0.2 | 0.02 | 0.05 | 0.01 | 3.52 | 0.01 | 0.01 | 0.14 | 0.26 | 0.10 | 0.05 | 0.01 | 0.01 | 0.01 | 0.07 | 0.31 | 21.7 | −196.9 | −26.1 | 99.76 | 124.33 | |
| 17 | Apricot | BN | 0.3 | 2.9 | 1.4 | 0.4 | 1.6 | 0.001 | 0.001 | 0.03 | 1.2 | 0.7 | 3.8 | 0.1 | 8.1 | 0.9 | 12.9 | 0.1 | 0.6 | 0.0 | 15.7 | 1.4 | 4.8 | 1.0 | 0.1 | 0.1 | 0.02 | 0.01 | 0.01 | 0.01 | 0.57 | 0.01 | 0.01 | 0.02 | 0.14 | 0.07 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.02 | 16.3 | −197.8 | −26.2 | 100.51 | 126.61 | |
| 18 | Apple | BN | 0.3 | 4.9 | 1.2 | 1.4 | 2.8 | 0.01 | 0.02 | 0.1 | 0.2 | 1.6 | 80.7 | 1.0 | 13.8 | 2.9 | 24.5 | 0.7 | 0.1 | 0.1 | 15.5 | 0.2 | 14.0 | 15.6 | 0.1 | 0.1 | 3.7 | 0.00 | 0.02 | 0.01 | 0.36 | 0.01 | 0.01 | 0.05 | 0.14 | 0.05 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.05 | 14.8 | −206.2 | −27.9 | 96.83 | 123.12 | |
| 19 | Plum | BN | 0.3 | 3.8 | 1.2 | 1.1 | 1.4 | 0.1 | 0.2 | 0.1 | 0.1 | 1.7 | 11.0 | 0.2 | 126.2 | 2.4 | 14.5 | 0.1 | 0.0 | 0.1 | 14.8 | 150.0 | 5.7 | 18.4 | 0.1 | 0.05 | 3.7 | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 | 0.05 | 0.19 | 0.05 | 0.02 | 0.01 | 0.02 | 0.00 | 0.00 | 0.05 | 20.5 | −204.7 | −27.6 | 99.93 | 127.38 | |
| 20 | Manufacture | Apricot | SM | 2.1 | 2.7 | 4.8 | 0.2 | 12.8 | 0.02 | 1.7 | 0.1 | 0.2 | 1.6 | 12.4 | 0.2 | 5.8 | 0.8 | 69.2 | 0.1 | 0.9 | 0.5 | 2.1 | 97.1 | 10.5 | 4.7 | 3.1 | 0.1 | 0.7 | 0.01 | 0.01 | 0.01 | 0.95 | 0.01 | 0.01 | 0.03 | 0.16 | 0.08 | 0.03 | 0.01 | 0.11 | 0.01 | 0.00 | 0.03 | 20.6 | −218.7 | −23 | 101.07 | 127.69 |
| 21 | Pear | SM | 3.2 | 5.0 | 2.2 | 0.2 | 2.8 | 0.1 | 0.1 | 0.1 | 0.1 | 3.1 | 7.4 | 0.2 | 7.1 | 0.9 | 64.8 | 2.1 | 0.1 | 0.1 | 19.8 | 3.3 | 48.6 | 21.9 | 1.2 | 0.1 | 1.3 | 0.01 | 0.00 | 0.01 | 0.33 | 0.01 | 0.01 | 0.05 | 0.17 | 0.07 | 0.05 | 0.01 | 0.02 | 0.01 | 0.00 | 0.02 | 10.1 | −228 | −27.7 | 97.25 | 122.61 | |
| 22 | Plum | B | 4.9 | 1.3 | 4.5 | 0.2 | 4.2 | 0.01 | 0.2 | 0.1 | 0.3 | 2.8 | 7.0 | 0.1 | 3.5 | 0.9 | 49.8 | 3.5 | 0.5 | 0.1 | 1.2 | 13.0 | 10.5 | 20.3 | 0.8 | 0.1 | 1.2 | 0.00 | 0.01 | 0.01 | 1.20 | 0.01 | 0.01 | 0.03 | 0.25 | 0.08 | 0.03 | 0.01 | 0.03 | 0.00 | 0.01 | 0.03 | 13.7 | −207.3 | −26.9 | 97.9 | 127.4 | |
| 23 | Apricot | CV | 0.1 | 5.8 | 0.5 | 0.1 | 10.8 | 0.01 | 0.001 | 0.2 | 0.002 | 1.3 | 3.0 | 0.2 | 4.0 | 1.4 | 18.1 | 0.1 | 0.1 | 0.0 | 4.5 | 0.8 | 8.3 | 4.6 | 0.3 | 0.02 | 1.0 | 0.00 | 0.01 | 0.01 | 0.90 | 0.01 | 0.00 | 0.02 | 0.19 | 0.07 | 0.02 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 | 20.9 | −185.5 | −26.8 | 99.42 | 126.06 | |
| 24 | Others | Quince | CJ | 0.2 | 11.6 | 0.5 | 0.1 | 4.1 | 0.01 | 0.2 | 0.1 | 0.1 | 1.3 | 40.8 | 0.2 | 5.3 | 0.7 | 11.1 | 0.0 | 0.1 | 0.2 | 28.7 | 0.6 | 3.4 | 18.4 | 0.3 | 0.2 | 0.9 | 0.03 | 0.03 | 0.03 | 0.66 | 0.01 | 0.00 | 0.01 | 0.16 | 0.08 | 0.03 | 0.01 | 0.01 | 0.01 | 0.00 | 0.21 | 10.9 | −202.8 | −26.1 | 98.14 | 127.46 |
| 25 | Plum | SJ | 0.6 | 2.4 | 1.4 | 0.2 | 8.7 | 0.001 | 0.1 | 0.1 | 0.1 | 6.3 | 12.8 | 0.1 | 3.5 | 0.8 | 36.8 | 0.5 | 0.1 | 0.1 | 2.3 | 20.8 | 5.0 | 19.4 | 0.1 | 0.2 | 1.2 | 0.00 | 0.01 | 0.01 | 0.13 | 0.00 | 0.00 | 0.03 | 0.43 | 0.13 | 0.03 | 0.01 | 0.01 | 0.00 | 0.00 | 0.05 | 14.1 | −188 | −27.6 | 97.84 | 123.69 | |
| 26 | Plum | CL | 0.3 | 1.4 | 2.0 | 0.4 | 7.7 | 0.1 | 0.1 | 0.2 | 0.1 | 2.9 | 10.0 | 0.2 | 4.7 | 1.3 | 10.0 | 0.8 | 0.1 | 0.2 | 2.7 | 0.2 | 26.6 | 21.2 | 0.1 | 0.1 | 1.4 | 0.01 | 0.01 | 0.01 | 1.29 | 0.01 | 0.01 | 0.05 | 0.24 | 0.11 | 0.03 | 0.01 | 0.01 | 0.01 | 0.00 | 0.08 | 20.6 | −191.7 | −26.8 | 97.77 | 124.69 | |
References
- Coldea, T.E.; Socaciu, C.; Moldovan, Z.; Mudura, E. Minor Volatile Compounds in Traditional Homemade Fruit Brandies from Transylvania-Romania, as Determined by GC-MS Analysis. Not. Bot. Horti Agrobo. 2014, 42, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Strojnik, L.; Hladnik, J.; Weber, N.C.; Koron, D.; Stopar, M.; Zlati, E.; Kokalj, D.; Strojnik, M.; Ogrinc, N. Construction of IsoVoc Database for the Authentication of Natural Flavours. Foods 2021, 10, 1550. [Google Scholar] [CrossRef]
- Bauer-Christoph, C.; Christoph, N.; Aguilar-Cisneros, B.O.; Lopez, M.G.; Richling, E.; Rossmann, A.; Schreier, P. Authentication of tequila by gas chromatography and stable isotope ratio analyses. Eur. Food Res. Technol. 2003, 217, 438–443. [Google Scholar] [CrossRef]
- Aguilar-Cisneros, B.O.; Lopez, M.G.; Richling, E.; Heckel, F.; Schreier, P. Tequila authenticity assessment by headspace SPME-HRGC-IRMS analysis of 13C/12C and 18O/16O ratios of ethanol. J. Agric. Food Chem. 2002, 50, 7520–7523. [Google Scholar] [CrossRef]
- Pissinatto, L.; Martinelli, L.A.; Victoria, R.L.; De Camargo, P.B. Stable carbon isotopic analysis and the botanical origin of ethanol in Brazilian brandies. Food Res. Int. 2000, 32, 665–668. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Magdas, D.A. Application of Raman spectroscopy and Machine Learning algorithms for fruit distillates discrimination. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Advances in the Detection of the Adulteration of Alcoholic Beverages Including Unrecorded Alcohol. In Advances in Food Authenticity Testing; Downey, G., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Duxford, UK, 2016; pp. 565–584. [Google Scholar] [CrossRef]
- Dippong, T.; Avram, A.; Mihali, C. Gas-chromatography assessments of the major volatile compounds in traditional fruit brandies throughout fruit and wood maturation. Studia Univ. Babes-Bolyai Chem. 2019, LXIV, 141–155. [Google Scholar] [CrossRef]
- Jamin, E.; Guerin, R.; Retif, M.; Lees, M.; Martin, G.J. Improved detection of added water in orange juice by simultaneous determination of the oxygen-18/oxygen-16 isotope ratios of water and ethanol derived from sugars. J. Agric. Food Chem. 2003, 51, 5202–5206. [Google Scholar] [CrossRef]
- Perini, M.; Camin, F. δ18O of Ethanol in Wine and Spirits for Authentication Purposes. J. Food Sci. 2013, 78, C839–C844. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for stable isotope ratio analysis 2013 (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal Component Analysis. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Amadi, A.N.; Yisa, J.; Ogbonnaya, I.C.; Dan-Hassan, M.A.; Jacob, J.O.; Alkali, Y.B. Quality evaluation of River Chanchaga using metal pollution index and Principal Component Analysis. J. Geogr. Geol. 2012, 4, 13–21. [Google Scholar] [CrossRef]
- Magdas, D.A.; Puscas, R. Stable isotopes determination in some Romanian fruit juices. Isot. Environ. Health Stud. 2011, 47, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Winterová, R.; Mikulíková, R.; Mazáč, J.; Havelec, P. Assessment of the authenticity of fruit spirits by gas chromatography and stable isotope ratio analyses. Czech J. Food Sci. 2008, 26, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Figueira, R.; Venturini Filho, W.G.; Ducatti, C.; Nogueira, A.M.P. Isotope analysis (δ13C) of pulpy whole apple juice. Food Sci. Technol. 2011, 31, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.P.; Santos, M.C.; Lemos, S.G.; Ferreira, M.M.C.; Nogueira, A.R.A.; Nobrega, J.A. Pattern recognition applied to mineral characterization of Brazilian coffees and sugar-cane spirits. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 717–724. [Google Scholar] [CrossRef]
- Magdas, D.A.; Feher, I.; Dehelean, A.; Cristea, G.; Magdas, T.M.; Puscas, R.; Marincas, O. Isotopic and elemental markers for geographical origin and organically carrots discrimination. Food Chem. 2018, 267, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Beroldi, D.; Barbero, A.; Camin, F.; Caligiani, A.; Larcher, R. Multielemental fingerprinting and geographical traceability of Theobroma cacao beans and cocoa products. Food Control 2016, 65, 46–53. [Google Scholar] [CrossRef]
- Magdas, D.A.; Dehelean, A.; Feher, I.; Radu, S. Isotopic and multielemental fingerprinting of organically and conventionally grown potatoes. Isot. Environ. Health Stud. 2017, 53, 610–619. [Google Scholar] [CrossRef]
- Cristea, G.; Feher, I.; Voica, C.; Radu, S.; Magdas, D.A. Isotopic and elemental profiling alongside with chemometric methods for vegetable differentiation. Isot. Environ. Health Stud. 2020, 56, 69–82. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Puscas, R.; Vigouroux, A.; Gaillard, L.; Dehelean, A.; Feher, I.; Cristea, G. Applications of emerging stable isotopes and elemental markers for geographical and varietal recognition of Romanian and French honeys. Food Chem. 2021, 334, 127599. [Google Scholar] [CrossRef] [PubMed]
- Monsallier-Bitea, C.; Jamin, E.; Lees, M.; Zhang, B.L.; Martin, G.J. Study of the influence of alcoholic fermentation and distillation on the oxygen-18/oxygen-16 isotope ratio of ethanol. J. Agric. Food Chem 2006, 54, 279–284. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in Mineral Nutrition Transport and Signal Transduction in Rosaceae Fruit Quality and Postharvest Storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef]
- Ganie, M.A.; Akhter, F.; Bhat, M.A.; Malik, A.R.; Junaid, J.M.; Abas Shah, M.; Bhat, A.H.; Bhat, T.A. Boron—A critical nutrient element for plant growth and productivity with reference to temperate fruits. Curr. Sci. 2013, 104, 76–85. [Google Scholar]
- Lee, S.H.; Kim, W.S.; Han, T.H. Effects of post-harvest foliar boron and calcium applications on subsequent seasons pollen germination and pollen tube growth of pear (Pyrus pyrifolia). Sci. Hortic. 2009, 122, 77–82. [Google Scholar] [CrossRef]
- Coetzee, P.P.; van Jaarsveld, F.P.; Vanhaecke, F. Intraregional classification of wines via ICP-MS elemental fingerprint. Food Chem. 2014, 164, 485–492. [Google Scholar] [CrossRef]
- Kelly, S.; Baxter, M.; Chapman, S.; Rhodes, C.; Dennis, J.; Brereton, P. The application of isotopic and elemental analysis to determine the geographical origin of premium long grain rice. Eur. Food Res. Technol 2002, 214, 72–78. [Google Scholar] [CrossRef]
- Aguilar, M.V.; Martinez, M.C.; Masoud, T.A. Arsenic content in some Spanish wines. Influence of the wine-making technique on arsenic content in musts and wines. Z. Lebensm. Unters. Forsch. 1987, 185, 185–187. [Google Scholar] [CrossRef]
- Bontempo, L.; Camin, F.; Ziller, L.; Perini, M.; Nicolini, G.; Larcher, R. Isotopic and elemental composition of selected types of Italian honey. Measurement 2017, 98, 283–289. [Google Scholar] [CrossRef]
- Hegedûs, A.; Papp, N.; Stefanovits-Bányai, É. A review of nutritional value and putative health-effects of quince (Cydonia oblonga Mill.) fruit. Int. J. Hortic. Sci. 2013, 19, 29–32. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdas, D.A.; Cristea, G.; Pîrnau, A.; Feher, I.; Hategan, A.R.; Dehelean, A. Authentication of Transylvanian Spirits Based on Isotope and Elemental Signatures in Conjunction with Statistical Methods. Foods 2021, 10, 3000. https://doi.org/10.3390/foods10123000
Magdas DA, Cristea G, Pîrnau A, Feher I, Hategan AR, Dehelean A. Authentication of Transylvanian Spirits Based on Isotope and Elemental Signatures in Conjunction with Statistical Methods. Foods. 2021; 10(12):3000. https://doi.org/10.3390/foods10123000
Chicago/Turabian StyleMagdas, Dana Alina, Gabriela Cristea, Adrian Pîrnau, Ioana Feher, Ariana Raluca Hategan, and Adriana Dehelean. 2021. "Authentication of Transylvanian Spirits Based on Isotope and Elemental Signatures in Conjunction with Statistical Methods" Foods 10, no. 12: 3000. https://doi.org/10.3390/foods10123000







