The Physicochemical Properties of Starch Are Affected by Wxlv in Indica Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and the Preparation of Samples
2.2. Analysis of Rice Grain Composition
2.3. Analysis of Rice Pasting and Thermal Profiles
2.4. Analysis of the Fine Structure of Starch
2.5. Analysis of the Crystalline Structure of Starch
2.6. Statistical Analysis
3. Results and Discussion
3.1. Rice Grain Quality Profiles
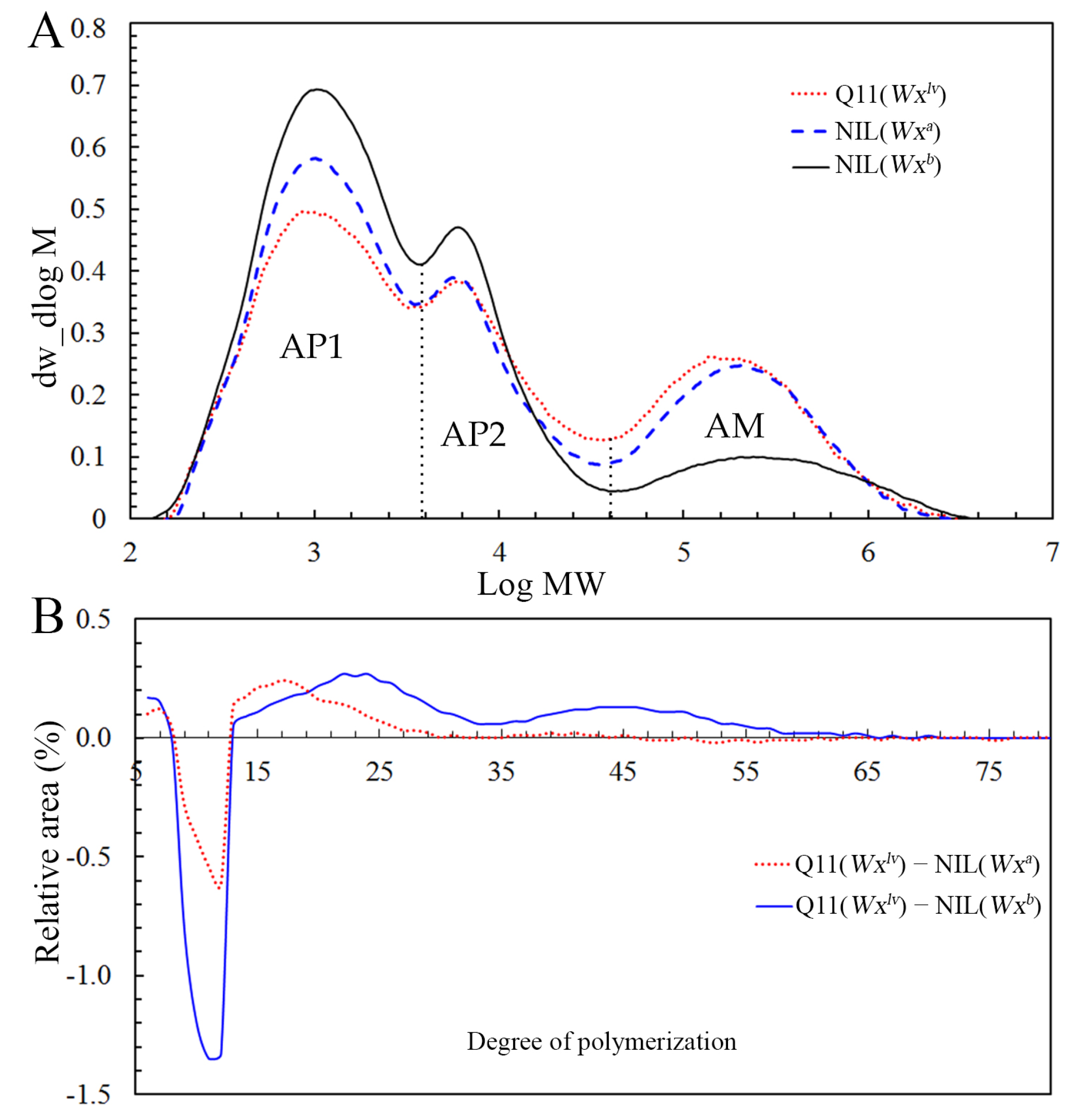
3.2. Starch Fine Structure
3.3. Starch Fine Structure in the Developing Endosperm
3.4. Crystalline Structure of Starches from Different NILs
3.5. Rice Flour and Starch Pasting Properties
3.6. Rice Starch Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Lu, Y.; Zhang, Y.D.; Zhang, C.Q.; Zhao, L.; Yao, S.; Sun, X.C.; Chen, T.; Zhu, Z.; Zhao, C.F.; et al. Characteristics of grain quality and starch fine structure of japonica rice kernels following preharvest sprouting. J. Cereal Sci. 2020, 95, 103023. [Google Scholar] [CrossRef]
- Li, H.; Gilbert, R.G. Starch molecular structure: The basis for an improved understanding of cooked rice texture. Carbohyd. Polym. 2018, 195, 9–17. [Google Scholar] [CrossRef]
- Adegoke, T.V.; Wang, Y.F.; Chen, L.J.; Wang, H.M.; Liu, W.N.; Liu, X.Y.; Cheng, Y.C.; Tong, X.H.; Ying, J.Z.; Zhang, J. Posttranslational modification of waxy to genetically improve starch quality in rice grain. Int. J. Mol. Sci. 2021, 22, 4845. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.J.; Dhital, S. High-amylose starches to bridge the “fiber gap”: Development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Q.; Zhu, J.H.; Chen, S.J.; Liu, Q.Q. Wxlv, the ancestral allele of rice waxy gene. Mol. Plant. 2019, 36, 140. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Wu, Z.L.; Xing, Y.Y.; Zheng, F.G.; Guo, X.L.; Zhang, W.G.; Hong, M.M. Nucleotide sequence of rice waxy gene. Nucleic Acids Res. 1990, 18, 5898. [Google Scholar] [CrossRef] [Green Version]
- Hiroyuki, S.; Yasuhiro, S.; Makoto, S.; Tokio, I. Molecular characterization of Wx-mq, a novel mutant gene for low-amylose content in endosperm of rice (Oryza sativa L.). Breed. Sci. 2002, 52, 131–135. [Google Scholar]
- Mikami, I.; Uwatoko, N.; Ikeda, Y.; Yamaguchi, J.; Hirano, H.Y.; Suzuki, Y.; Sano, Y. Allelic diversification at the wx locus in landraces of asian rice. Theor. Appl. Genet. 2008, 116, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.D.; Park, W.D. Association of waxy gene single nucleotide polymorphisms with starch characteristics in rice (Oryza sativa L.). Mol. Breed. 2003, 12, 335–339. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; Zhao, D.; Li, Y.H.; Li, P.B.; Wu, B.; Gao, G.J.; Zhang, Q.L.; Wang, G.W.; Xiao, J.H.; et al. The origin of Wxla provides new insights into the improvement of grain quality in rice. J. Integr. Plant Biol. 2020, 63, 878–888. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Yang, Y.; Chen, S.J.; Liu, X.J.; Zhu, J.H.; Zhou, L.H.; Lu, Y.; Li, Q.F.; Fan, X.L.; Tang, S.Z.; et al. A rare waxy allele coordinately improves rice eating and cooking quality and grain transparency. J. Integr. Plant Biol. 2020, 63, 889–901. [Google Scholar] [CrossRef]
- Ando, I.; Sato, H.; Aoki, N.; Suzuki, Y.; Hirabayashi, H.; Kuroki, M.; Shimizu, H.; Ando, T.; Takeuchi, Y. Genetic analysis of the low-amylose characteristics of rice cultivars oborozuki and hokkai-pl9. Breed. Sci. 2010, 60, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Wanchana, S.; Toojinda, T.; Tragoonrung, S.; Vanavichit, A. Duplicated coding sequence in the waxy allele of tropical glutinous rice (Oryza sativa L.). Plant Sci. 2003, 165, 1193–1199. [Google Scholar] [CrossRef]
- Teng, B.; Zeng, R.Z.; Wang, Y.C.; Liu, Z.Q.; Zhang, Z.M.; Zhu, H.T.; Ding, X.H.; Li, W.T.; Zhang, G.Q. Detection of allelic variation at the Wx locus with single-segment substitution lines in rice (Oryza sativa L.). Mol. Breed. 2011, 30, 583–585. [Google Scholar] [CrossRef]
- Zhou, L.J.; Sheng, W.T.; Jun, W.U.; Zhang, C.Q.; Liu, Q.Q.; Deng, Q.Y. Differential expressions among five Waxy alleles and their effects on the eating and cooking qualities in specialty rice cultivars. J. Integr. Agric. 2015, 14, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.X.; Jobling, S.A.; Millar, A.; Morell, M.K.; Li, Z.Y. Allelic effects on starch structure and properties of six starch biosynthetic genes in a rice recombinant inbred line population. Rice 2015, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Q.; Chen, S.J.; Ren, X.Y.; Lu, Y.; Liu, D.R.; Cai, X.L.; Li, Q.F.; Gao, J.P.; Liu, Q.Q. Molecular Structure and physicochemical properties of starches from rice with different amylose contents resulting from modification of OsGBSSI activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Crofts, N.; Itoh, A.; Abe, M.; Miura, S.; Oitome, N.F.; Bao, J.; Fujita, N. Three major nucleotide polymorphisms in the Waxy gene correlated with the amounts of extra-long chains of amylopectin in rice cultivars with s or l-type amylopectin. J. Appl. Glycosci. 2019, 66, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.F.; Li, J.X.; Yu, S.B.; Xing, Y.Z.; Xu, C.G.; Zhang, Q. The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, shanyou 63. Theor. Appl. Genet. 1999, 99, 642–648. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, Q.Q.; Sang, Y.J.; Gu, M.H.; Shi, Y.C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 2009, 120, 94–100. [Google Scholar] [CrossRef]
- Cai, J.W.; Man, J.M.; Huang, J.; Liu, Q.Q.; Wei, W.X.; Wei, C.X. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohyd. Polym. 2015, 125, 35–44.2002. [Google Scholar] [CrossRef]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.H.; Bergman, C.J.; Pinson, S.; Fjellstrom, R. Waxy gene haplotypes: Associations with pasting properties in an international rice germplasm collection. J. Cereal Sci. 2008, 48, 781–788. [Google Scholar] [CrossRef]
- Traore, K.; Mcclung, A.M.; Chen, M.H.; Fjellstrom, R. Inheritance of flour paste viscosity is associated with a rice Waxy gene exon 10 SNP marker. J. Cereal Sci. 2011, 53, 37–44. [Google Scholar] [CrossRef]
- Hanashiro, I.; Abe, J.I.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Yangcheng, H.; Blanco, M.; Gardner, C.; Li, X.H.; Jane, J. Dosage effects of Waxy gene on the structures and properties of corn starch. Carbohyd. Polym. 2016, 149, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.C.; Tan, H.Y.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 5, 100237. [Google Scholar] [CrossRef]
- Wang, W.T.; Cui, W.P.; Xu, K.; Gao, H.; Wei, H.Y.; Zhang, H.C. Effects of early- and late-sowing on starch accumulation and associated enzyme activities during grain filling stage in rice. Rice Sci. 2021, 28, 191–199. [Google Scholar]
- Lin, L.S.; Huang, J.; Zhang, L.; Liu, Q.; Wei, C. Effects of inhibition of starch branching enzymes on starch ordered structure and component accumulation in developing kernels of rice. J. Cereal Sci. 2020, 91, 102884. [Google Scholar] [CrossRef]
- Tetlow, I.J.; Bertoft, E. A review of starch biosynthesis in relation to the building block-backbone model. Int. J. Mol. Sci. 2020, 21, 7011. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Application of small-angle x-ray and neutron scattering techniques to the characterisation of starch structure: A review. Carbohyd. Polym. 2011, 85, 281–293. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. i. structural aspects provide insight into crystallinity characteristics and gelatinisation behaviour of granular starch. J. Cereal Sci. 2003, 38, 43–52. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Yang, Y.; Chen, Z.Z.; Chen, F.; Pan, L.X.; Lu, Y.; Li, Q.F.; Fan, X.L.; Sun, Z.Z.; Liu, Q.Q. Characteristics of grain physicochemical properties and the starch structure in rice carrying a mutated ALK/SSIIa gene. J. Agric. Food Chem. 2020, 68, 13950–13959. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lai, H.M. Characteristics of the starch fine structure and pasting properties of waxy rice during storage. Food Chem. 2014, 152, 432–439. [Google Scholar] [CrossRef]
- Li, H.T.; Dhital, S.; Slade, A.J.; Yu, W.W.; Gilbert, R.G.; Gidley, M.J. Altering starch branching enzymes in wheat generates high-amylose starch with novel molecular structure and functional properties. Food Hydrocoll. 2019, 92, 51–59. [Google Scholar] [CrossRef]
- Li, C.; Wu, A.; Yu, W.W.; Hu, Y.M.; Li, E.P.; Zhang, C.Q.; Liu, Q.Q. Parameterizing starch chain-length distributions for structure-property relations. Carbohyd. Polym. 2020, 241, 116390. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice starch diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Kong, X.L.; Zhu, P.; Sui, Z.Q.; Bao, J.S. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]



| Samples | MC (%) | PC (%) | AAC (%) | GC (mm) | TSC (%) |
|---|---|---|---|---|---|
| Q11(Wxlv) | 10.76 ± 0.26a | 7.14 ± 0.24a | 26.58 ± 0.16a | 104.75 ± 9.84a | 86.44 ± 0.86a |
| NIL(Wxa) | 11.03 ± 0.43a | 7.08 ± 0.31a | 25.04 ± 0.13b | 36.28 ± 5.43c | 86.15 ± 0.54a |
| NIL(Wxb) | 10.92 ± 0.51a | 6.98 ± 0.26a | 15.42 ± 0.08c | 80.56 ± 0.12b | 85.92 ± 0.63a |
| Lines | AP1 | AP2 | AM | AP1/AP2 |
|---|---|---|---|---|
| Q11(Wxlv) | 43.06 ± 0.09c | 25.50 ± 0.03b | 31.44 ± 0.06a | 1.69 ± 0.00c |
| NIL(Wxa) | 46.78 ± 0.31b | 24.08 ± 0.31b | 29.15 ± 0.01b | 1.95 ± 0.02b |
| NIL(Wxb) | 60.85 ± 0.14a | 26.44 ± 0.12a | 12.72 ± 0.27c | 2.31 ± 0.00a |
| Lines | RC (%) | Imax (Counts) | D (nm) |
|---|---|---|---|
| Q11(Wxlv) | 20.37 ± 0.01c | 289.17 ± 3.25c | 10.47 ± 0.00a |
| NIL(Wxa) | 21.21 ± 0.06b | 310.28 ± 6.68b | 10.17 ± 0.04b |
| NIL(Wxb) | 23.79 ± 0.14a | 355.56 ± 10.72a | 9.86 ± 0.01c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Lu, C.; Yang, Y.; Lu, Y.; Li, Q.; Huang, L.; Fan, X.; Liu, Q.; Zhang, C. The Physicochemical Properties of Starch Are Affected by Wxlv in Indica Rice. Foods 2021, 10, 3089. https://doi.org/10.3390/foods10123089
Feng L, Lu C, Yang Y, Lu Y, Li Q, Huang L, Fan X, Liu Q, Zhang C. The Physicochemical Properties of Starch Are Affected by Wxlv in Indica Rice. Foods. 2021; 10(12):3089. https://doi.org/10.3390/foods10123089
Chicago/Turabian StyleFeng, Linhao, Chenya Lu, Yong Yang, Yan Lu, Qianfeng Li, Lichun Huang, Xiaolei Fan, Qiaoquan Liu, and Changquan Zhang. 2021. "The Physicochemical Properties of Starch Are Affected by Wxlv in Indica Rice" Foods 10, no. 12: 3089. https://doi.org/10.3390/foods10123089







