Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Tea Sample
2.2. PEF Treatment
2.3. IPL Treatment
2.4. SWE Treatment
2.5. High-Performance Liquid Chromatography
2.6. Data Analysis
2.7. Environmental Scanning Electron Microscopy (ESEM)
3. Results
3.1. HPLC Analysis of Tea Catechins
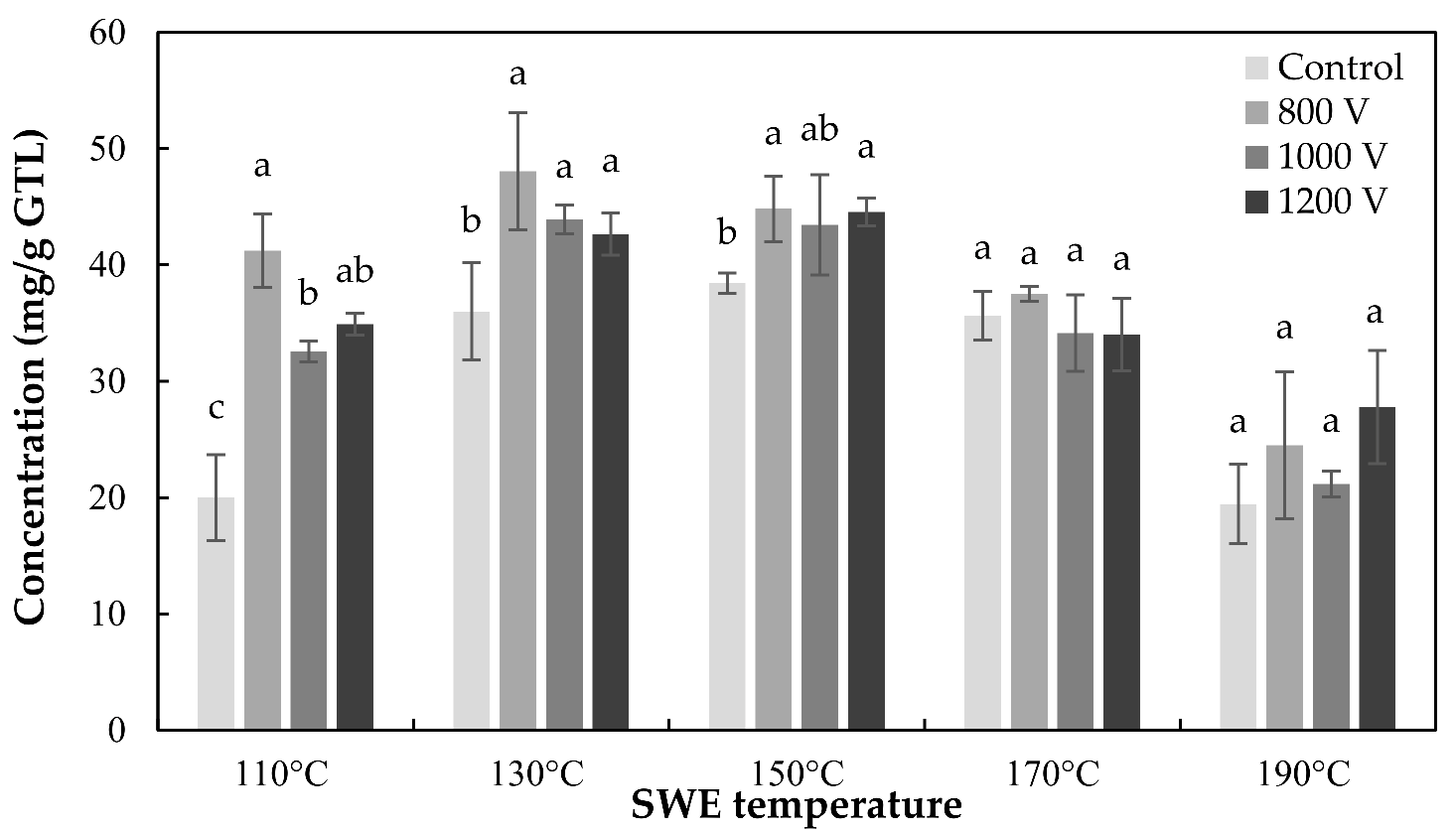
3.2. Effects of the Combined Treatment of PEF and SWE
3.2.1. Effects of SWE
3.2.2. Effects of PEF
3.3. Effects of the Combined Treatment of IPL and SWE
3.3.1. Effects of SWE
3.3.2. Effects of IPL
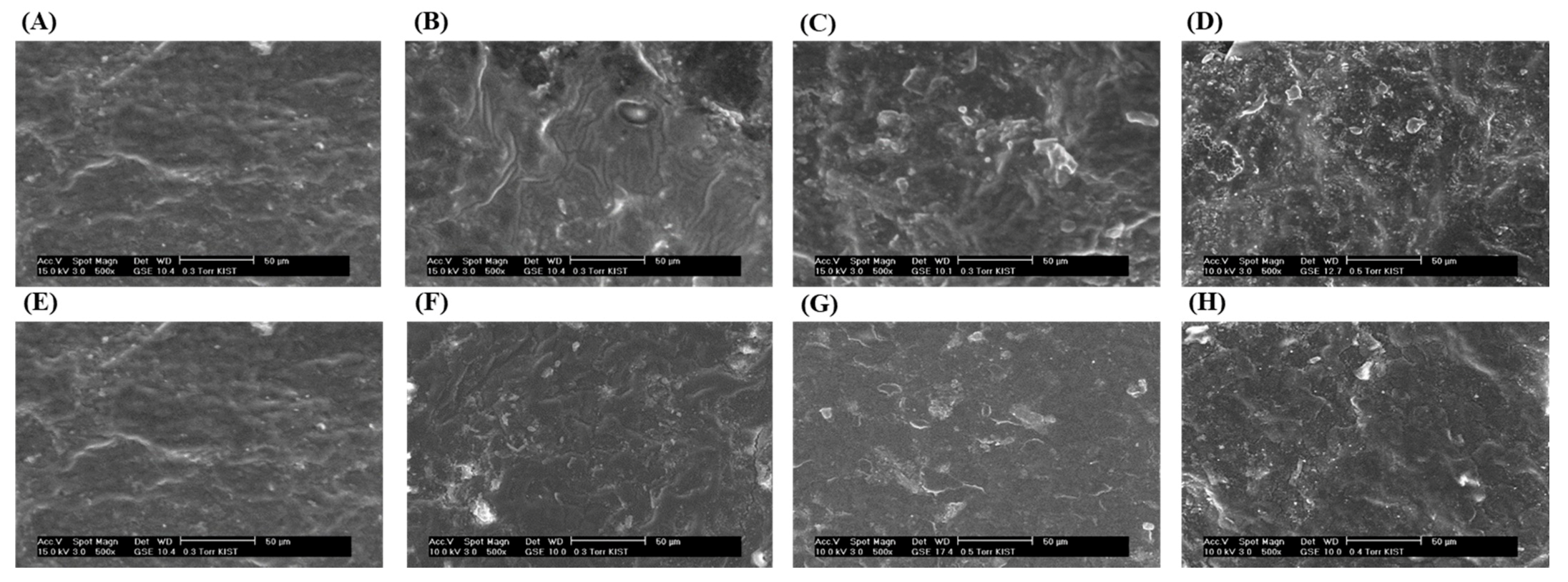
3.4. Surface Morphology Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollman, P.C.; Feskens, E.J.; Katan, M.B. Tea flavonols in cardiovascular disease and cancer epidemiology. Proc. Soc. Exp. Biol. Med. 1999, 220, 198–202. [Google Scholar] [PubMed] [Green Version]
- Stensvold, I.; Tverdal, A.; Solvoll, K.; Foss, O.P. Tea consumption. Relationship to cholesterol, blood pressure, and coronary and total mortality. Prev. Med. 1992, 21, 546–553. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; He, R.; Cheng, S.; Wenjuan, X. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989, 14, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. Process Intensif. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food. Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food. Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Reygaert, W.C. An update on the health benefits of green tea. Beverages 2013, 3, 6. [Google Scholar] [CrossRef]
- Subramani, C.; Natesh, R.K. Molecular mechanisms and biological implications of green tea polyphenol,(-)-epigallocatechin-3-gallate. Int. J. Pharma Bio Sci. Technol. 2013, 1, 54–63. [Google Scholar]
- Khokhar, S.; Magnusdottir, S. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food. Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Optimization of subcritical water extraction of flavanols from green tea leaves. J. Agric. Food. Chem. 2014, 62, 6828–6833. [Google Scholar] [CrossRef]
- King, J.W. Development and potential of critical fluid technology in the nutraceutical industry. In Supercritical Fluid Technology for Drug Product Development; Peter, Y., Uday, B.K., Boris, Y.S., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 579–614. [Google Scholar]
- Budrat, P.; Shotipruk, A. Enhanced recovery of phenolic compounds from bitter melon (Momordica charantia) by subcritical water extraction. Sep. Purif. Technol. 2009, 66, 125–129. [Google Scholar] [CrossRef]
- García, V.; Godoy, P.; Daniels, C.; Hurtado, A.; Ramos, J.L.; Segura, A. Functional analysis of new transporters involved in stress tolerance in Pseudomonas putida DOT-T1E. Environ. Microbiol. Rep. 2010, 2, 389–395. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Validation of green-solvent extraction combined with chromatographic chemical fingerprint to evaluate quality of Stevia rebaudiana Bertoni. J. Sep. Sci. 2009, 32, 613–622. [Google Scholar] [CrossRef]
- Miller, D.J.; Hawthorne, S.B.; Gizir, A.M.; Clifford, A.A. Solubility of polycyclic aromatic hydrocarbons in subcritical water from 298 K to 498 K. J. Chem. Eng. Data. 1998, 43, 1043–1047. [Google Scholar] [CrossRef]
- Ibanez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, G.; Hawthorne, S.B. Subcritical water extraction of antioxidant compounds from rosemary plants. J. Agric. Food. Chem. 2003, 51, 375–382. [Google Scholar] [CrossRef]
- Ko, M.J.; Cheigh, C.I.; Cho, S.W.; Chung, M.S. Subcritical water extraction of flavonol quercetin from onion skin. J. Food Eng. 2011, 102, 327–333. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Fincan, M.; DeVito, F.; Dejmek, P. Pulsed electric field treatment for solid–liquid extraction of red beetroot pigment. J. Food Eng. 2004, 64, 381–388. [Google Scholar] [CrossRef]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crops Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- Takaki, K.; Hatayama, H.; Koide, S.; Kawamura, Y. Improvement of polyphenol extraction from grape skin by pulse electric field. In Proceedings of the 2011 IEEE Pulsed Power Conference, Chicago, IL, USA, 19–23 June 2011; pp. 1262–1265. [Google Scholar]
- Zderic, A.; Zondervan, E.; Meuldijk, J. Breakage of cellular tissue by pulsed electric field: Extraction of polyphenols from fresh tea leaves. Chem. Eng. 2013, 32, 1795–1800. [Google Scholar]
- Mandal, R.; Mohammadi, X.; Wiktor, A.; Singh, A.; Anubhav, P.S. Applications of Pulsed Light Decontamination Technology in Food Processing: An Overview. Appl. Sci. 2020, 10, 3606. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Impact of pulsed light treatments on quality characteristics and oxidative stability of freshcut avocado. LWT Food Sci. Technol. 2014, 59, 320–326. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Charles, F.; Renard, C.M.; Page, D.; Carlin, F. Pulsed light effects on surface decontamination, physical qualities and nutritional composition of tomato fruit. Postharvest Biol. Technol. 2013, 86, 29–36. [Google Scholar] [CrossRef]
- Pereira, R.N.; Vicente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Bazhal, M.; Lebovka, N.; Vorobiev, E. Optimisation of pulsed electric field strength for electroplasmolysis of vegetable tissues. Biosyst. Eng. 2003, 86, 339–345. [Google Scholar] [CrossRef]
- Jo, H.L.; Hwang, H.J.; Chung, M.S. Inactivation of Bacillus subtilis spores at various germination and outgrowth stages using intense pulsed light. Food Microbiol. 2019, 82, 409–415. [Google Scholar] [CrossRef]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Miklavčič, D. In Vitro electroporation detection methods–An overview. Bioelectrochem. 2018, 120, 166–182. [Google Scholar] [CrossRef]
- Hossain, M.B.; Aguiló-Aguayo, I.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Effect of pulsed electric field and pulsed light pre-treatment on the extraction of steroidal alkaloids from potato peels. Innovative Food Sci. Emerg. Technol. 2015, 29, 9–14. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innovative Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.P.; Bruins, M.E. Pulsed electric field as an alternative pre-treatment for drying to enhance polyphenol extraction from fresh tea leaves. Food Bioprocess. Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, V.S.; Dou, J.; Chen, R.J.; Lin, R.S.; Lee, M.R.; Tzen, J.T. Massive accumulation of gallic acid and unique occurrence of myricetin, quercetin, and kaempferol in preparing old oolong tea. J. Agric. Food. Chem. 2008, 56, 7950–7956. [Google Scholar] [CrossRef]
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Jun, X.; Shuo, Z.; Bingbing, L.; Rui, Z.; Ye, L.; Deji, S.; Guofeng, Z. Separation of major catechins from green tea by ultrahigh pressure extraction. Int. J. Pharm. 2010, 386, 229–231. [Google Scholar] [CrossRef]
- Jun, X.; Deji, S.; Ye, L.; Rui, Z. Micromechanism of ultrahigh pressure extraction of active ingredients from green tea leaves. Food Control 2011, 22, 1473–1476. [Google Scholar] [CrossRef]
- Agüero, M.V.; Jagus, R.J.; Martín-Belloso, O.; Soliva-Fortuny, R. Surface decontamination of spinach by intense pulsed light treatments: Impact on quality attributes. Postharvest Biol. Technol. 2016, 121, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.M.; Silva, E.O.; Canuto, K.M.; Silva, L.M.; Gallão, M.I.; Urban, L.; Ayala-Zavala, J.F.; Miranda, M.R.A. Low fluence pulsed light enhanced phytochemical content and antioxidant potential of ‘Tommy Atkins’ mango peel and pulp. Innov. Food Sci. Emerg. Technol. 2016, 33, 216–224. [Google Scholar] [CrossRef]
- Pataro, G.; Sinik, M.; Capitoli, M.M.; Donsì, G.; Ferrari, G. The influence of post-harvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage. Innov. Food Sci. Emerg. Technol. 2015, 30, 103–111. [Google Scholar] [CrossRef]
- Hong, G.; Wang, J.; Zhang, Y.; Hochstetter, D.; Zhang, S.; Pan, Y.; Shi, Y.; Xu, P.; Wang, Y. Biosynthesis of catechin components is differentially regulated in dark-treated tea (Camellia sinensis L.). Plant. Physiol. Biochem. 2014, 78, 49–52. [Google Scholar] [CrossRef]
- Llano, K.R.A.; Marsellés-Fontanet, A.R.; Martín-Belloso, O.; Soliva-Fortuny, R. Impact of pulsed light treatments on antioxidant characteristics and quality attributes of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2016, 33, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Cleaner Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-J.; Kim, Y.-G.; Chung, M.-S. Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment. Foods 2021, 10, 3092. https://doi.org/10.3390/foods10123092
Hwang H-J, Kim Y-G, Chung M-S. Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment. Foods. 2021; 10(12):3092. https://doi.org/10.3390/foods10123092
Chicago/Turabian StyleHwang, Hee-Jeong, Yu-Gyeong Kim, and Myong-Soo Chung. 2021. "Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment" Foods 10, no. 12: 3092. https://doi.org/10.3390/foods10123092





